Treatment of Clozapine-Induced Hypersalivation With Ipratropium Bromide: A Randomized, Double-Blind, Placebo-Controlled Crossover Study
Objective: Clozapine-induced hypersalivation (CIH) occurs in up to 57% of treated patients and can be the source of considerable subjective distress. Previous open-label studies suggest that sublingual ipratropium bromide may be effective in treating CIH.
Method: We conducted a randomized, double-blind, placebo-controlled crossover trial to evaluate the efficacy of ipratropium in 20 individuals with CIH between September 2006 and August 2007. This study was 5 to 6 weeks in duration, based on the participants’ clozapine blood-monitoring schedule, and it consisted of two 2-week crossover phases separated by a 1- or 2-week washout period. Primary outcome measures included the reduction in the Toronto Nocturnal Hypersalivation Scale (TNHS) and the Clinical Global Impressions-Severity of Illness (CGI-S) and -Improvement (CGI-I) scales. Secondary outcomes included visual analog scales assessing hypersalivation severity (VAS-S) and distress (VAS-D).
Results: No significant reduction in CIH was found on the TNHS (P = .379), CGI-S (P = .266), or CGI-I (P = .599). Moreover, no difference was noted between study groups on the VAS-S (P = .969) and VAS-D (P = .527). There was no difference in the number of CIH responders at the conclusion of the 2-week placebo (40%, n = 8) and ipratropium (45%, n = 9) study phases (45%, n = 9) according to the TNHS. Randomization order did not have a significant effect on TNHS, CGI-S, or CGI-I scores. Tolerability was comparable between groups, with dry mouth occurring in 1 placebo group subject and 2 ipratropium group subjects.
Conclusions: Despite the reports of some preliminary studies that ipratropium is an efficacious treatment for CIH, ipratropium failed to demonstrate significant clinical effect in comparison to placebo. Further research should explore the efficacy of other locally acting anticholinergic agents or other classes of medications.
Trial Registration: clinicaltrials.gov Identifier: NCT00381589
J Clin Psychiatry 2009;70(8):1114-1119
© Copyright 2009 Physicians Postgraduate Press, Inc.
Submitted: June 22, 2008; accepted September 10, 2008 (doi:10.4088/JCP.08m04495).
Corresponding author: Sanjeev Sockalingam, MD, University Health Network, Toronto General Hospital 8EN-225, 200 Elizabeth St, Toronto, Ontario M5G 2C4, Canada ([email protected]).
Despite its efficacy in treatment-resistant schizophrenia, clozapine remains hindered by its problematic side effects.1-4 Hypersalivation, also called sialorrhea or ptyalism, represents one of clozapine’s more common adverse events, occurring in up to 57% of treated patients and primarily evident through the night.4,5 Studies examining the relationship between clozapine dose or plasma level and hypersalivation are inconclusive.5,6 Clozapine-induced hypersalivation (CIH) has been linked to both medical and psychological consequences. Severe hypersalivation can result in aspiration pneumonia, swelling of salivary glands, parotitis, and skin irritation.7-9 CIH can reduce tolerability and, in so doing, compromise adherence. An effective treatment for CIH would be welcomed by both patients and clinicians trying to provide relief from this troublesome side effect.
Clozapine acts as an antagonist at dopaminergic (D1-D5), serotoninergic (5-HT2, 5-HT4), histaminergic (H1), adrenergic (α1 and α2) and muscarinic receptors (M1-M3, M5), with agonistic properties at the M4 muscarinic receptor.10 Its dual antagonistic and agonistic properties are believed to cause its paradoxical hypersalivation effect. This theory is supported by reports of olanzapine-induced hypersalivation because of its M4 agonism and attenuation of CIH following administration of pirenzepine, an M4 muscarinic receptor antagonist.11,12 Moreover, such factors as disrupted deglutition, α2-antagonism and increased saliva flow rates have been implicated as potential mechanisms of CIH on the basis of preliminary studies.13-15
Currently, nonpharmacologic and pharmacologic treatments of CIH are limited. Behavioral strategies, such as placing a towel on one’s pillow and replacing bed sheets, can be troublesome, and they yield minimal improvement in quality of life. Moreover, the efficacy of pharmacologic treatments of CIH is based predominantly on case series and small uncontrolled trials.16,17 Much of the evidence has focused on anticholinergic agents, namely benztropine, glycopyrrolate, pirenzepine, trihexyphenidyl, scopolamine, amitriptyline, biperiden, atropine eye drops, and ipratropium bromide.12,18-31 Other CIH treatments have included α2-adrenergic agents, such as clonidine, guanfacine, and lofexidine, and antipsychotic agents, such as amisulpride and sulpiride.32-36 These agents were found to be beneficial and well tolerated in small open-label studies and case series. Only amisulpride and pirenzepine have been studied in double-blind, randomized, controlled cross-over trials.15,35 These trials demonstrated a significant reduction in nocturnal CIH scores with amisulpride but not pirenzepine in comparison to placebo. Furthermore, several agents, including trihexyphenidyl and benztropine, are limited by the risk of additive systemic anticholinergic effects and can result in severe complications, such as colon perforation secondary to obstruction.37
