One-Year Treatment Continuation in Patients Switched to Paliperidone Palmitate: A Retrospective Study
To the Editor: Paliperidone palmitate is a long-acting injectable (LAI) antipsychotic, approved for treatment of schizophrenia in adults.1 It is the major active metabolite of risperidone and, in its injectable form, has several potential advantages over other LAIs. It reaches steady state within 4 to 8 days, so patients are not required to take oral supplementation at initiation of therapy.2 It can be administered monthly2 and can be given up to 7 days before or after the usual date of administration, allowing flexibility to avoid missed doses.3
Given these advantages, clinicians may be inclined to switch patients from other LAIs to paliperidone palmitate. However, there are limited data on the outcome of patients switched to paliperidone palmitate over extended durations, with most studies following patients for 13 weeks or less.4-8 Of the 3 longer-term studies,9-11 2 excluded many of the patients seen in real-world practice (including involuntary patients, those with substance dependence, and those at risk of suicidal behavior).9,10 There is only 1 previously published naturalistic paliperidone palmitate switching study, and it demonstrated a discontinuation rate of 35% over 1 year.11
The aim of our study was to retrospectively follow 45 patients of a mental health service for 52 weeks after they were switched from their antipsychotic to paliperidone palmitate and to explore risk factors for discontinuing the treatment. Understanding these factors may help clinicians to more appropriately select patients for treatment with paliperidone palmitate.
Method. The study was performed at an area mental health service in Melbourne, Australia, that provides inpatient and outpatient services. Data were retrospectively collected from medical records of all patients started on paliperidone palmitate between December 2010 and April 2011. This time period was selected because it coincided with a program funded by Janssen-Cilag12 that subsidized paliperidone palmitate (for up to 2 years) for patients over 18 years of age and with a diagnosis of DSM-IV-TR schizophrenia. All doctors working in the mental health service could choose to prescribe paliperidone palmitate to eligible patients. At that time, paliperidone palmitate was not approved under the Pharmaceutical Benefits Scheme (PBS)—a government program that subsidizes medications—thus, patients would have otherwise had to pay full price.
There was no financial incentive to the service, clinicians, or patients for participating in the program,12 and the cost of paliperidone palmitate for patients was no different than the cost of their previous medications (as these were subsidized under PBS).
