First-Trimester Exposure to Methylphenidate:
A Population-Based Cohort Study
ABSTRACT
Objective: The use of methylphenidate to treat attention-deficit/hyperactivity disorder has risen dramatically in Western countries, and it is increasingly used by adults, including women of childbearing age. Very little is known about potential hazards of in utero exposure to methylphenidate. We conducted this study to estimate the risk of major congenital malformations following first-trimester in
utero exposure to methylphenidate.
Method: Data from 2005 to 2012 were
extracted from the Danish National Patient Register, the Danish National Prescription Registry, the Medical Birth Registry, and the Danish Civil Registration System. Exposure was defined as having redeemed 1 or more prescriptions for methylphenidate within a time window defined as 14 days before the beginning of the first trimester up to the end of the first trimester. Each exposed subject was propensity score–matched to 10 unexposed subjects with respect to maternal age, smoking status, body mass index, length of education, calendar year of completion of pregnancy, and concomitant use of antipsychotics, antidepressants, anxiolytics, and nonsteroidal anti-inflammatory drugs.
Results: We included 222 exposed and 2,220 unexposed pregnancies in the analysis. There was no statistically significant increase in major malformations (point prevalence ratio = 0.8; 95% CI, 0.3–1.8) or cardiac malformations (point prevalence ratio = 0.9; 95% CI, 0.2–3.0). Sensitivity analyses using different definitions of exposure or previous users of methylphenidate as the unexposed comparison cohort yielded comparable results.
Conclusions: First-trimester in utero exposure to methylphenidate does not appear to be associated with a substantially (ie, more than 2-fold) increased overall risk of major congenital malformations.
J Clin Psychiatry 2014;75(1):e88–e93
© Copyright 2014 Physicians Postgraduate Press, Inc.
Submitted: July 30, 2013; accepted October 24, 2013 (doi:10.4088/JCP.13m08708).
Corresponding author: Anton Pottegård, MScPharm, Department of Clinical Pharmacology, University of Southern Denmark, JB Winsløwsvej 19, 2, 5000 Odense C, Denmark ([email protected]).
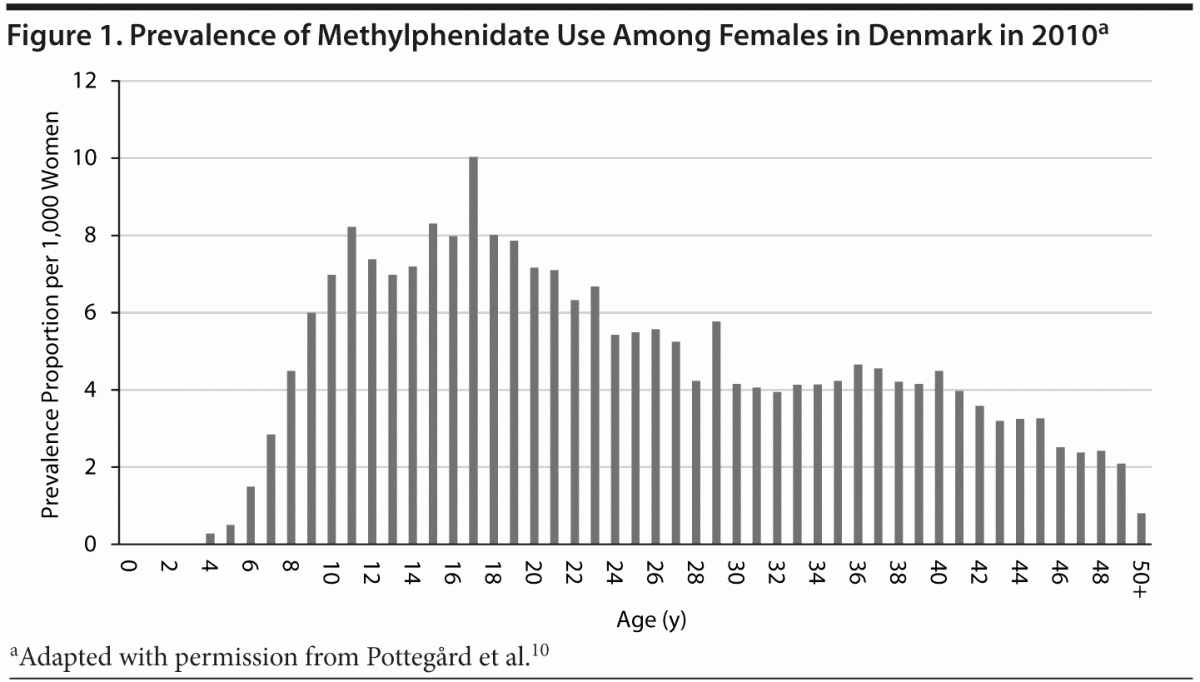
The issue of methylphenidate use during pregnancy has become increasingly relevant as the prevalence of attention-deficit/hyperactivity disorder (ADHD) among adults has risen over the last decade.1,2 It is estimated that between 30% and 70% of children with ADHD will experience symptoms as adults.3 Estimates of the prevalence of adult ADHD have been reported to be around 3%–4%, ranging from 1% to 7% in different countries, with the highest prevalence among developed countries.4,5 Methylphenidate was approved for use in adults by the US Food and Drug Administration in 2008, but it does not yet hold this indication in Europe.6,7 Some guidelines recommend the use of methylphenidate in adults suffering from ADHD,8 including a recommendation on off-label use from the National Institute for Health and Clinical Excellence in the United Kingdom.9 A recent study10 showed that in Denmark many women in the fertile age range are prescribed methylphenidate: Among women aged 18–40 years, 4 to 8 per 1,000 persons use methylphenidate (Figure 1).