Although much of the literature has focused on systemic anticholinergic drugs in treating CIH, locally acting agents offer advantages related to their more favorable side effect profiles. Atropine possesses such features, but it is limited by complicated administration (rinsing the oral cavity with the solution), which may reduce efficacy in this patient population. Ipratropium has shown benefit in small open-label and case series studies, with minimal side effects and easy intranasal or sublingual administration.19,22,28 It is structurally similar to atropine, and its mechanism of action is nonspecific muscarinic receptor blockade with minimal nicotinic receptor activity. Furthermore, ipratropium has minimal central nervous system penetration and has less than 10% systemic absorption when administered intranasally.38 In contrast, atropine is rapidly absorbed by the gastrointestinal tract and crosses the blood-brain barrier, thus increasing its propensity for systemic and central nervous system adverse effects. The ipratropium nasal spray is manufactured in a 0.03% and 0.06% form, has a 1.6 hour terminal half-life, and is approved for the treatment of perennial allergic rhinitis and rhinorrhea. Studies using ipratropium for treating CIH have used between 2 to 4 sprays per day of both concentrations and have demonstrated benefit.19,22,28 Two case series involving 9 and 10 patients demonstrated a clinically relevant reduction in CIH in 7 and 10 patients, respectively.22,28 Calderon and colleagues19 reported an improvement in a clinician-rated hypersalivation scale in 60% of patients. Given these favorable results in uncontrolled studies, ipratropium has been purported to be an effective treatment alternative for CIH.
However, no randomized controlled trials have been conducted evaluating the efficacy of ipratropium in the treatment of CIH. To determine the efficacy of ipratropium in the management of nocturnal CIH, we undertook a randomized, controlled, double-blind, crossover trial. Congruent with previous uncontrolled studies, we hypothesized that ipratropium nasal spray, used sublingually, would demonstrate benefit in reducing CIH in clozapine-treated patients.
METHOD
Participants
The trial was conducted from September 1, 2006, to August 1, 2007, at the Centre for Addiction and Mental Health (CAMH) in Toronto, Ontario, Canada. Twenty patients followed by the CAMH Schizophrenia Program were enrolled in the study, with 19 subjects being treated as outpatients and 1 subject as an inpatient. Institutional review board approval was obtained from CAMH and the University of Toronto. Written consent was obtained from subjects once competency to consent to the study was established using the MacArthur Competence Assessment Tool for Clinical Research.39
Participants were included if they (1) were between the ages of 18 and 65 years, (2) met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision, criteria for schizophrenia or schizoaffective disorder, (3) had been treated with clozapine for a minimum of 60 days, (4) had had no change in the dose of clozapine and other concomitant medications during the previous 14 days, (5) were experiencing at least moderate hypersalivation (defined by a score of 2 or greater on the Toronto Nocturnal Hypersalivation Scale [TNHS]), and (6) were able to provide voluntary, informed consent. Study exclusion criteria included (1) the presence of concurrent medical conditions contributing to hypersalivation (eg, idiopathic Parkinson’s disease, cerebral palsy); (2) a history of narrow-angle glaucoma, prostatic hypertrophy, or bladder obstruction; and (3) a previous ipratropium treatment trial. Individuals who had been treated with anticholinergic agents and continued to experience CIH were allowed to participate in the study provided they continued to suffer from CIH of at least moderate severity. No changes in clozapine dose were permitted during the course of the study.
Medication
The study employed a fixed dose of 2 sprays of ipratropium 0.03% sublingually at bedtime. The 0.03% ipratropium concentration was selected on the basis of previous studies demonstrating efficacy and the reduced likelihood of side effects at this concentration. Both placebo and ipratropium sprays involved identical canisters, and they were prepared by a provincial accredited pharmacy. The placebo spray solution consisted of a normal saline solution with an identical appearance to the ipratropium solution.
