Long-acting injectable antipsychotics (LAIs) are among the most effective treatments in psychiatry, yet they remain underutilized in clinical practice. Although LAIs are typically used to maintain treatment adherence in patients with chronic schizophrenia, recent research has suggested that they may also provide an effective treatment strategy for patients with early-phase or first-episode disease. In October 2015, a group of 8 experts on the management of schizophrenia and LAIs met to evaluate the evidence surrounding the efficacy, safety, and cost-effectiveness of LAIs and to develop practical recommendations regarding the clinical use, education, and unmet needs related to LAIs. Participants were also asked to rate the importance of several patient characteristics when choosing an LAI versus an oral antipsychotic, from the perspectives of 4 different stakeholder groups: patients, health care professionals, families, and payers. The evidence review demonstrated that LAIs are superior to placebo for acute and maintenance treatment of schizophrenia and, in general, appear to be similar to one another in terms of schizophrenia relapse prevention. Study design impacts the demonstrated efficacy of LAIs versus oral antipsychotics, but recent database and randomized controlled studies favor the use of LAIs in early-phase schizophrenia patients. LAIs vary considerably in their propensity to cause certain adverse effects, including weight gain, metabolic effects, extrapyramidal symptoms, and prolactin elevation, and these differences can be used to help guide LAI selection. Some studies, but not all, have demonstrated significant reductions in health care utilization or overall costs with LAIs. The expert panel identified several barriers to LAI use in current practice, including clinician lack of knowledge, negative attitudes about LAIs, and resource and cost issues. The participants also identified a number of additional factors that should be considered when weighing the use of LAI therapy, including medication adherence, relapse risk and severity, cognitive impairment, ease of use, substance misuse, access and cost, stigma, social support, patient autonomy, control over medication dosing, fear of needles, and the potential for patient harm due to relapses and associated loss of functioning. This evidence review, discussion, and summary recommendations may help clinicians, patients, families, payers, and other stakeholders to better characterize the role of LAIs in the treatment of schizophrenia.
See letters by Stip and Stip and reply by Correll et al
Long-acting injectable antipsychotics (LAIs) are among the most effective treatments in psychiatry, yet they remain underutilized in clinical practice. Although LAIs are typically used to maintain treatment adherence in patients with chronic schizophrenia, recent research has suggested that they may also provide an effective treatment strategy for patients with early-phase or first-episode disease. In October 2015, a group of 8 experts on the management of schizophrenia and LAIs met to evaluate the evidence surrounding the efficacy, safety, and cost-effectiveness of LAIs and to develop practical recommendations regarding the clinical use, education, and unmet needs related to LAIs. Participants were also asked to rate the importance of several patient characteristics when choosing an LAI versus an oral antipsychotic, from the perspectives of 4 different stakeholder groups: patients, health care professionals, families, and payers. The evidence review demonstrated that LAIs are superior to placebo for acute and maintenance treatment of schizophrenia and, in general, appear to be similar to one another in terms of schizophrenia relapse prevention. Study design impacts the demonstrated efficacy of LAIs versus oral antipsychotics, but recent database and randomized controlled studies favor the use of LAIs in early-phase schizophrenia patients. LAIs vary considerably in their propensity to cause certain adverse effects, including weight gain, metabolic effects, extrapyramidal symptoms, and prolactin elevation, and these differences can be used to help guide LAI selection. Some studies, but not all, have demonstrated significant reductions in health care utilization or overall costs with LAIs. The expert panel identified several barriers to LAI use in current practice, including clinician lack of knowledge, negative attitudes about LAIs, and resource and cost issues. The participants also identified a number of additional factors that should be considered when weighing the use of LAI therapy, including medication adherence, relapse risk and severity, cognitive impairment, ease of use, substance misuse, access and cost, stigma, social support, patient autonomy, control over medication dosing, fear of needles, and the potential for patient harm due to relapses and associated loss of functioning. This evidence review, discussion, and summary recommendations may help clinicians, patients, families, payers, and other stakeholders to better characterize the role of LAIs in the treatment of schizophrenia.
(J Clin Psychiatry 2016;77[suppl 3]:1-24)
dx.doi.org/10.4088/JCP.15032su1
Given the high frequency of suboptimal adherence to oral antipsychotics and the strong link between nonadherence and relapse, long-acting injectable antipsychotics (LAIs) are among the most effective treatments in psychiatry, yet they remain underutilized in clinical practice.1-4 LAIs have traditionally been used in patients with chronic schizophrenia who have frequent relapses accompanied by marked social and occupational disabilities. However, it is likely that LAIs may benefit patients beyond the population of those with a history of poor treatment adherence. Recent research has focused in particular on the efficacy of LAIs in early-phase or first-episode schizophrenia. Although patients with a first episode of psychosis often respond very well to initial antipsychotic therapy, few are able to attain long-lasting symptom remission or functional recovery.5 LAIs may provide an important treatment option for helping patients remain on therapy and reduce relapse risk and disease progression. As this supplement will demonstrate, LAIs may be underused for many reasons, including lack of familiarity among many physicians, inaccurate perceptions about safety and efficacy, cost and access to treatment, and negative perceptions of injectable therapy among patients, families, and providers.
This supplement was developed from a consensus roundtable that was held October 31, 2015. The goal of this roundtable was to examine current evidence regarding the role of LAIs in the treatment of schizophrenia in order to develop specific, practical recommendations for their use in clinical practice. The panel also identified areas in which additional research is needed to better understand LAI use for schizophrenia as well as health care policy and education goals to improve appropriate implementation of LAIs.
METHODS
A group of 8 experts on the management of schizophrenia and LAIs met to evaluate the evidence and to develop a set of recommendations regarding the clinical use, education, and unmet research needs related to LAIs. Attendees included representatives from academic and community psychiatry settings, the National Alliance on Mental Illness, and a commercial insurance company. Six attendees provided brief presentations related to LAIs, including efficacy, safety, considerations in clinical trial design, value and cost-effectiveness, patient selection, and optimizing LAI use. Participants discussed the information presented and used the information to develop recommendations for treatment, research, and policy.