Safety data on the use of methylphenidate during pregnancy are scarce and give little guidance for the prescribing physician. Labeling for use during pregnancy is “C” (“animal data have shown adverse effect on the fetus”) in the United States,6 while the UK Summary of Product Characteristics7 states that “there is a limited amount of data from the use of methylphenidate in pregnant women” and that “methylphenidate is not recommended for use during pregnancy unless a clinical decision is made that postponing treatment may pose a greater risk to the pregnancy.” A recent review1 identified 180 first-trimester exposed pregnancies from various uncontrolled or small controlled studies, with no apparent excess risk of congenital malformations. No large controlled study of malformations following in utero methylphenidate exposure exists, and we thus performed a register-based nationwide cohort study of first-trimester use of methylphenidate in Denmark from 2005 to 2012.
METHOD
The analysis was performed as a cohort study. We compared the prevalence proportion of malformations in a cohort of pregnancies exposed to methylphenidate in the first trimester to a propensity score–matched cohort of pregnancies during which the mother had never used psychostimulants.
Data Sources
We used data from 4 Danish nationwide registries: the Danish National Patient Register, the Danish National Prescription Registry, the Danish Medical Birth Registry, and the Danish Civil Registration System. Data were obtained for the period of January 1, 1995, to December 31, 2012.
The Danish National Patient Register11 contains data on all hospitalizations in Denmark since 1977 and outpatient visits since 1995. Discharge diagnoses are coded according to ICD-10 since 1994. Virtually all medical care in Denmark is furnished by the national health authorities, whereby this data resource allows true population-based studies, covering all inhabitants of Denmark.
The Medical Birth Registry12 contains information on all pregnancies in Denmark, both hospital births and home births. Information stored in the registry includes basic data on the children (gestational age, weight, height, Apgar score, referral to neonatal care unit, etc), complications, and procedures performed during the delivery. Until 1997, data were collected through birth reports sent to the National Board of Health. From 1997, the registry is primarily based on the Danish National Patient Register, but supplied with birth reports on home births and stillborn children.
The Danish National Prescription Registry13 contains data on all prescription drugs dispensed in Denmark since 1995. Prescription data include the date of dispensing, substance, brand name, and quantity. Drugs are categorized according to the Anatomical Therapeutic Chemical code, a hierarchical classification system developed by the World Health Organization for purposes of drug use statistics.14
The Danish Civil Registration System15 contains data on vital status (date of death) and migration to and from Denmark, which we used for censoring purposes. All data sources were linked by use of the personal identification number, a unique identifier assigned to all Danish individuals. All linkage was performed anonymously within Statistics Denmark, a governmental institution that collects and maintains electronic records for a broad spectrum of statistical and scientific purposes.16,17
Pregnancies
Pregnancies were identified using the Medical Birth Registry. As this registry includes only pregnancies resulting in births, either stillborn or live born, pregnancies terminated prior to birth, that is, both spontaneous and elective abortions, were not included in our material. As malformations are incompletely registered in stillborn children, we included only pregnancies resulting in live births (the infant survived ≥ 1 day). Exploratory analysis showed that maternal use
of methylphenidate was very rare (1 subject) prior to January 1, 2005. Also, we required the possibility of 6 months of follow-up for the child after birth, to be able to assess malformations that were not detected at birth. Therefore, we included only pregnancies completed after January 1, 2005, and before July 1, 2012. Children who died within the first 6 months were, however, still included in the analysis. To assess drug exposure, we defined a period corresponding to the first trimester for each pregnancy. We did so using information from the Danish Medical Birth Registry: We took the date of completion of the pregnancy and subtracted the gestational age at completion as estimated in the registry. The resulting date estimates the date of the end of the last menstruation. We therefore defined the first trimester as an interval from the 15th day to the 84th day from this date. We excluded multiple gestation pregnancies, pregnancies in which the mother migrated to or from Denmark within 5 years prior to the initiation of the pregnancy, and pregnancies in which the child migrated from Denmark within 6 months after being born. Additionally, we excluded pregnancies during which the mother had used certain drugs that are rarely used during pregnancy but known to be teratogenic. These drugs were vitamin K antagonists (B01AA), tetracyclines (J01AA), angiotensin-converting enzyme inhibitors (C09A and C09B), retinoids (D05BB, D10AD, and D10BA), and selected antiepileptic drugs: carbamazepine (N03AF01), oxcarbazepine (N03AF02), phenytoin (N03AB02), valproic acid (N03AG01), and phenobarbital (N03AA02).18,19 Use of these drugs was defined as having redeemed a prescription within 180 days before pregnancy to the end of the pregnancy.