Study Design
The study was a randomized, double-blind, placebo-controlled, crossover, fixed-dose study of ipratropium. The duration of the trial was 5 or 6 weeks for subjects on weekly or biweekly clozapine blood monitoring, respectively. Study subjects were randomly assigned to ipratropium or placebo for 2 weeks following their baseline assessment and crossed over to the alternate study arm following either a 1- or 2-week washout period corresponding to their clozapine monitoring frequency. At the start of each 2-week period, subjects were taught how to correctly self-administer the spray sublingually, and a study investigator (S.S.) observed their spray technique prior to dispensing the study spray. Moreover, subjects were given a treatment adherence record both to serve as a reminder to use the correct spray dose and to monitor their study adherence. Subjects reporting less than 80% compliance on their daily dose monitoring logs were excluded from the final data analysis.
The screening and baseline assessment included a medical and psychiatric history, a review of the medical chart, and assessment of baseline hypersalivation using the TNHS and the Clinical Global Impressions-Severity of Illness scale (CGI-S)40 for hypersalivation.
Efficacy Measures
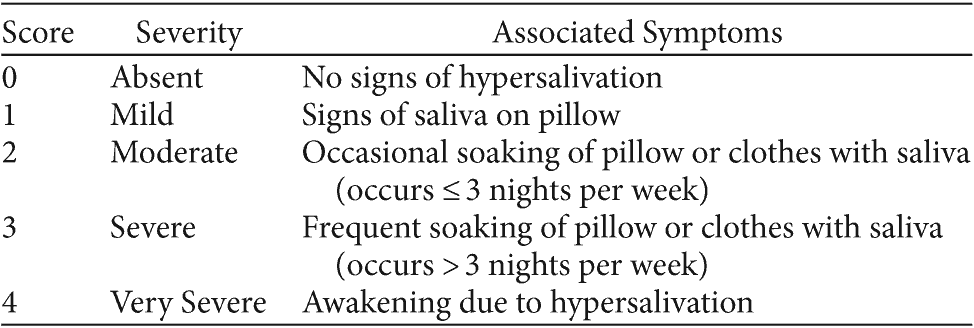
Table 1. Toronto Nocturnal Hypersalivation Scale
© Copyright 2009 Sanjeev Sockalingam, Chekkera Shammi, and Gary Remington, Toronto, Ontario, Canada. Reproduced with permission.
Click figure to enlarge
Hypersalivation study measures employed at baseline and 2-week visits for each phase included the TNHS, the CGI-S40 for hypersalivation, the CGI-Improvement scale (CGI-I)40 for hypersalivation, the Simpson-Angus Scale (SAS),41 a visual analog scale for hypersalivation severity (VAS-S),42 and a visual analog scale for hypersalivation distress (VAS-D).42 The TNHS is a composite version of 2 studied hypersalivation scales, the Drooling Severity Scale (DSS)43 and the Nocturnal Hypersalivation Rating Scale (NHRS),27,35 which have been used in several studies to evaluate hypersalivation (Table 1).27,35,43 The TNHS builds on the NHRS and DSS by incorporating defined anchors for hypersalivation frequency and severity from each of these scales. The CGI-S and CGI-I measured the severity and improvement of hypersalivation, respectively, over the course of each 2-week study arm.
The primary study outcomes were the difference in the TNHS, CI CGI-S, and CGI-I hypersalivation scores following the 2-week exposure to ipratropium and placebo. The secondary outcome measures included the VAS-D and VAS-S scores at the conclusion of each 2-week study arm. The SAS was used to account for antipsychotic-induced parkinsonism, which could confound hypersalivation measures. In addition, the SAS includes one question assessing hypersalivation and measures daytime hypersalivation exclusively.
Adverse effects in each crossover phase were evaluated using the CGI-Side Effect scale.40 Clozapine and norclozapine levels were recorded at the conclusion of each 2-week study period to account for differences in clozapine compliance and for potential drug interactions.
Statistics
The study was designed to have sufficient statistical power (≥ 80%) to detect a difference of 1.02 points on the TNHS between treatment and control groups, assuming a standard deviation of 1.10 and a 2-tailed significance level of 0.05. This power calculation resulted in a sample size of 20 subjects (10 per group), which corresponds to the sample size utilized in the only other published randomized, controlled crossover study evaluating the treatment of CIH with amisulpride.35
Descriptive statistics are given as means ± SD and comparison of qualitative data used the Pearson χ2 test. Paired sample t tests were used to determine statistical significance of the change in hypersalivation scores for the ipratropium and placebo study phases. The statistical significance of randomization order on the differences in hypersalivation scores between the groups was determined using an independent sample t test. For all t tests, significance was determined by a P value < .05. Pearson correlations were computed to determine the relationship between clozapine and norclozapine levels and hypersalivation as per the TNHS. All statistical tests were performed using SPSS software version 15.0 (SPSS Inc, Chicago, Illinois).