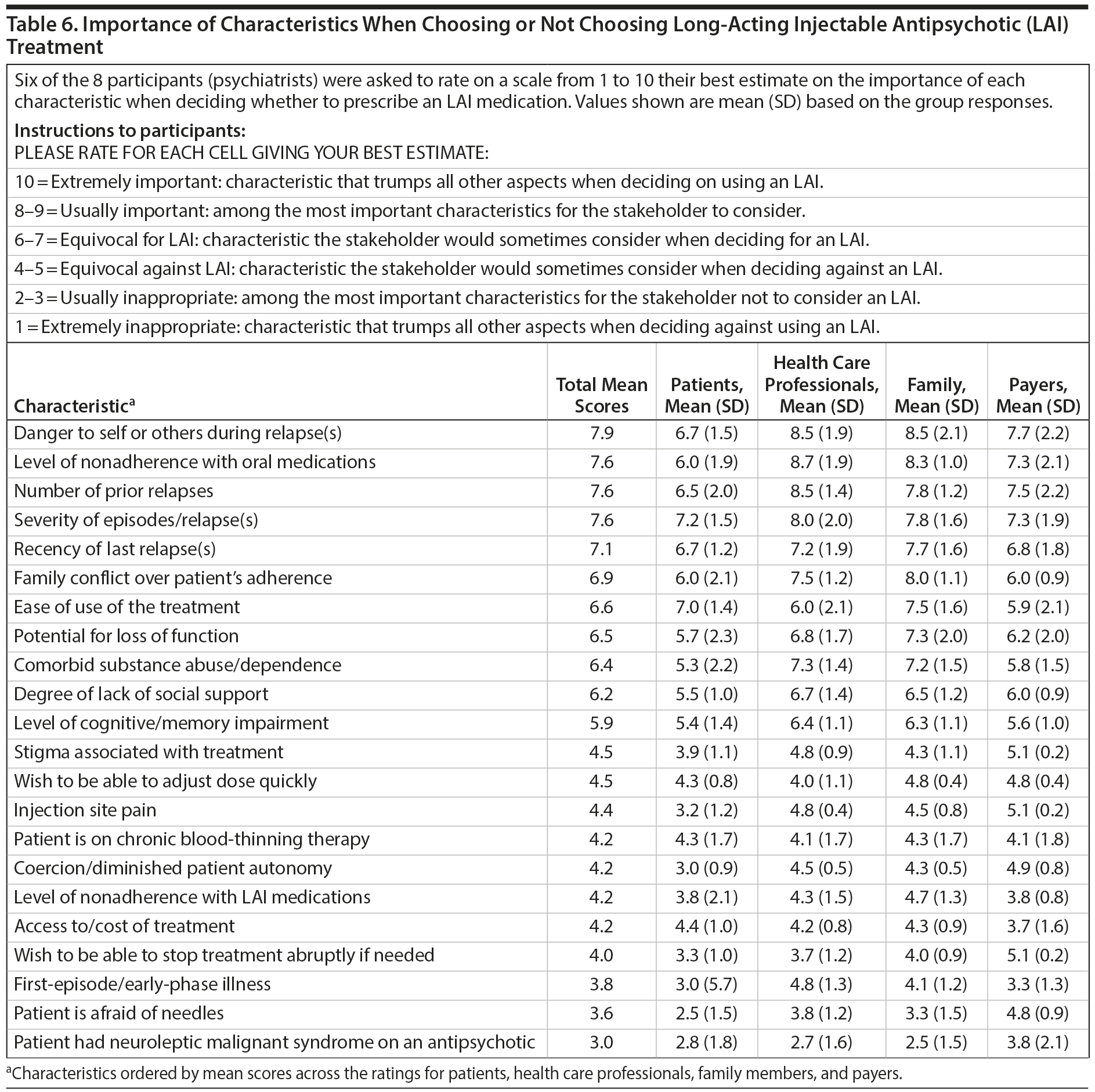
Participants were also asked to rate the importance of several patient characteristics when choosing an LAI versus an oral antipsychotic. They rated the importance of each characteristic from the perspectives of 4 different stakeholder groups: patients, health care professionals, families, and payers. The importance of each characteristic and stakeholder group was evaluated using a 10-point scale, in which 10 = an extremely important characteristic or a characteristic that trumps all other aspects when deciding to use an LAI and 1 = an extremely inappropriate characteristic or a characteristic