Cohorts
To be included in the cohort of pregnancies exposed to methylphenidate in the first trimester, the mother was required to redeem 1 or more prescriptions for methylphenidate (ATC, N06BA04) within a time window defined as 14 days before the beginning of the first trimester up to the end of the first trimester. The unexposed cohort included pregnancies in which the mother had not redeemed a prescription for a psychostimulant (ATC, N06BA) at any time point prior to the end of the pregnancy. Using the above definitions, some pregnancies failed to be included in either cohort. This was intentional, as to avoid bias from misclassification, and was subject to sensitivity analyses (see Analysis).
Matching
The limiting factor in terms of statistical precision would be the number of first-trimester exposed women. From preliminary analyses of age-specific methylphenidate user prevalences and age-specific birth rates, we had calculated the expected number of exposed women to be on the order of 400. As the expected number of outcomes would therefore be low, we chose to analyze the data by use of propensity scores,20 which estimate the likelihood of being treated, conditional on other covariates. For each pregnancy in the exposed cohort, we matched 10 pregnancies from the unexposed cohort. We used sequential balanced nearest-neighbor matching technique21 to match exposed and unexposed subjects on propensity score, allowing a maximum caliper of 0.05. This ensures that for each exposed subject, half of the individually matched unexposed subjects have propensity scores above the index subject, and half have propensity scores below. The following variables were included in the calculation of the propensity score: maternal age, maternal smoking status during first trimester, maternal body mass index (5% missing values were handled by imputation of mean value), calendar year of completion of pregnancy, length of education (grouped into 7–10 years, 11–12 years, ≥ 13 years, and unknown), and concomitant use of each of the following drugs: antipsychotics (N05A), antidepressants (N06A), anxiolytics (N05B), and nonsteroidal anti-inflammatory drugs (M01A excluding M01AX). Concomitant use of these drugs was defined as 1 or more prescriptions for the given drug within 40 days before the beginning of the first trimester up to the end of the first trimester.
Endpoints
The 2 study endpoints were major malformations and major cardiac malformations. We classified major malformations and subgroupings according to the European Surveillance of Congenital Anomalies (EUROCAT) system guide v1.3,22 based on ICD-10 discharge diagnoses from the Danish National Patient Register. Furthermore, we calculated the mean gestational age and birth weight.
Analysis
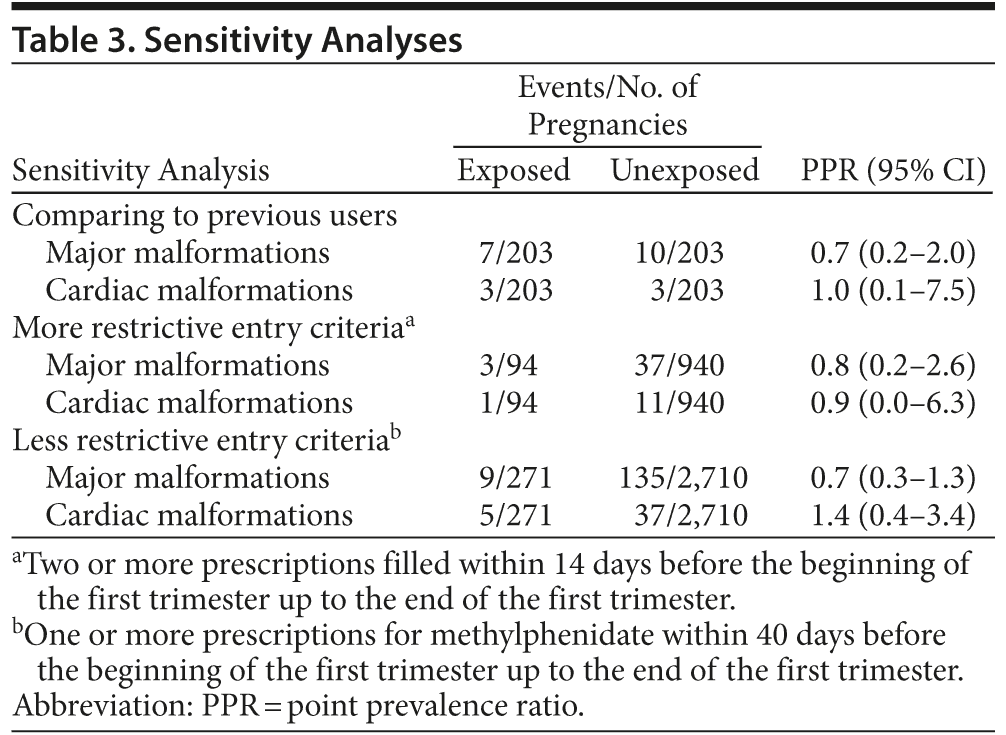
Our main outcome measure was the point prevalence ratio, equivalent to the risk ratio, for major malformations and major cardiac malformations, calculated by comparing the exposed to the unexposed pregnancies. We furthermore applied a range of supplementary/sensitivity analyses. First, we performed subgroup analyses, stratifying by maternal age and by excluding pregnancies with concomitant use of psychostimulants other than methylphenidate (ATC, N06BA) or any of the abovementioned confounder drugs. Second, we changed the eligibility criteria for entering the unexposed cohort from never-use to previous use of methylphenidate. Previous use was defined as having redeemed 2 or more prescriptions for methylphenidate at any time prior to 180 days before the beginning of the first trimester, but no prescriptions for any psychostimulant (ATC, N06BA), within a time window defined as 180 days before the beginning of the first trimester up to the end of the pregnancy. In this analysis, we matched exposed to unexposed in a ratio of 1:1. Last, we applied alternative criteria to define pregnancies that entered the exposed cohort: (1) more restrictive criteria, requiring 2 or more prescriptions within 14 days before the beginning of the first trimester up to the end of the first trimester, and (2) less restrictive criteria, requiring 1 or more prescriptions for methylphenidate within 40 days before the beginning of the first trimester up to the end of the first trimester.