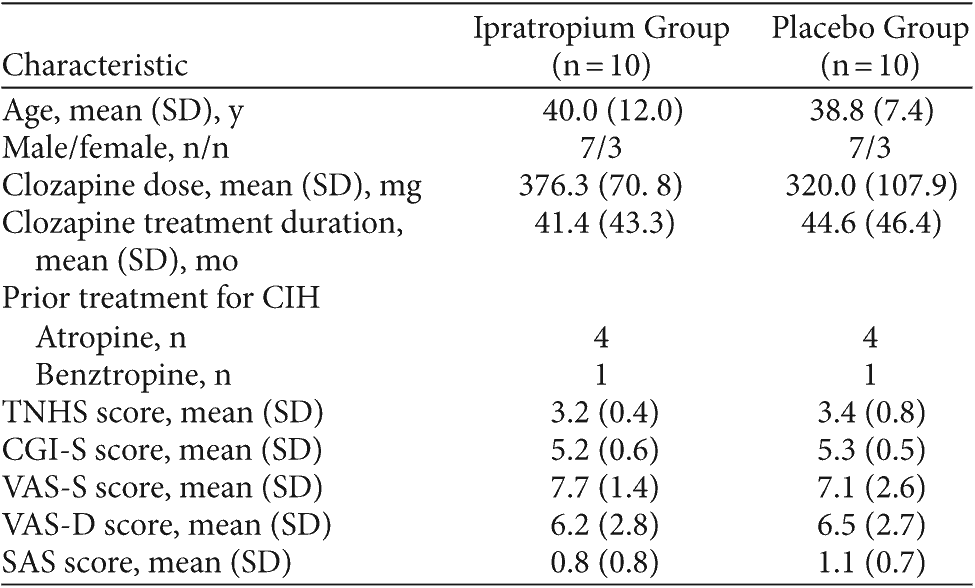
Table 2. Baseline Demographic and Clinical Characteristics of Subjects Treated With Ipratropium and Placebo for Clozapine-Induced Hypersalivation (CIH)
Abbreviations: CGI-S = Clinical Global Impressions-Severity of Illness scale, CIH = clozapine-induced hypersalivation, SAS = Simpson-Angus Scale, TNHS = Toronto Nocturnal Hypersalivation Scale, VAS-D = visual analog scale for distress, VAS-S = visual analog scale for severity.
Click figure to enlarge
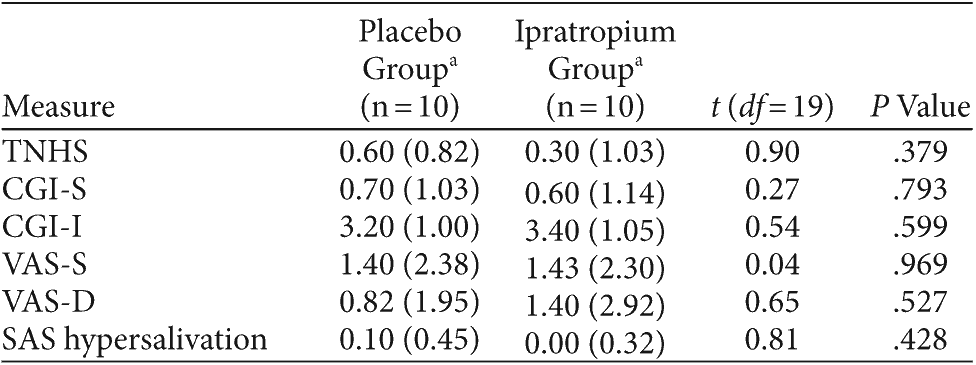
Table 3. Reduction in Hypersalivation Measures From Baseline in Subjects Treated With Ipratropium and Placebo for Clozapine-Induced Hypersalivation
aValues presented as mean (SD).
Abbreviations: CGI-I = Clinical Global Impressions-Improvement scale, CGI-S = Clinical Global Impressions-Severity of Illness scale, SAS = Simpson-Angus Scale, TNHS = Toronto Nocturnal Hypersalivation Scale, VAS-D = visual analog scale for distress, VAS-S = visual analog scale for severity.
Click figure to enlarge
RESULTS
Subject Characteristics
Between September 2006 and June 2007, 21 patients were screened, and 20 subjects were included in the study. All 20 subjects completed the study; demographic data are summarized in Table 2.