Data collected included demographics, treatment setting at the time of initiation of paliperidone palmitate, the patient’s mental state prior to switching to paliperidone palmitate, reason for switching, time until discontinuation, and reason for discontinuation. Ethics for this study was granted by the health service’s research ethics committee.
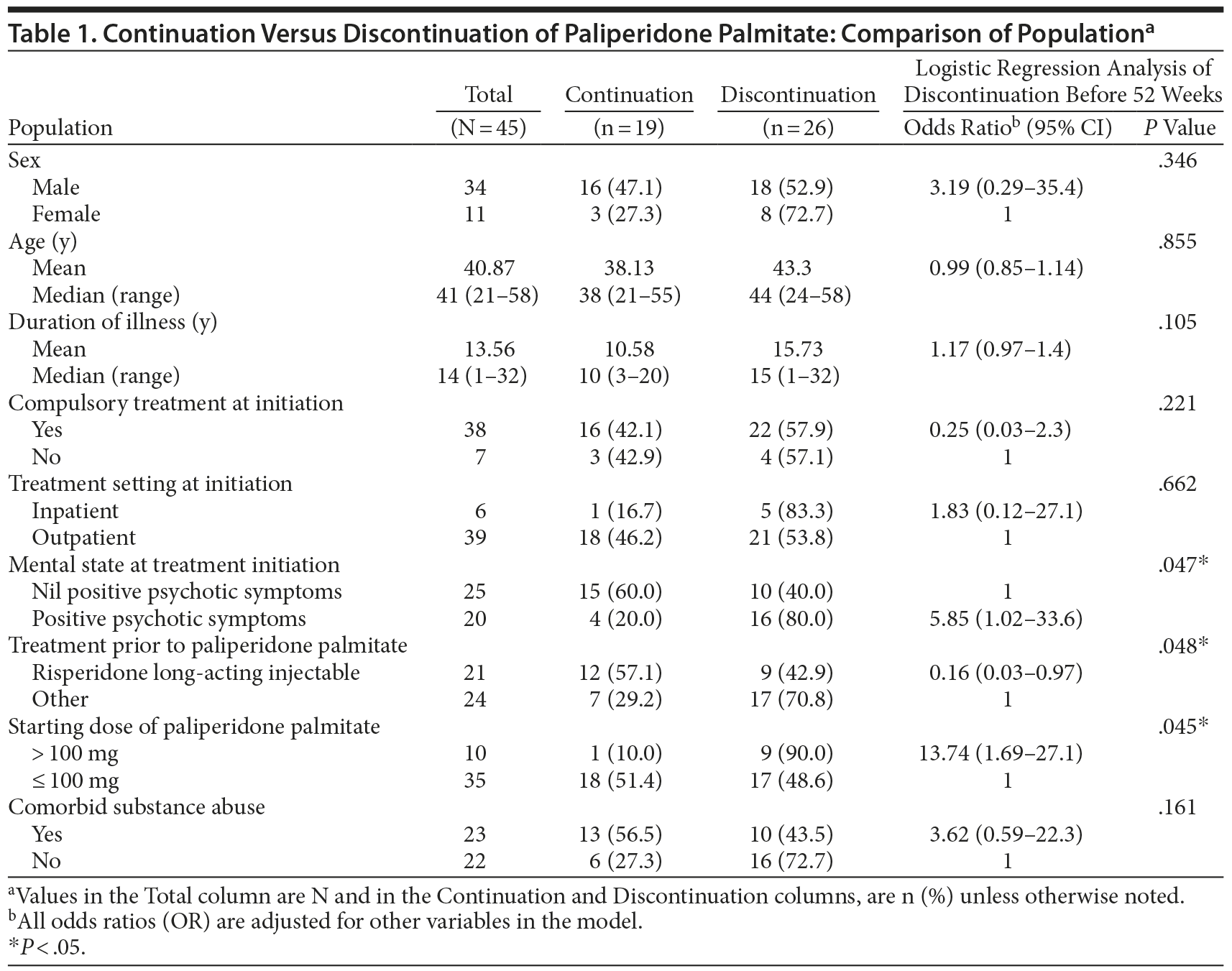
Results. Table 1 compares the demographic and clinical profile of patients who continued paliperidone palmitate for 52 weeks with those who discontinued treatment. Twenty-six patients (57.8%) discontinued treatment within the 52-week study period—3 due to adverse effects, 3 due to patient refusal, and 20 due to deterioration in their mental state (7 of whom required an acute admission). Of those who discontinued treatment prior to 52 weeks, 9 discontinued within 3 months, 7 discontinued between 3 and 6 months, and 10 discontinued between 6 and 12 months.
Logistic regression analysis found that discontinuation of paliperidone palmitate was significantly associated with the presence of positive psychotic symptoms at treatment initiation (P = .047), switching to paliperidone palmitate from treatments other than risperidone LAI (P = .048), and starting on a paliperidone palmitate dose > 100 mg (P = .045).
This study demonstrated a relatively high rate (57.8%) of discontinuation with paliperidone palmitate within 52 weeks, with the main reason for discontinuation being deterioration in patient mental state (77%). The results suggest that more severely ill patients with an incomplete response to their previous antipsychotic were more likely to experience deterioration in their mental state after a switch to paliperidone palmitate and subsequently discontinue with treatment.
Those who switched to paliperidone palmitate from treatments other than risperidone LAI were also more likely to discontinue treatment (71%). A number of factors may have contributed to this high discontinuation rate. Four of the patients who discontinued paliperidone palmitate had previously been unsuccessfully treated with risperidone. Given that paliperidone is the active metabolite of risperidone, a history of resistance to risperidone suggests that paliperidone palmitate would also be ineffective and that these patients were unsuited to be switched. In addition, Janssen-Cilag provides specific dosing information for patients switching from risperidone LAI1 but not for patients switching from other antipsychotics. This lack of information may have contributed to inadequate initial or maintenance dosing and the subsequent high discontinuation rate.
There are limited data in the literature about the outcomes of patients who have been taking paliperidone palmitate for longer than 13 weeks. In this study, only 6 of the 20 patients who discontinued treatment due to deterioration in their mental state did so within 13 weeks, which suggests that 13 weeks is an inadequate follow-up period to determine whether paliperidone palmitate is effective.