that trumps all other aspects when deciding against using an LAI. The consensus opinion on the value of LAIs for each scenario and stakeholder group was presented with mean and standard deviation of the pooled ratings.
The first portion of this supplement reviews the efficacy and safety of LAIs, while the second part examines practical issues in LAI use. A series of recommendations is provided regarding treatment and patient selection, education about LAIs for patients and providers, and additional topics for research. Finally, the supplement concludes with recommendations for identifying patients who should be considered for LAI therapy.
Part 1: Efficacy and Safety of LAIs
Efficacy of LAIs Versus Placebo, Versus Oral Antipsychotics, and Among LAIs
LAIs versus placebo. Although LAIs are typically advocated for relapse prevention in patients with chronic schizophrenia,6 data from placebo-controlled, randomized controlled trials (RCTs) show that these agents also reduce symptoms when administered as first-line therapy in acutely ill patients.7-9
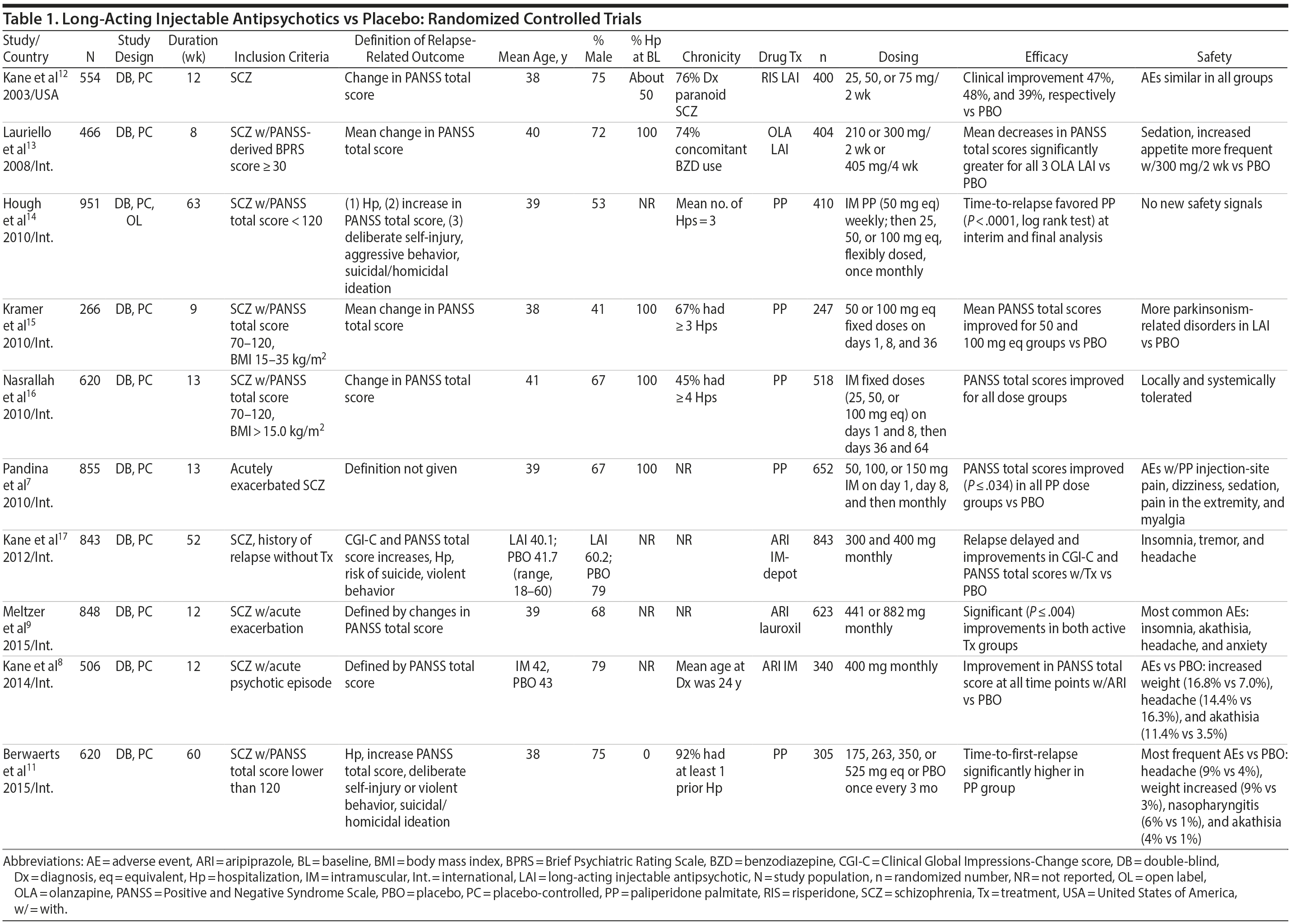
Placebo-controlled RCTs have also demonstrated reduction in relapses with long-acting formulations of second-generation antipsychotics (SGAs) administered at intervals