Efficacy
There was no significant difference in TNHS scores between placebo and ipratropium groups after 2 weeks (P = .379). The TNHS findings were consistent with CGI-S (P = .266) and CGI-I (P = .599) scores, which also failed to demonstrate a treatment effect with ipratropium. Overall, there was no significant association between the group allocation and the number of responders, as defined by a 1-point reduction in the TNHS (ipratropium = 45%, placebo = 40%; χ2 = 0.10, df = 1, P = .749) or CGI-I (placebo = 45%, ipratropium = 40%; χ2 = 0.10, df = 1, P = .749).
The results of the VAS-S, VAS-D, and the SAS hypersalivation scores are summarized in Table 3. There was no significant difference in subject reports of hypersalivation severity and distress on the VAS-S (P = .969) and VAS-D (P = .527), respectively, between the 2 groups. With regard to daytime CIH, the mean SAS subscore for hypersalivation in the placebo and ipratropium groups did not differ significantly over the 2-week period (P = .428). Total SAS scores were identical to the SAS hypersalivation scores, as no subjects scored on the remaining SAS items.
Independent t tests to determine the effect of randomization order on CIH study measures indicated no significant effect on TNHS (P = .240), CGI-S (P = .300), or CGI-I (P = .438) scores.
Safety and Tolerability
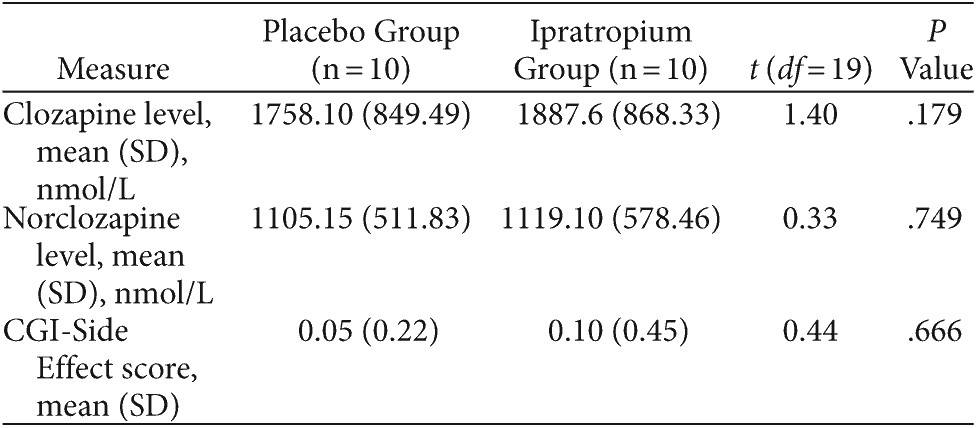
Table 4 summarizes subjects’ serum clozapine/norclozapine levels and reported side effects as per the CGI-Side Effect scale. No subjects withdrew over the 2 study phases. Ipratropium was well tolerated, and the placebo and ipratropium groups did not differ in terms of adverse effects (P = .666). One subject in the placebo group and 2 subjects in the ipratropium group experienced dry mouth. Subjects reported no other adverse effects. There were no significant differences in serum clozapine (P = .179) and norclozapine (P = .749) levels at the conclusion of each 2-week study phase. Furthermore, 2-week TNHS scores were not significantly correlated with clozapine or norclozapine levels in the placebo (Pearson value = −0.01, P = .969 and Pearson value = −0.20, P = .453, respectively) and ipratropium (Pearson value = −0.15, P = .570 and Pearson value = −0.18, P = .494, respectively) study arms.
DISCUSSION
CIH remains a common and problematic side effect of clozapine treatment. Initial open-label, uncontrolled studies and case series suggested that ipratropium could be a nascent treatment alternative for this condition; however, our results, which represent the only controlled trial investigating ipratropium’s value in this regard, failed to substantiate these findings The lack of effect was consistent across multiple hypersalivation measures, including the TNHS, CGI-S, CGI-I, VAS-S, and VAS-D. Moreover, the percentage of responders as denoted by at least a 1-point reduction in the TNHS was not markedly different between the placebo and ipratropium treatment phases.