RESULTS
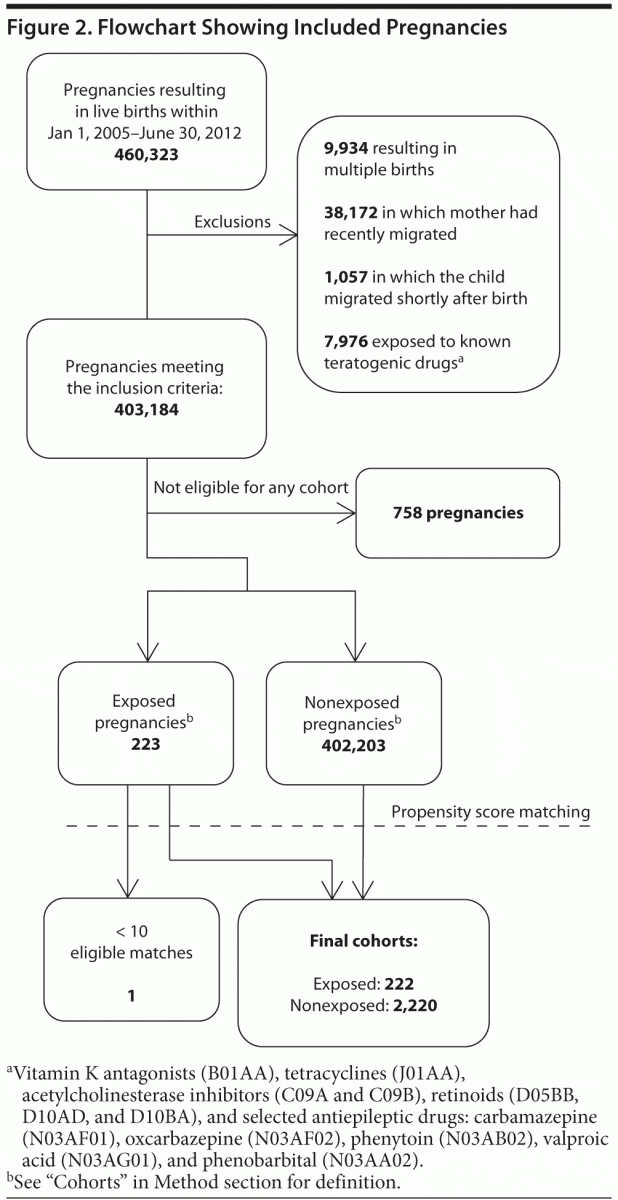
From 460,323 pregnancies resulting in live births, we identified 240 exposed pregnancies in the period. Of these,
7 were excluded due to recent migration, 10 were excluded from analysis due to concomitant treatment with drugs with documented teratogenic potential, and 1 was excluded from analysis because she could not be matched with 10 unexposed subjects within the allowed caliper. A total of
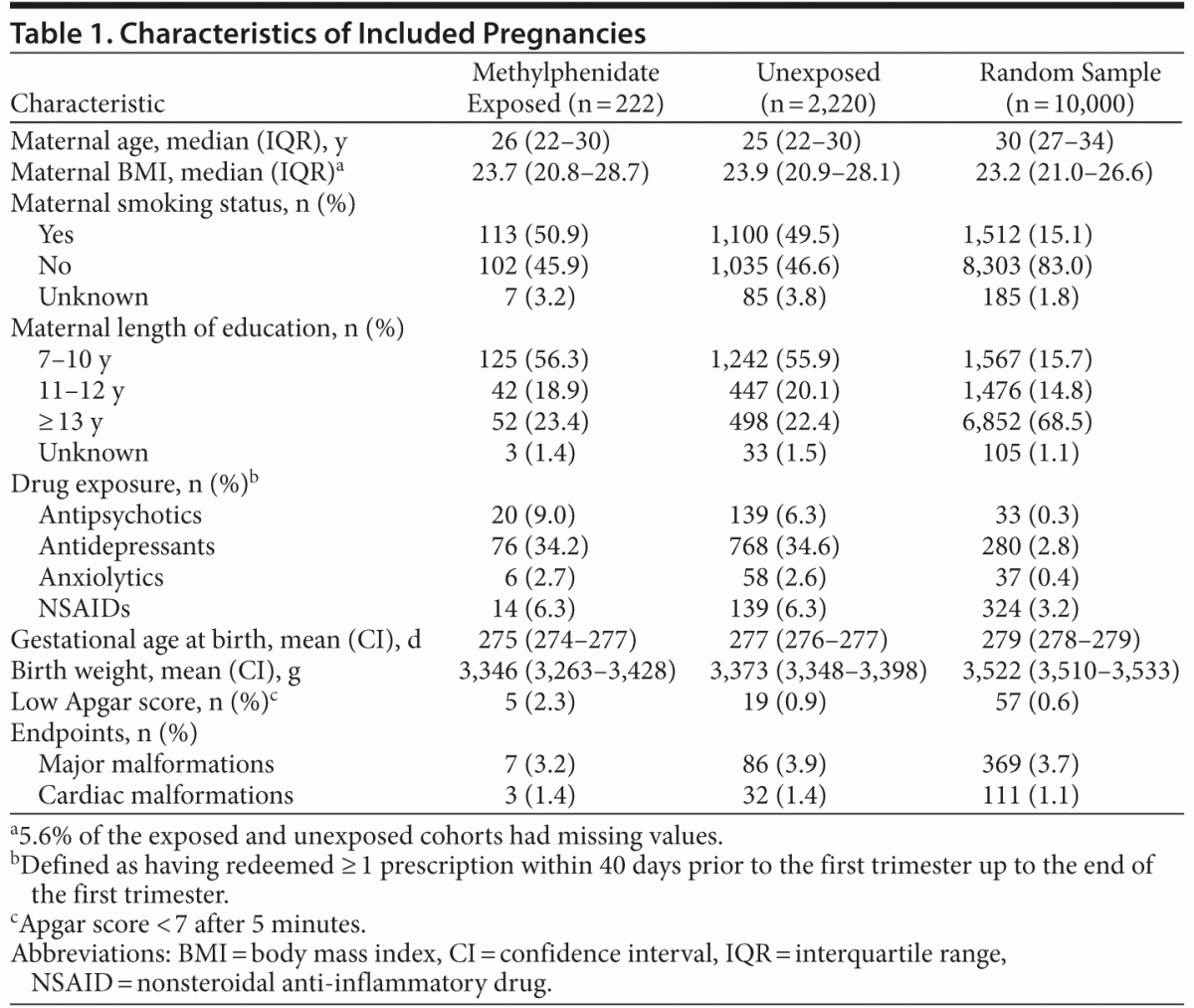
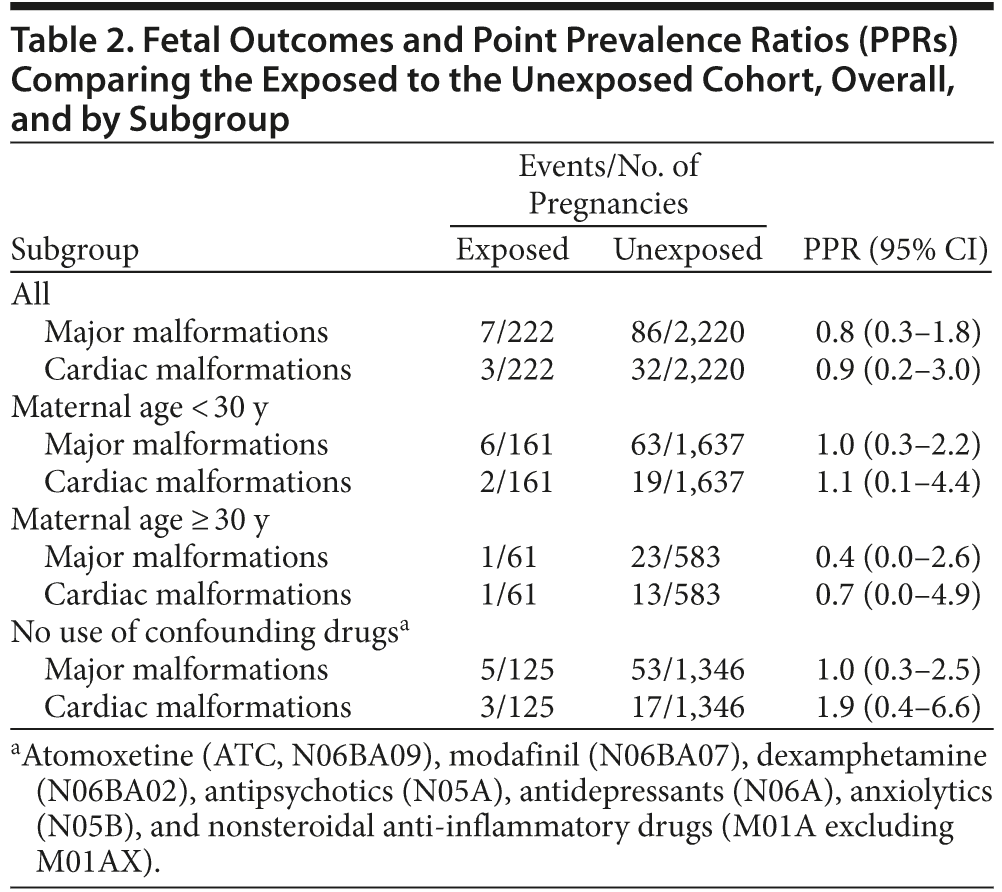
222 subjects exposed to methylphenidate in the first trimester were matched to 2,220 unexposed subjects (Figure 2). Among the 222 exposed pregnancies, 6 women each contributed with 2 pregnancies. The exposed and unexposed cohorts were well balanced on all variables included in the propensity score model (Table 1), with the exception of a slightly higher prevalence of use of antipsychotics among the exposed. Compared to a random sample of 10,000 unexposed pregnancies, the cohorts were significantly younger, were less educated, smoked more, and had substantially higher consumption of drugs (ie, antipsychotics, antidepressants, anxiolytics, nonsteroidal anti-inflammatory drugs). Among the exposed, we observed 7 (3.2%) major malformations, 3 (1.4%) of which were cardiac. Among the unexposed, the corresponding numbers were 86 (3.9%) and 32 (1.4%). These rates are comparable to those observed in the random sample of unexposed pregnancies (3.7% and 1.1%, respectively). Comparison of the exposed and unexposed cohorts yielded overall point prevalence ratios of 0.8 (95% CI, 0.3–1.8) for all major malformations and 0.9 (95% CI, 0.2–3.0) for cardiac malformations (Table 2). There was no sign of aggregation of single malformations.