There are a number of limitations in this study. As a retrospective study, it is likely that the data about patients’ mental state (at initiation and discontinuation of treatment) were not as reliable as those that would have been collected in a prospective study using validated rating scales. The sample size in this study was small and limited the likelihood of finding significant associations between patient or treatment factors and discontinuation.
Despite the limitations of this study and the potential advantages of paliperidone palmitate, given the relatively high discontinuation rate in this study, a cautious approach is recommended when deciding whether to switch a patient to paliperidone palmitate. Further long-term studies are required to determine the benefit of switching patients to paliperidone palmitate, to determine whether there are specific patient groups that are less suitable to be switched (eg, those who have responded poorly to other antipsychotics and those taking antipsychotics other than risperidone), and to clarify appropriate dosing when switching to paliperidone palmitate from medications other than risperidone.
References
1. Invega Sustenna . New South Wales, Australia. Janssen-Cilag Pty, Ltd. 2010. www.janssen.com.au/files/Products/Invega_Sustenna_PI.pdf. Updated April 9, 2013. Accessed November 15, 2013.
2. Bishara D. Once-monthly paliperidone injection for the treatment of schizophrenia. Neuropsychiatr Dis Treat. 2010;6:561-572. PubMed doi:10.2147/NDT.S8505
3. Samtani MN, Gopal S, Kern Silwa J, et al. Switching to paliperidone palmitate from other antipsychotics: guidance based on pharmacokinetic modeling and simulations. Poster II-62. Poster presented at the 49th Annual New Clinical Drug Evaluation Unit Meeting; June 29-July 2, 2009; Hollywood, FL.
4. Kramer M, Litman R, Hough D, et al. Paliperidone palmitate, a potential long-acting treatment for patients with schizophrenia. Results of a randomized, double-blind, placebo-controlled efficacy and safety study. Int J Neuropsychopharmacol. 2010;3(5):635-647. PubMed doi:10.1017/S1461145709990988
5. Gopal S, Hough D, Xu H, et al. Efficacy and safety of paliperidone palmitate in adult patients with acutely symptomatic schizophrenia: a randomized, double-blind, placebo-controlled dose-response study. Int Clin Psychopharmacol. 2010;25(5):247-256. PubMed doi:10.1097/YIC.0b013e32833948fa
6. Nasrallah HA, Gopal S, Gassman-Mayer C, et al. A controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophrenia. Neuropsychopharmacology. 2010;35(10):2072-2082. PubMed doi:10.1038/npp.2010.79
7. Pandina GJ, Lindenmayer JP, Lull J, et al. A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychopharmacol. 2010;30(3):235-244. PubMed doi:10.1097/JCP.0b013e3181dd3103
8. Pandina G, Lane R, Gopal S, et al. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):218-226. PubMed doi:10.1016/j.pnpbp.2010.11.008
9. Fleischhacker W, Gopal S, Lane R, et al. A randomized trial of paliperidone palmitate and risperidone long-acting injectable in schizophrenia. Int J Neuropsychopharmacol. 2012;15(1):107-118. PubMed doi:10.1017/S1461145711001076
10. Gopal S, Vijapurkar U, Lim P, et al. A 52-week open-label study of the safety and tolerability of paliperidone palmitate in patients with schizophrenia. J Psychopharmacol. 2011;25(5):685-697. PubMed doi:10.1177/0269881110372817
11. Attard A, Olofinjana O, Cornelius V, et al. Paliperidone palmitate long-acting injection: prospective year-long follow-up of use in clinical practice. Acta Psychiatr Scand. 2013;n/a. PubMed doi:10.1111/acps.12201
12. Jannsen-Cilag. Invega Sustenna Product Familiarisation Program: Program Reference Guide. Sydney, Australia: Jannsen-Cilag; 2010.
Author affiliations: Department of Psychiatry, Austin Health, University of Melbourne, Melbourne, Victoria, Australia.
Potential conflicts of interest: None reported.
Funding/support: Janssen-Cilag provided subsidized access to paliperidone palmitate to patients in this study.
Role of the sponsor: Other than the provision of subsidized access to medication, the sponsor had no role in the design and conduct of the study; collection, management, and analysis of the data; or preparation, review, and approval of the manuscript.
J Clin Psychiatry 2014;75(11):1267-1269 (doi:10.4088/JCP.13l08866).
© Copyright 2014 Physicians Postgraduate Press, Inc.