The present study represents the first randomized, double-blind, placebo-controlled crossover study to evaluate efficacy of ipratropium in the treatment of CIH, and its negative findings are in line with studies of other CIH treatment agents, for example pirenzepine, where benefits in open-label, uncontrolled trials were not replicated in a trial employing more rigorous methodology.12,18
Potential study limitations cannot be ignored. Despite our study design’s being adequately powered to detect a 1-point difference in the TNHS scale, it is possible that a larger reduction in the TNHS is needed to determine a clinical effect using self-report measures, warranting a larger sample size. Furthermore, we did not utilize objective measures of hypersalivation, such as measurement of the wet area on a pillow using tissue paper, unstimulated or stimulated saliva collection, or collection with a Salivette. Although measurement of CIH can be enhanced with direct saliva collection, these measurements represent daytime hypersalivation, which is less common than nocturnal hypersalivation during clozapine treatment. Moreover, calculation of saliva area on pillows with wax paper or measurement of pillow cases is associated with its own limitations, including the need for closely monitored study settings (ie, inpatient units) to collect daily pillow measurements and disruption of sleep behavior or positioning, resulting in poor distribution of saliva on the pillow. In this study, we used multiple measures of CIH to strengthen the validity of our qualitative measurements. Evaluation included clinician-rated scales such as the TNHS and CGI, as well as self-report measures such as the VAS. The TNHS incorporated fixed anchors and was based on previously used measures of hypersalivation in other research studies.
In addition, subjects did not undergo directly supervised administration of the study treatments and may have been vulnerable to noncompliance or improper administration of the study treatments. Subjects were trained on how to administer the ipratropium and placebo sprays at the start of each 2-week study phase. In addition, study subjects were compliant with clozapine treatment according to their serum clozapine levels despite their hypersalivation; it was our sense that they would be more likely to be compliant with a study medication that could potentially alleviate the distressing symptoms of hypersalivation. Although we chose to study the efficacy of the 0.03% ipratropium spray, one retrospective study reported benefit and minimal side effects with ipratropium 0.06%, 2 sprays up to 3 times daily.22 It is therefore possible that our study could be limited by its conservative dosing strategy. It is of note, though, that favorable results were reported in a published case series and uncontrolled trial evaluating the efficacy of ipratropium in CIH using the ipratropium concentration and dosing schedule equivalent to those in our study.19,28 Further research determining the clinical significance of varying ipratropium spray concentrations and dosing strategies is warranted. Lastly, there was a high placebo effect in our study, with 40% of subjects experiencing at least a 1-point reduction in TNHS, potentially limiting our ability to identify an ipratropium treatment response.
In summary, our study has 2 salient clinical implications. First, although ipratropium is a well-tolerated drug and has shown some benefit in reducing CIH in less rigorous studies, our study did not confirm its utility in treating CIH. Second, the TNHS had comparable utility to other self-report and clinician-rated measures in our study and had, as an advantage, clearly defined symptom anchors. With its brevity and ease of administration, the TNHS could be a useful assessment tool for CIH; however, future studies comparing the TNHS with the NHRS and other objective hypersalivation measures are needed to determine the psychometric properties of the TNHS in various patient populations.
Our study findings highlight the difficulty in treating CIH. Hypersalivation remains a challenging burden to patients treated with clozapine, and ongoing efforts to identify effective treatments for CIH must continue. Future randomized controlled trials will be necessary to determine the efficacy of other locally acting anticholinergic agents, such as atropine, or novel classes of medications that appear promising based on anecdotal evidence. In the interim, behavioral interventions (eg, using a towel on one’s pillow for nocturnal hypersalivation and chewing gum for diurnal hypersalivation) offer strategies that can be used in conjunction with existing pharmacologic approaches.
Drug names: atropine (Atropen and others), benztropine (Cogentin and others), biperiden (Akineton), clonidine (Catapres, Duraclon, and others), clozapine (FazaClo, Clozaril, and others), glycopyrrolate (Robinule and others), guanfacine (Tenex and others), ipratropium (Atrovent and others), olanzapine (Zyprexa), scopolamine (Transderm Scop).
Author affiliations: Program in Medical Psychiatry, University Health Network (Dr Sockalingam); Centre for Addiction and Mental Health (Dr Shammi); Schizophrenia Program (Drs Shammi and Remington), Department of Psychiatry, University of Toronto (Drs Sockalingam, Shammi, and Remington); and Medication Assessment Program for Schizophrenia (Dr Remington), University of Toronto, Toronto, Ontario, Canada.
Financial disclosure: Dr Shammi has received grant/research support from Novartis and is a member of the speakers/advisory boards for Novartis and AstraZeneca. Dr Remington has received grant/research support from Novartis and Merck Germany. Dr Sockalingam reports no financial or other relationships relevant to the subject of this article.
Funding/support: This study was funded in part by Novartis Canada.