https://www.psychiatrist.com/wp-content/uploads/2024/02/v75n0113F1-scaled.gif
Sensitivity analyses stratifying by maternal age and excluding users of potentially confounding drugs did not
result in materially different risk estimates (Table 2). Covariates were still well balanced among exposed and unexposed subjects within these subgroups (data not shown). Changing the comparison to previous users or applying other entry criteria to the exposed cohort also returned similar risk estimates (Table 3).
DISCUSSION
This is the first population-based study of the use of methylphenidate in pregnancy. We identified 240 women exposed to methylphenidate in the first trimester of pregnancy in Denmark from 2005 to 2012, among whom 222 entered the final cohort for analysis. We found no indication of increased overall risk of major congenital malformations. This finding is in accordance with 4 previously published studies that in total evaluated 180 women exposed in the first trimester and found 4 major malformations, corresponding to a rate of 2.2% (95% CI, 0.6–5.6).1 These previously reported cohort data, the majority of which are reported in the Swedish Birth Registry comprising 104 in utero exposed women, are mainly uncontrolled and thus have little or no potential to address confounding.18,23–25 The data in the Swedish Birth Registry have recently been updated and now comprise a total of 220 first-trimester exposed children, among whom 5 congenital malformations are described.23
We used propensity score–matched controls and included maternal age, smoking status, body mass index, calendar year of birth date, length of education, and concomitant use of antipsychotics, antidepressants, antiepileptics, anxiolytics, and nonsteroidal anti-inflammatory drugs in the first trimester. Our analyses were robust, as various sensitivity analyses using less or more restrictive criteria for definition of exposure to methylphenidate or previous methylphenidate users for comparison did not change the results. We found a somewhat lower rate of exposure to methylphenidate during pregnancy than expected from total population estimates of methylphenidate use. On the basis of population estimates of the use of methylphenidate in women between the ages of 18 and 40 years between 2005 and 2012, about 400 exposed cases were expected.10 However, it is quite plausible that many women, on their own initiative or on the basis of physicians’ advice, who are contemplating pregnancy or are accidentally exposed in the first trimester of pregnancy will discontinue treatment and not redeem prescriptions. The source of drug information most commonly used by health care professionals during pregnancy in Denmark states that, due to insufficient safety data, methylphenidate should be avoided during pregnancy.26
With the addition of our data to the previously reported cohort data and the updated data from the Swedish Birth Registry, 518 first-trimester exposed pregnancies have now been reported, with no signs of excess risk of major congenital malformations.1 The European Medicines Agency guidelines suggest that at least 1,000 first-trimester exposed women be evaluated with no sign of excess risk before a reassuring statement can be included in the Summary of Product Characteristics.27 While the quantity and quality of the data at hand are short of this regulatory requirement, they still offer some reassurance to the treating clinician and the pregnant woman receiving methylphenidate.