of biweekly to once every 3 months, including paliperidone palmitate (39-156 mg every 4 weeks or 273-819 mg every 3 months), olanzapine pamoate (150 or 300 mg every 2 weeks or 405 mg every 4 weeks), aripiprazole monohydrate (400 mg every 4 weeks), and aripiprazole lauroxil (441, 662, or 882 every 4 weeks or 881 mg every 6 weeks).10,11 RCTs that have compared LAI antipsychotics with placebo in patients with schizophrenia are summarized in Table 1.7-9,11-17
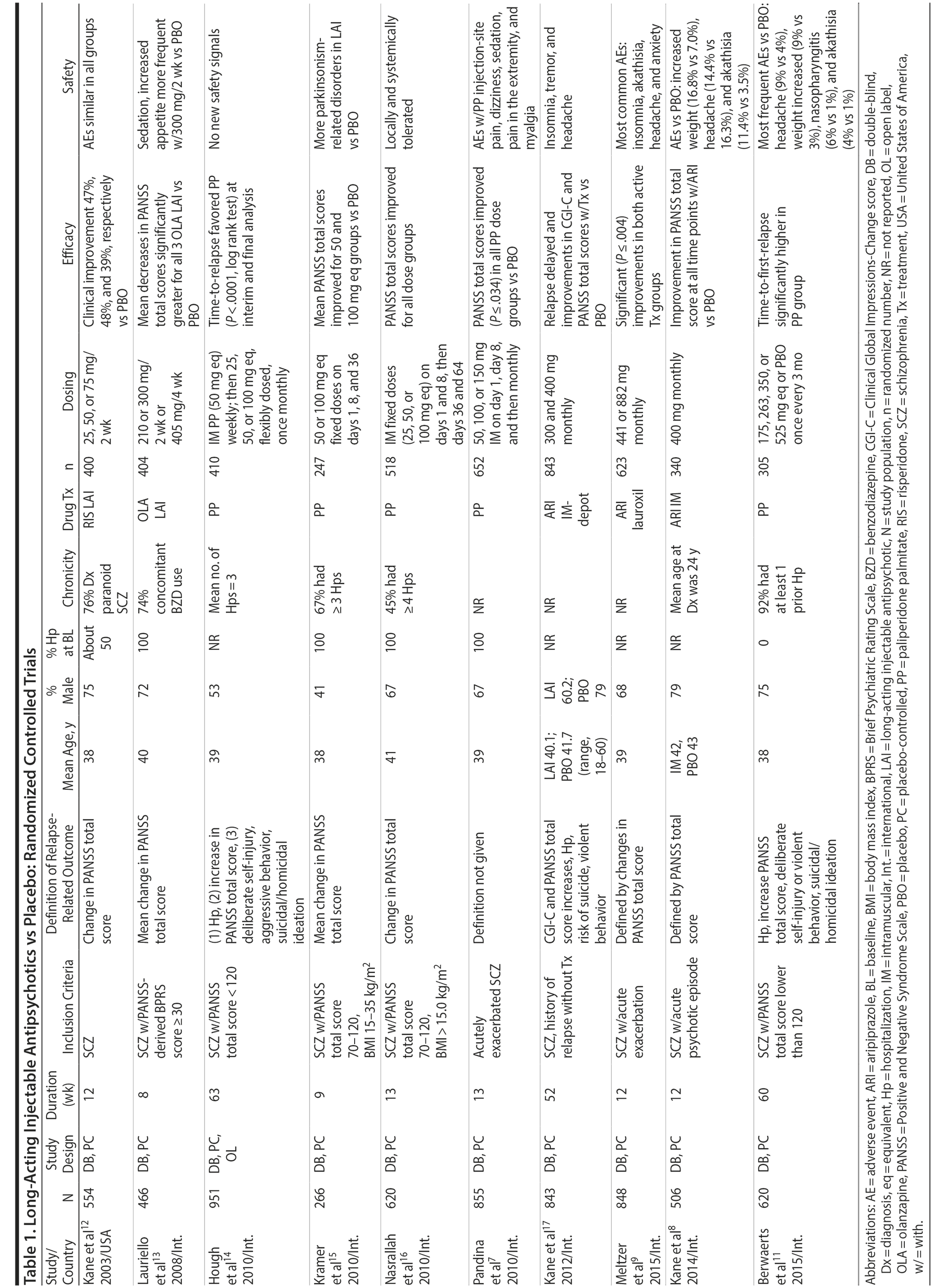
Notably, newer studies of SGA LAIs were sometimes discontinued prematurely on the basis of interim analyses demonstrating efficacy.11,17 