REFERENCES
1. Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789-796. PubMed
2. McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600-610. PubMed doi:10.1176/appi.ajp.163.4.600
3. Honigfeld G. Effects of the clozapine national registry system on incidence of deaths related to agranulocytosis. Psychiatr Serv. 1996;47(1):52-56. PubMed
4. Safferman A, Lieberman JA, Kane JM, et al. Update on the clinical efficacy and side effects of clozapine. Schizophr Bull. 1991;17(2):247-261. PubMed
5. Yusufi B, Mukherjee S, Flanagan R, et al. Prevalence and nature of side effects during clozapine maintenance treatment and the relationship with clozapine dose and plasma concentration. Int Clin Psychopharmacol. 2007;22(4):238-243. PubMed doi:10.1097/YIC.0b013e32819f8f17
6. Khan AY, Preskorn SH. Examining concentration-dependent toxicity of clozapine: role of therapeutic drug monitoring. J Psychiatr Pract. 2005;11(5):289-301. PubMed doi:10.1097/00131746-200509000-00003
7. Hinkes R, Quesada T, Currier MB, et al. Aspiration pneumonia possibly secondary to clozapine-induced sialorrhea. J Clin Psychopharmacol. 1996;16(6):462-463. PubMed doi:10.1097/00004714-199612000-00013
8. McCarthy RH, Terkelsen KG. Esophageal dysfunction in two patients after clozapine treatment. J Clin Psychopharmacol. 1994;14(4):281-283. PubMed doi:10.1097/00004714-199408000-00012
9. Pearlman C. Clozapine, nocturnal sialorrhea, and choking. (letter) J Clin Psychopharmacol. 1994;14(4):283. PubMed doi:10.1097/00004714-199408000-00013
10. Zorn SH, Jones SB, Ward KM, et al. Clozapine is a potent and selective muscarinic M4 receptor agonist. Eur J Pharmacol. 1994;269(3):R1-R2. PubMed doi:10.1016/0922-4106(94)90047-7
11. Perkins DO, McClure RK. Hypersalivation coincident with olanzapine treatment. Am J Psychiatry. 1998;155:993-994. PubMed
12. Schneider B, Weigmann H, Hiemke C, et al. Reduction of clozapine-induced hypersalivation by pirenzepine is safe. Pharmacopsychiatry. 2004;37(2):43-45. PubMed doi:10.1055/s-2004-815523
13. Baldessarini RJ, Frankenburg FR. Clozapine: a novel antipsychotic agent. N Engl J Med. 1991;324(11):746-754. PubMed
14. Ben-Aryeh H, Jungerman T, Szargel R, et al. Salivary flow-rate and composition in schizophrenic patients on clozapine: subjective reports and laboratory data. Biol Psychiatry. 1996;39:946-949. PubMed doi:10.1016/0006-3223(95)00296-0
15. Berlan M, Montastruc JL, Lafontan M. Pharmacological prospects for alpha 2-adrenoceptor antagonist therapy. Trends Pharmacol Sci. 1992;13(7):277-282. PubMed doi:10.1016/0165-6147(92)90085-K
16. Sockalingam S, Shammi C, Remington G. Clozapine-induced hypersalivation: a review of treatment strategies. Can J Psychiatry. 2007;52(6):377-384. PubMed
17. Praharaj SK, Arora M, Gandotra S. Clozapine-induced sialorrhea: pathophysiology and management strategies. Psychopharmacology (Berl). 2006;185(3):265-273. PubMed doi:10.1007/s00213-005-0248-4
18. Bai YM, Lin CC, Chen JY, et al. Therapeutic effect of pirenzepine for clozapine-induced hypersalivation: a randomized, double-blind, placebo-controlled, cross-over study. J Clin Psychopharmacol. 2001;21(6):608-611. PubMed doi:10.1097/00004714-200112000-00012
19. Calderon J, Rubin E, Sobota WL. Potential use of ipratropium bromide for the treatment of clozapine-induced hypersalivation: a preliminary report. Int Clin Psychopharmacol. 2000;15:49-52. PubMed doi:10.1097/00004850-200015010-00008
20. Comley C, Galletly C, Ash D. Use of atropine eye drops for clozapine-induced hypersalivation. Aust N Z J Psychiatry. 2000;34:1033-1034. PubMed doi:10.1080/000486700285
21. Copp PJ, Lament R, Tennent TG. Amitriptyline in clozapine-induced sialorrhoea. Br J Psychiatry. 1991;159:166. PubMed doi:10.1192/bjp.159.1.166a
22. Freudenreich O, Beebe M, Goff DC. Clozapine-induced sialorrhea treated with sublingual ipratropium spray: a case series. J Clin Psychopharmacol. 2004;24(1):98-100. PubMed doi:10.1097/01.jcp.0000106228.36344.2e