The strength of our study is that it comprises data from an entire national cohort of more than 460,000 pregnancies from 2005 to 2012, using high-quality and validated registry data for identification of exposure and outcome.12,13 More than 99% of all births in Denmark have been recorded in the Medical Birth Registry, and completeness of the National Prescription Registry has been estimated to be 98%.13,28 The diagnoses of malformations have been found to have a predictive value of 88% for congenital malformations, with a completeness of 90%, and misclassification was probably random.29 It is, however, conceivable that some newborns exposed to methylphenidate in utero will be more diligently scrutinized for malformations.30 If this is the case, it will only strengthen our conclusion that methylphenidate is safe in pregnancy. Our results are consistent and robust with respect to rates of malformations observed in a large sample of unmatched unexposed pregnancies and alterations in the definition of the exposure window. By the matching procedure, we could adjust for a variety of parameters that could affect the malformation rate, and we could exclude pregnancies in which the mother had used drugs with documented teratogenic potential. In the study period, the determination of length of pregnancy is based on a first-trimester nuchal translucency scan performed in weeks 11–14, so we expect the gestational age at birth and the calculated date of initiation of pregnancy to be quite precise. We had complete follow-up in national registers, as we excluded people who emigrated.
The weakness of this registry-based study is primarily that we have no information on whether pregnant women who redeemed a prescription for methylphenidate during the first trimester continuously took the medication or had 1 or more “drug holidays.” Such bias would result in a conservative bias toward the null. Our estimate on
cardiac malformations is inherently hampered by the sample size and outcome frequency. While we did not detect a statistically significant signal, the analysis is based on 3 cases of cardiac malformations in the exposed cohort. This specific malformation should be subject to future studies. Due to the number of exposed cases, the statistical power is insufficient for a meaningful stratified analysis with respect to other specific malformations.
CONCLUSION
Methylphenidate exposure during the first trimester of pregnancy does not appear to be associated with a substantially (ie, more than 2-fold) increased overall risk of congenital malformation. However, the current data are too sparse to allow risk estimates of specific malformations. While insufficient to exclude some excess risks of major malformations, our findings offer some reassurance to pregnant women who receive, and their treating physicians who prescribe, methylphenidate. Other possible unwanted outcomes such as excess risk of miscarriage, preterm birth, low birth weight, neonatal complications, and postnatal neurodevelopmental issues remain unaccounted for.
Drug names: atomoxetine (Strattera), carbamazepine (Carbatrol, Equetro, and others), methylphenidate (Focalin, Daytrana, and others), modafinil (Provigil), oxcarbazepine (Trileptal and others), phenytoin (Dilantin, Phenytek, and others), valproic acid (Depakene, Stavzor, and others).
Author affiliations: Department of Clinical Pharmacology, Institute of Public Health, University of Southern Denmark, Odense (Mr Pottegård, Ms Dideriksen, and Drs Hallas, Aagaard, and Damkier); Department of Clinical Chemistry & Pharmacology, Odense University Hospital, Odense (Mr Pottegård and Drs Hallas and Damkier); Department of Clinical Pharmacology, Copenhagen University Hospital Bispebjerg, Copenhagen (Dr Andersen); and Department of Obstetrics and Gynecology, Hillerød Hospital, University of Copenhagen, Hillerød (Dr Løkkegaard), Denmark.
Potential conflicts of interest: Dr Hallas has participated in research projects funded by and received teaching fees from Novartis. The remaining authors declare no conflict of interest.
Funding/support: None reported.
Acknowledgment: Anne Broe, BScMedicine, Department of Clinical Pharmacology, Institute of Public Health, University of Southern Denmark, Odense, Denmark, is acknowledged for contributions to data handling and declares no conflict of interest.
REFERENCES
1. Dideriksen D, Pottegård A, Hallas J, et al. First trimester in utero exposure to methylphenidate. Basic Clin Pharmacol Toxicol. 2013;112(2):73–76. doi:10.1111/bcpt.12034 PubMed
2. Bolea-Alamanac BM, Green A, Verma G, et al. Methylphenidate use in pregnancy and lactation, a systematic review of evidence [published online ahead of print April 18, 2013]. Br J Clin Pharmacol. doi:10.1111/bcp.12138 PubMed
3. Cantwell DP. Hyperactive children have grown up: what have we learned about what happens to them? Arch Gen Psychiatry. 1985;42(10):1026–1028. doi:10.1001/archpsyc.1985.01790330110013 PubMed
4. de Zwaan M, Gruss B, Müller A, et al. The estimated prevalence and correlates of adult ADHD in a German community sample. Eur Arch Psychiatry Clin Neurosci. 2012;262(1):79–86. doi:10.1007/s00406-011-0211-9 PubMed
5. Fayyad J, De Graaf R, Kessler RC, et al. Cross-national prevalence and correlates of adult attention-deficit hyperactivity disorder. Br J Psychiatry. 2007;190(5):402–409. doi:10.1192/bjp.bp.106.034389 PubMed
6. Concerta XL drug labeling. US Food and Drug Administration Drugs @ FDA Web site. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction=Search.Set_Current_Drug&ApplNo=021121&DrugName=CONCERTA&ActiveIngred=METHYLPHENIDATE%20HYDROCHLORIDE&SponsorApplicant=JANSSEN%20PHARMS&ProductMktStatus=1&goto=Search.DrugDetails Accessed June 30, 2013.