This early discontinuation may underestimate the true magnitude of improvement that would have been observed had all patients been followed for a longer period of time (eg, 1 year), as was more typical with older studies of first-generation antipsychotics (FGAs).
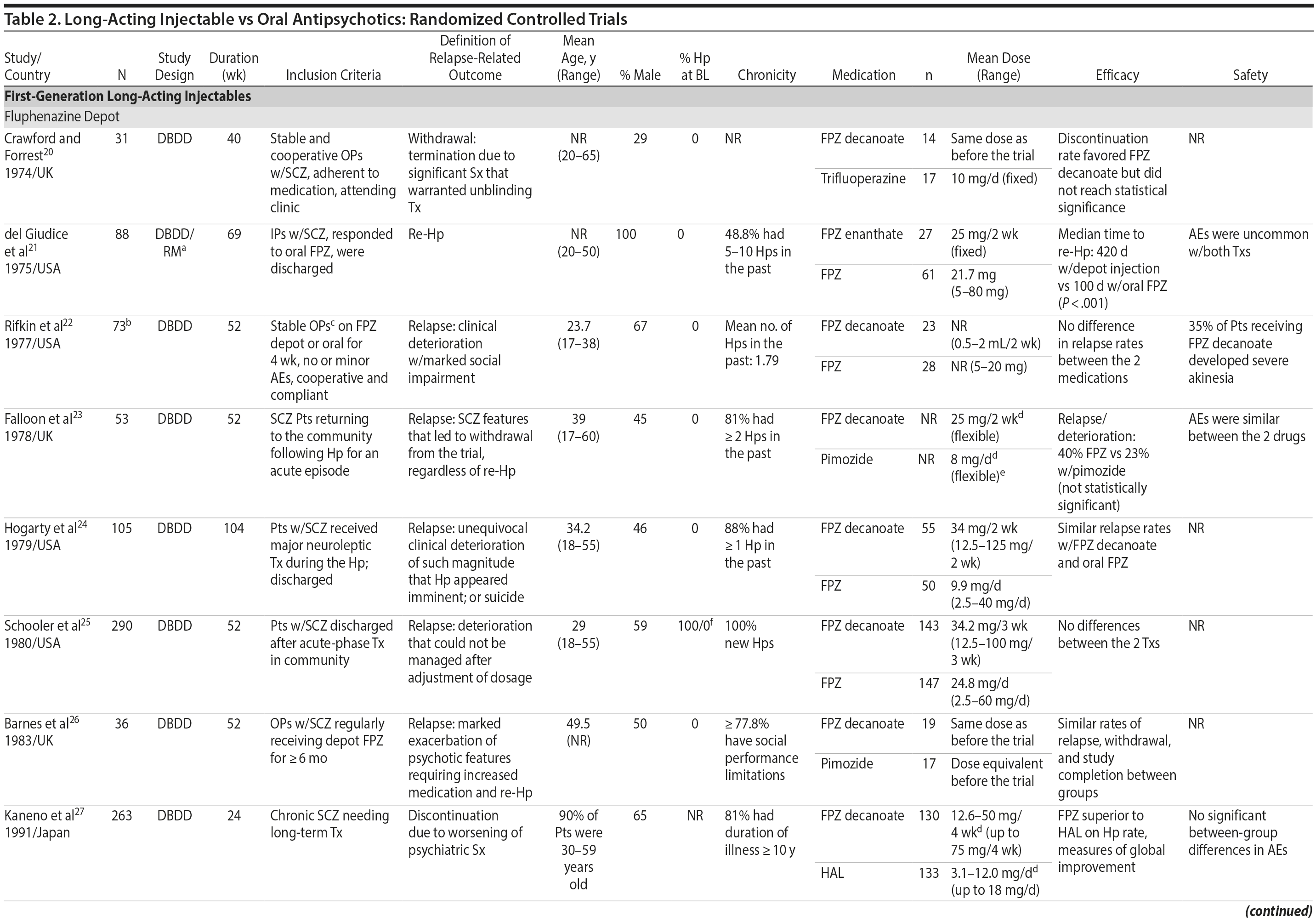
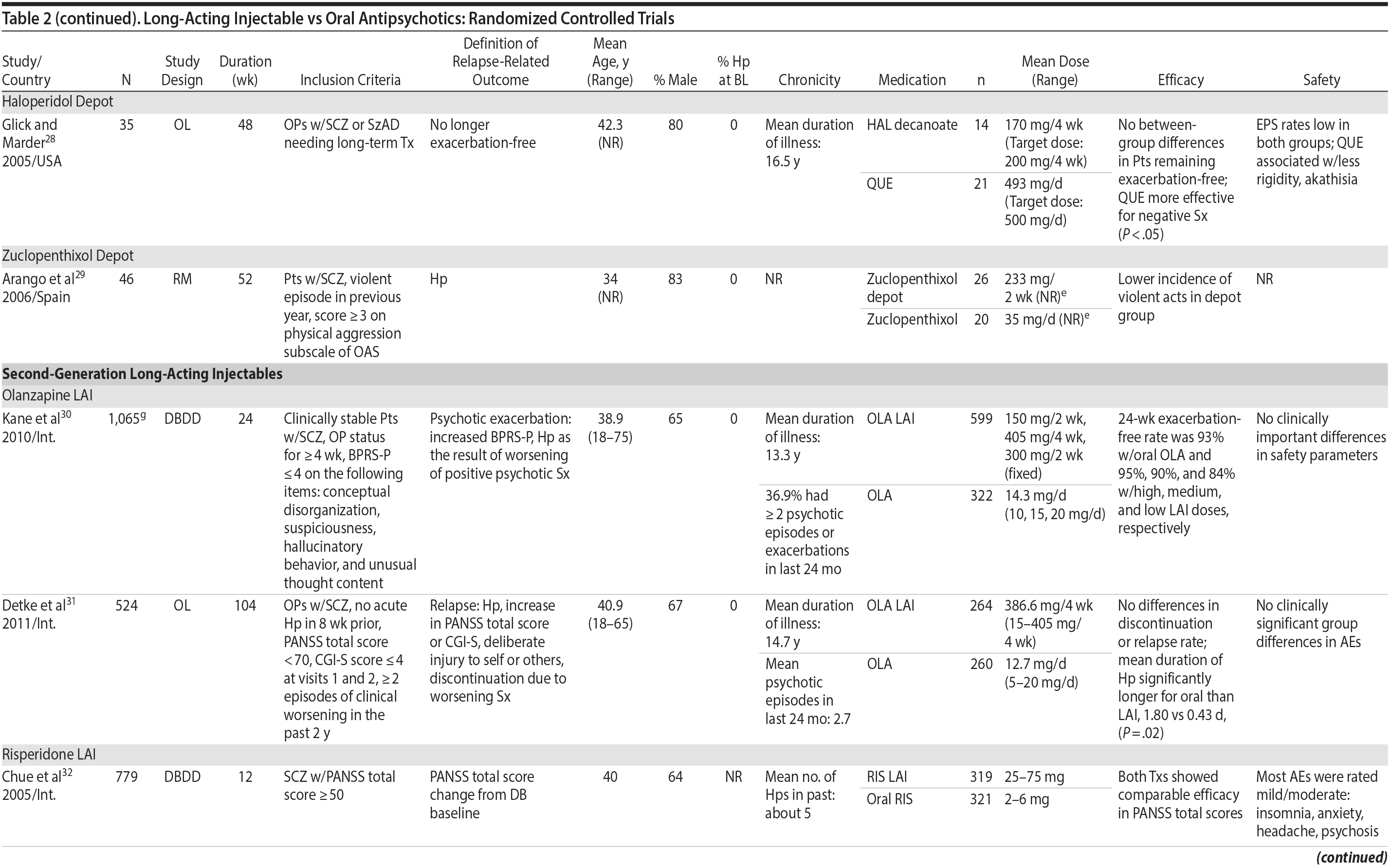
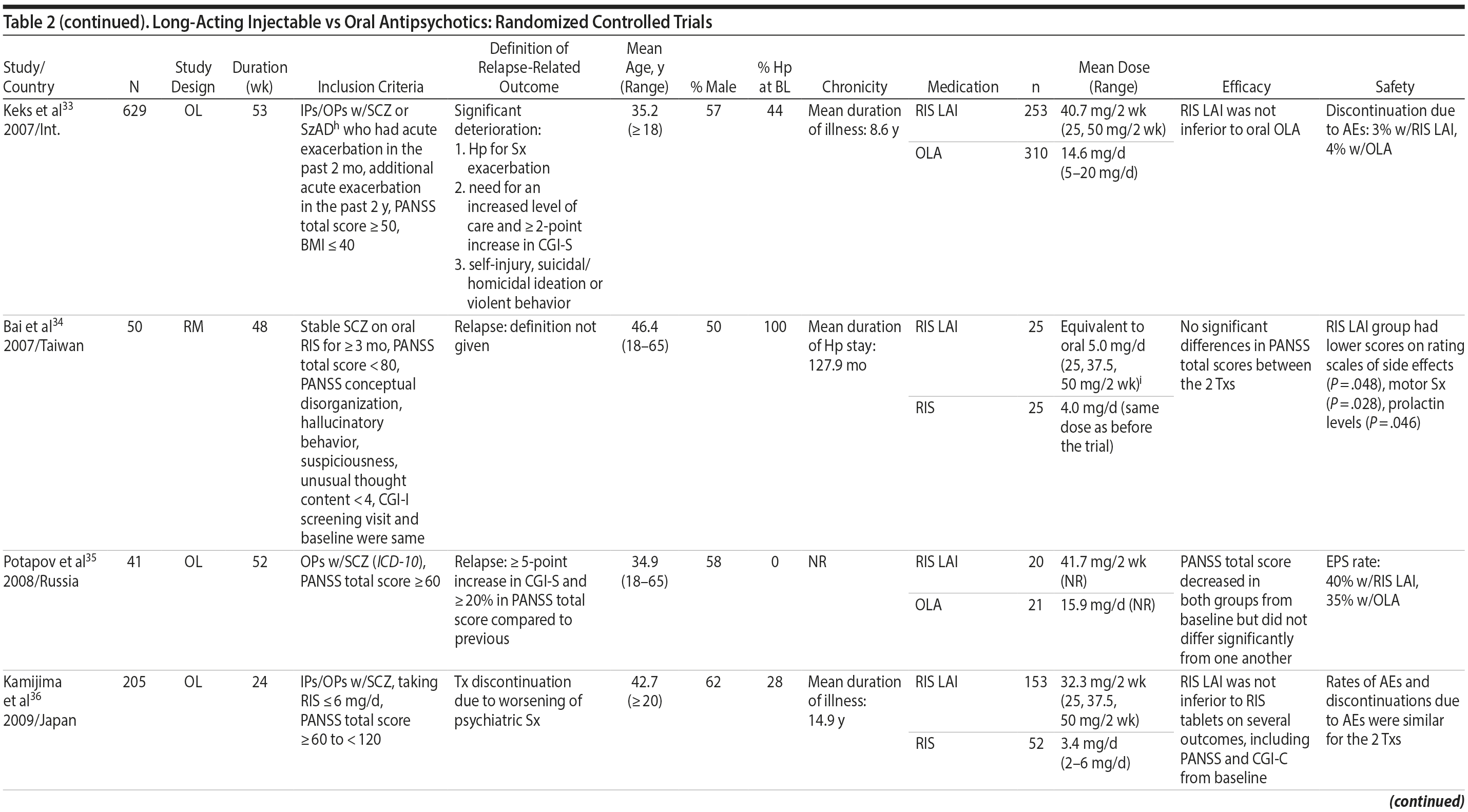
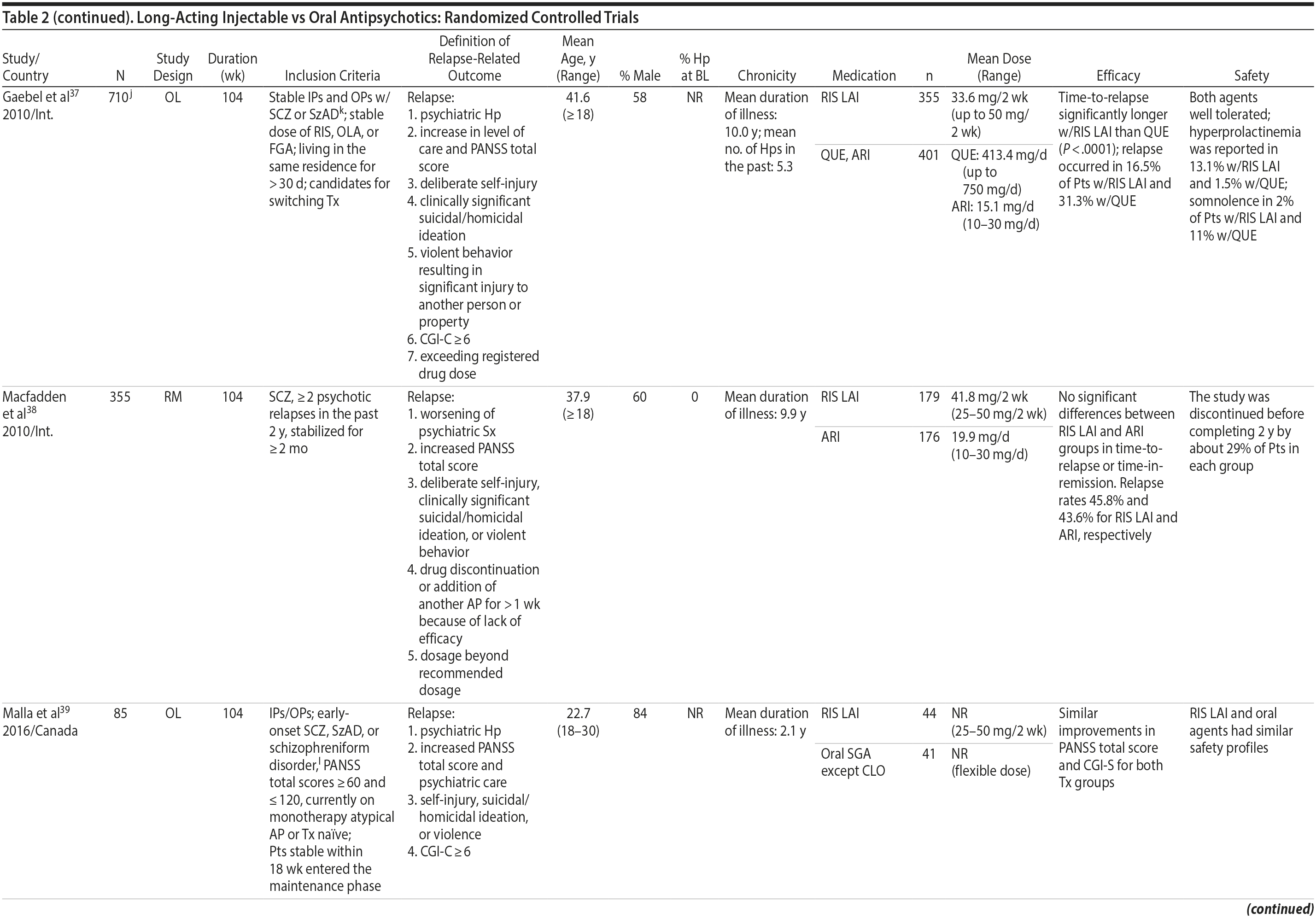
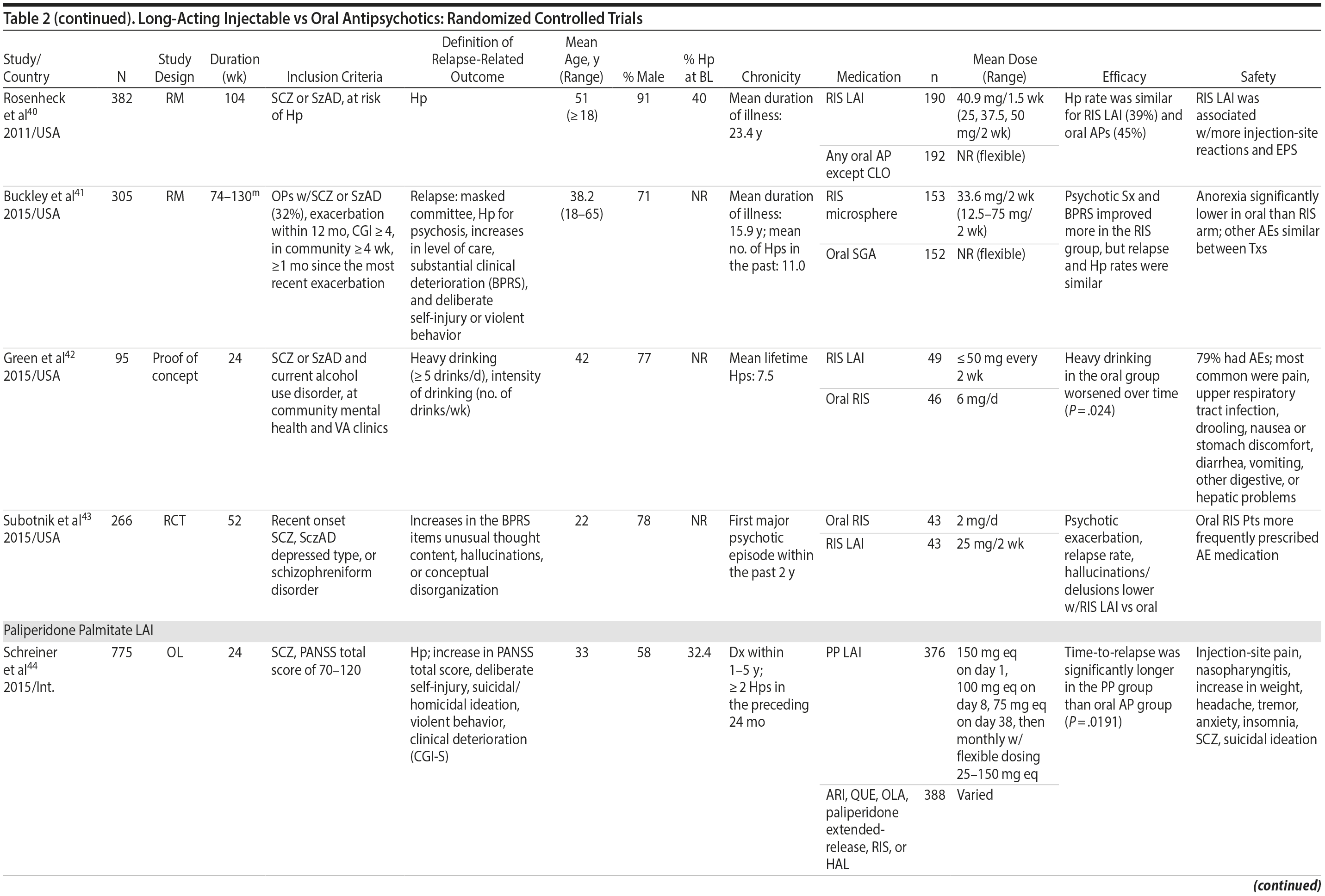
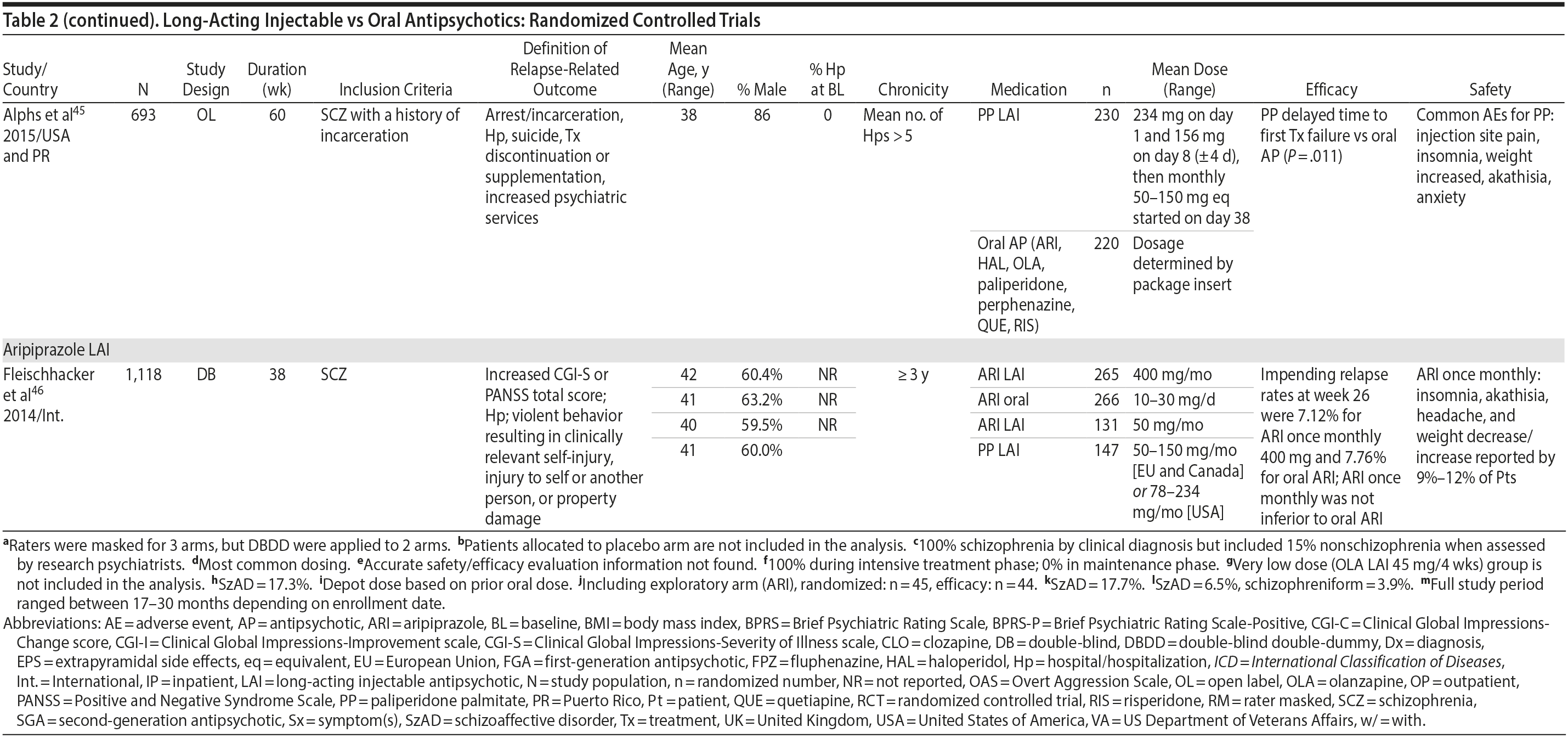
LAIs versus oral antipsychotics. Many trials and several meta-analyses have compared the efficacy of LAI versus oral