23. Gaftanyuk O, Trestman RL. Scopolamine patch for clozapine-induced sialorrhea. Psychiatr Serv. 2004;55(3):318. PubMed doi:10.1176/appi.ps.55.3.318
24. McKane JP, Hall C, Akram G. Hyoscine patches in clozapine-induced hypersalivation. (letter) Psychiatr Bull. 2001;25:277. doi:10.1192/pb.25.7.277-c
25. Reinstein MJ, Sirotovskaya LA, Chasanov MA, et al. Comparative efficacy and tolerability of benzatropine and terazosin in the treatment of hypersalivation secondary to clozapine. Clin Drug Investig. 1999;17(2):97-102. doi:10.2165/00044011-199917020-00003
26. Sharma A, Ramaswamy S, Dahl E, et al. Intraoral application of atropine sulfate ophthalmic solution for clozapine-induced sialorrhea. Ann Pharmacother. 2004;38(9):1538. PubMed
27. Spivak B, Adlersberg S, Rosen L, et al. Trihexyphenidyl treatment of clozapine-induced hypersalivation. Int Clin Psychopharmacol. 1997;12(4):213-215. PubMed doi:10.1097/00004850-199707000-00005
28. Tessier P, Antonello C. Clozapine and sialorrhea: update. J Psychiatry Neurosci. 2001;26(3):253. PubMed
29. Robb AS, Lee RH, Cooper EB, et al. Glycopyrrolate for treatment of clozapine-induced sialorrhea in three adolescents. J Child Adolesc Psychopharmacol. 2008;18(1):99-107. PubMed doi:10.1089/cap.2007.0037
30. Richardson C, Kelly DL, Conley RR. Biperiden for excessive sweating from clozapine. Am J Psychiatry. 2001;158(8):1329-1330. PubMed doi:10.1176/appi.ajp.158.8.1329-a
31. Fritze J, Elliger T. Pirenzepine for clozapine-induced hypersalivation. Lancet. 1995;346:1034. PubMed doi:10.1016/S0140-6736(95)91713-6
32. Croissant B, Hermann D, Olbrich R. Reduction of side effects by combining clozapine with amisulpride: case report and short review of clozapine-induced hypersalivation: a case report. Pharmacopsychiatry. 2005;38:38-39. PubMed doi:10.1055/s-2005-837771
33. Grabowski J. Clonidine treatment of clozapine-induced hypersalivation. J Clin Psychopharmacol. 1992;12(1):69-70. PubMed doi:10.1097/00004714-199202000-00026
34. Kreinin A, Epshtein S, Sheinkman A, et al. Sulpride addition for the treatment of clozapine-induced hypersalivation: preliminary study. Isr J Psychiatry Relat Sci. 2005;42(1):61-63. PubMed
35. Kreinin A, Novitski D, Weizman A. Amisulpride treatment of clozapine-induced hypersalivation in schizophrenia patients: a randomized, double-blind, placebo-controlled cross-over study. Int Clin Psychopharmacol. 2006;21:99-103. PubMed doi:10.1097/01.yic.0000188216.92408.69
36. Praharaj SK, Verma P, Roy D, et al. Is clonidine useful for treatment of clozapine-induced sialorrhea. J Psychopharmacol. 2005;19(4):426-428. PubMed doi:10.1177/0269881105053311
37. Freudenreich O, Goff DC. Colon perforation and peritonitis associated with clozapine. J Clin Psychiatry. 2000;61:950-951. PubMed
38. Laurikainen E, Koulu M, Kaila T, et al. Evaluation of the systemic anticholinergic activity of nasally administered ipratropium bromide. Rhinology. 1988;26(2):133-138. PubMed
39. Appelbaum PS, Grisso T. MacArthur Competence Tool for Clinical Research. Sarasota, FL: Professional Resource Press; 2001.
40. Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised DHEW Publication (ADM). Rockville, MD: National Institute of Mental Health; 1976:218-222.
41. Simpson GM, Angus JW. A rating scale for extrapyramidal side effects. Acta Psychiatr Scand Suppl. 1970;212:11-19. PubMed doi:10.1111/j.1600-0447.1970.tb02066.x
42. Ondo WG, Hunter C, Moore W. A double-blind placebo-controlled trial of botulinum toxin B for sialorrhea in Parkinson’s disease. Neurology. 2004;62:37-40. PubMed
43. Suskind DL, Titon A. Clinical study of botulinum: a toxin in the treatment of sialorrhea with children with cerebral palsy. Laryngoscope. 2002;112:73-81. PubMed doi:10.1097/00005537-200201000-00014