7. Concerta XL Summary of Product Characteristics. Medicines and Healthcare Products Regulatory Agency Web site. http://www.mhra.gov.uk. Accessed June 30, 2013.
8. Greenhill LL, Pliszka S, Dulcan MK, et al; American Academy of Child
and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults.
J Am Acad Child Adolesc Psychiatry. 2002;41(2, suppl):26S–49S. doi:10.1097/00004583-200210000-00003 PubMed
9. Attention deficit hyperactivity disorder. Clinical guidelines CG72. September 2008. National Institute for Health and Clinical Excellence
Web site. http://guidance.nice.org.uk/CG72. Accessed June 21, 2013.
10. Pottegård A, Bjerregaard BK, Glintborg D, et al. The use of medication against attention deficit hyperactivity disorder in Denmark: a drug use study from a national perspective. Eur J Clin Pharmacol. 2012;68(10):1443–1450. doi:10.1007/s00228-012-1265-y PubMed
11. Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(suppl):30–33. doi:10.1177/1403494811401482 PubMed
12. Knudsen LB, Olsen J. The Danish Medical Birth Registry. Dan Med Bull. 1998;45(3):320–323. PubMed
13. Kildemoes HW, Sørensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(suppl):38–41. doi:10.1177/1403494810394717 PubMed
14. WHO Collaborating Centre for Drug Statistics Methodology. Guidelines for ATC Classification and DDD Assignment 2013. Oslo, Norway: World Health Organization; 2012.
15. Pedersen CB. The Danish Civil Registration System. Scand J Public Health. 2011;39(suppl):22–25. doi:10.1177/1403494810387965 PubMed
16. Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39(suppl):103–105. doi:10.1177/1403494811405098 PubMed
17. Jensen VM, Rasmussen AW. Danish Education Registers. Scand J Public Health. 2011;39(suppl):91–94. doi:10.1177/1403494810394715 PubMed
18. Briggs GG, Freeman RK, Yaffe SJ. Drugs in Pregnancy and Lactation. 9th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2011.
19. Schardein JL. Chemically Induced Birth Defects. 3rd ed. New York, NY: Marcel Dekker Inc; 2000. doi:10.3109/9780203909904
20. Glynn RJ, Schneeweiss S, Stürmer T. Indications for propensity scores
and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol. 2006;98(3):253–259. doi:10.1111/j.1742-7843.2006.pto_293.x PubMed
21. Rassen JA, Shelat AA, Myers J, et al. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf. 2012;21
(suppl 2):69–80. doi:10.1002/pds.3263 PubMed
22. EUROCAT Guide 1.3 and reference documents. 2005. European Surveillance of Congenital Anomalies Web site. http://www.eurocat-network.eu. Accessed November 4, 2013.
23. Methylphenidate. Drugs and pregnancy [in Swedish]. Janusinfo Web site. http://www.janusinfo.se. Accessed October 15, 2013.
24. Wajnberg R, Diav-Citrin O, Shechtman S, et al. Pregnancy outcome after in-utero exposure to methylphenidate: a prospective comparative cohort study. Reprod Toxicol. 2011;13:255–268.
25. Heinonen OP, Slone D, Shapiro S. Birth Defects and Drugs in Pregnancy. Littleton, MA: Publishing Sciences Group; 1977:346–347.
26. Concerta. Pro.medicin.dk Web site. http://www.pro.medicin.dk. Accessed June 30, 2013.
27. Guideline on risk assessment of medical products on human reproduction and lactation: from data to labeling. EMEA/CHMP/203927/2005. European Medicines Agency Web site. http://www.ema.europa.eu. Accessed June 30, 2013.
28. Kristensen J, Langhoff-Roos J, Skovgaard LT, et al. Validation of the Danish Birth Registration. J Clin Epidemiol. 1996;49:893–897. PubMed
29. Larsen H, Nielsen GL, Bendsen J, et al. Predictive value and completeness
of the registration of congenital abnormalities in three Danish population-based registries. Scand J Public Health. 2003;31(1):12–16. doi:10.1080/14034940210134194 PubMed
30. Koren G. The effect of ascertainment bias in evaluating gestational antidepressant exposure. J Popul Ther Clin Pharmacol. 2011;18:e174–e175. PubMed