antipsychotics for the treatment of patients with schizophrenia. A meta-analysis of 21 RCTs (N = 5,176) found similar rates of relapse and all-cause discontinuation for patients treated with LAIs versus oral antipsychotics.18 Although the results of this analysis suggest that treatment outcomes appeared similar for LAIs and oral therapy, it should be recognized that the meta-analysis was associated with certain limitations. The studies varied in patient enrollment criteria and the ways in which relapse was defined. Most RCTs enrolled chronically ill patients with schizophrenia who had received antipsychotics for months or years. Most importantly, patients willing to be randomized to blinded treatment were likely less severely ill than those receiving LAIs in clinical care, and they also were more likely to be adherent to oral antipsychotics.18,19 It is also likely that trial participation itself had an impact on adherence to oral therapy, given enhanced attention as compared to usual practice. The results of studies that compared LAIs to oral antipsychotic agents are summarized in Table 2.20-46
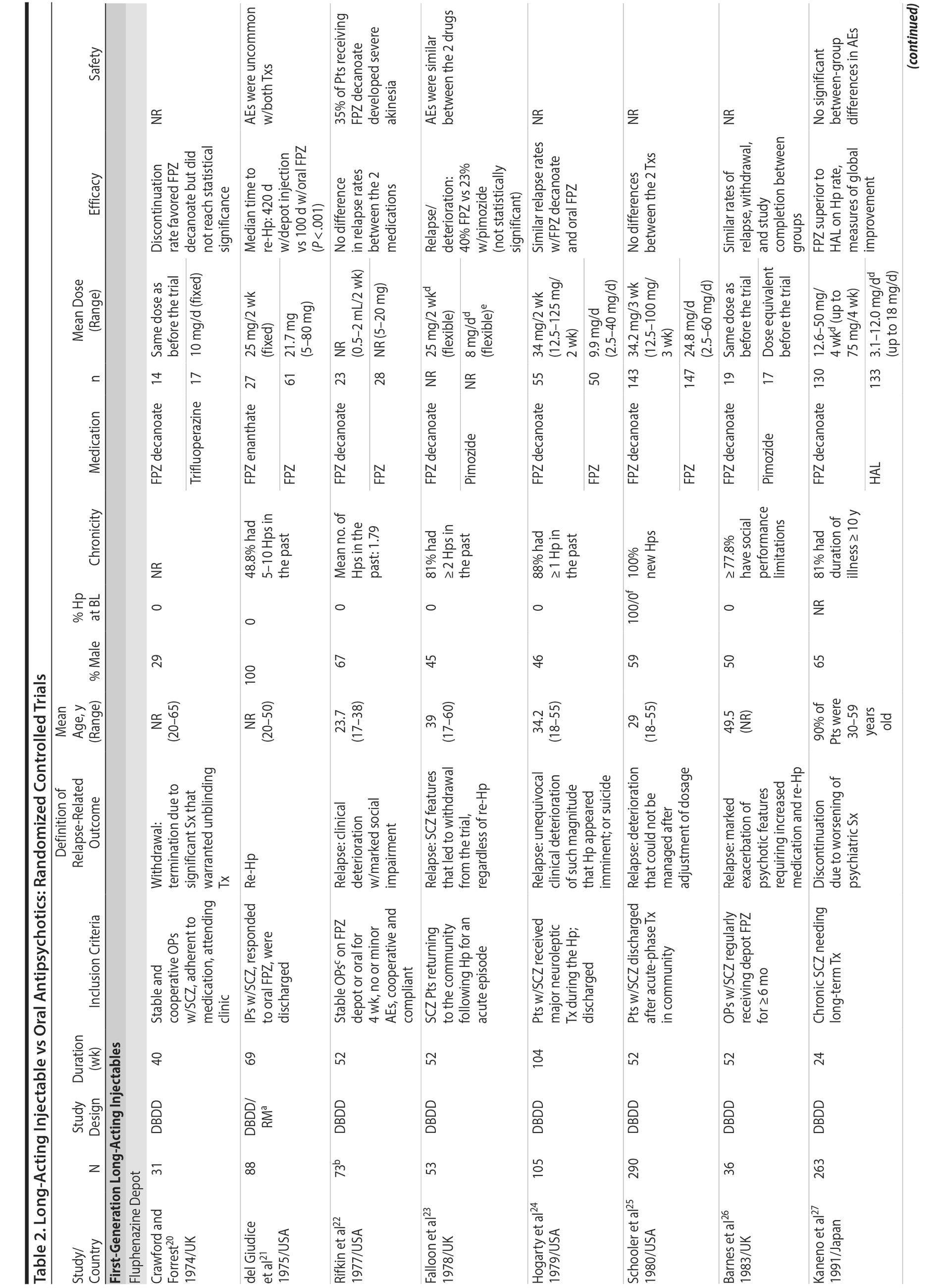
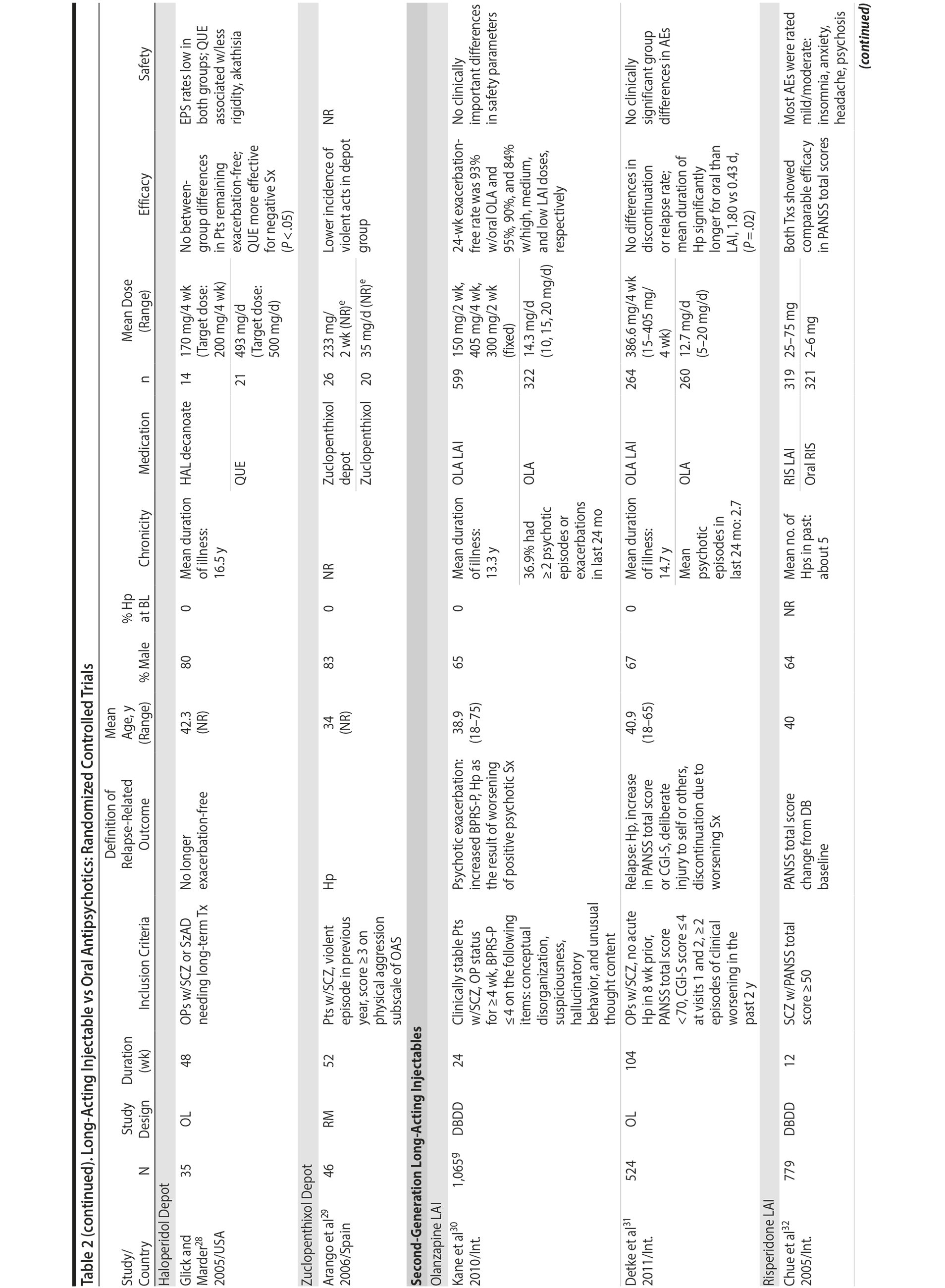
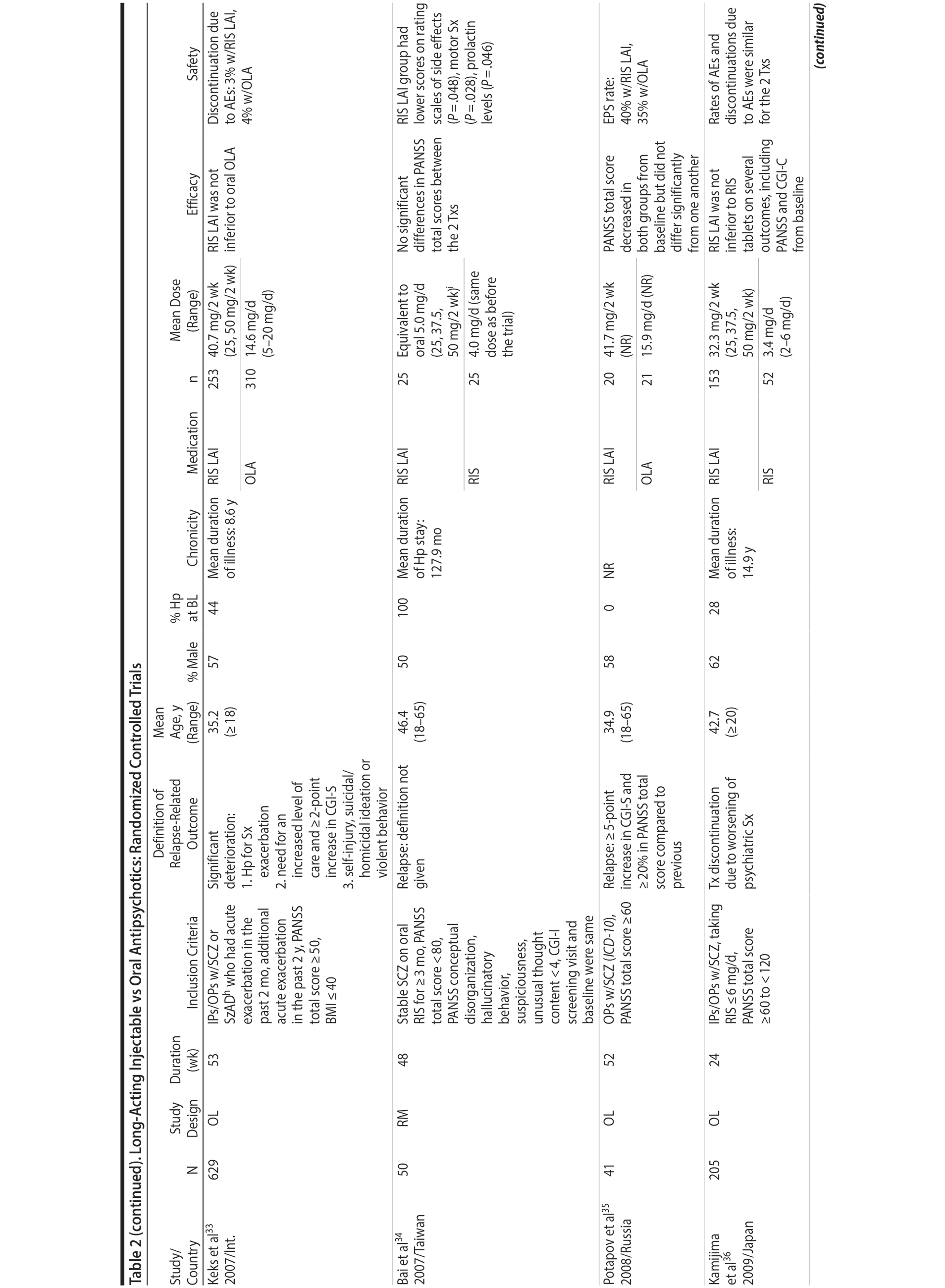
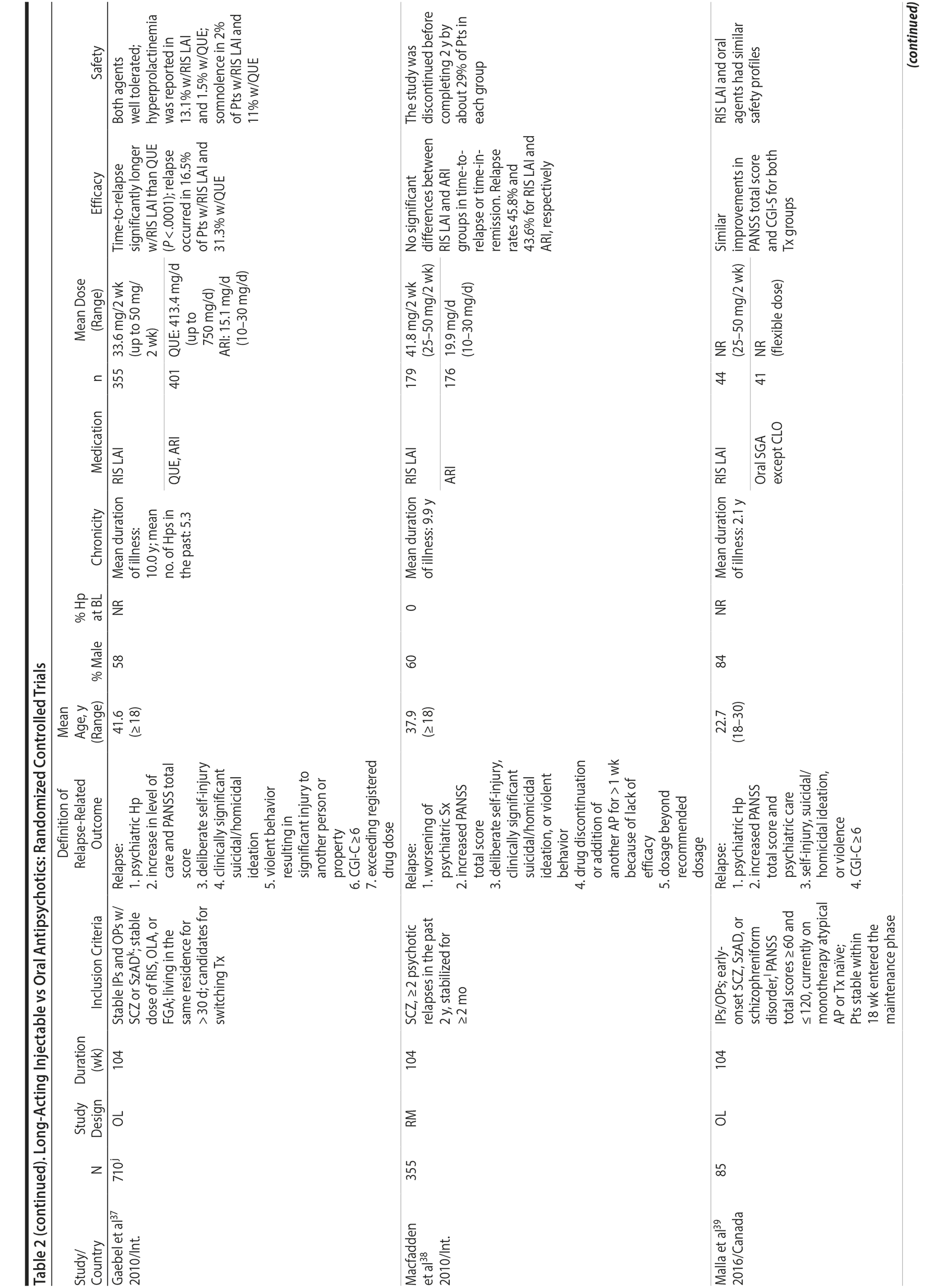
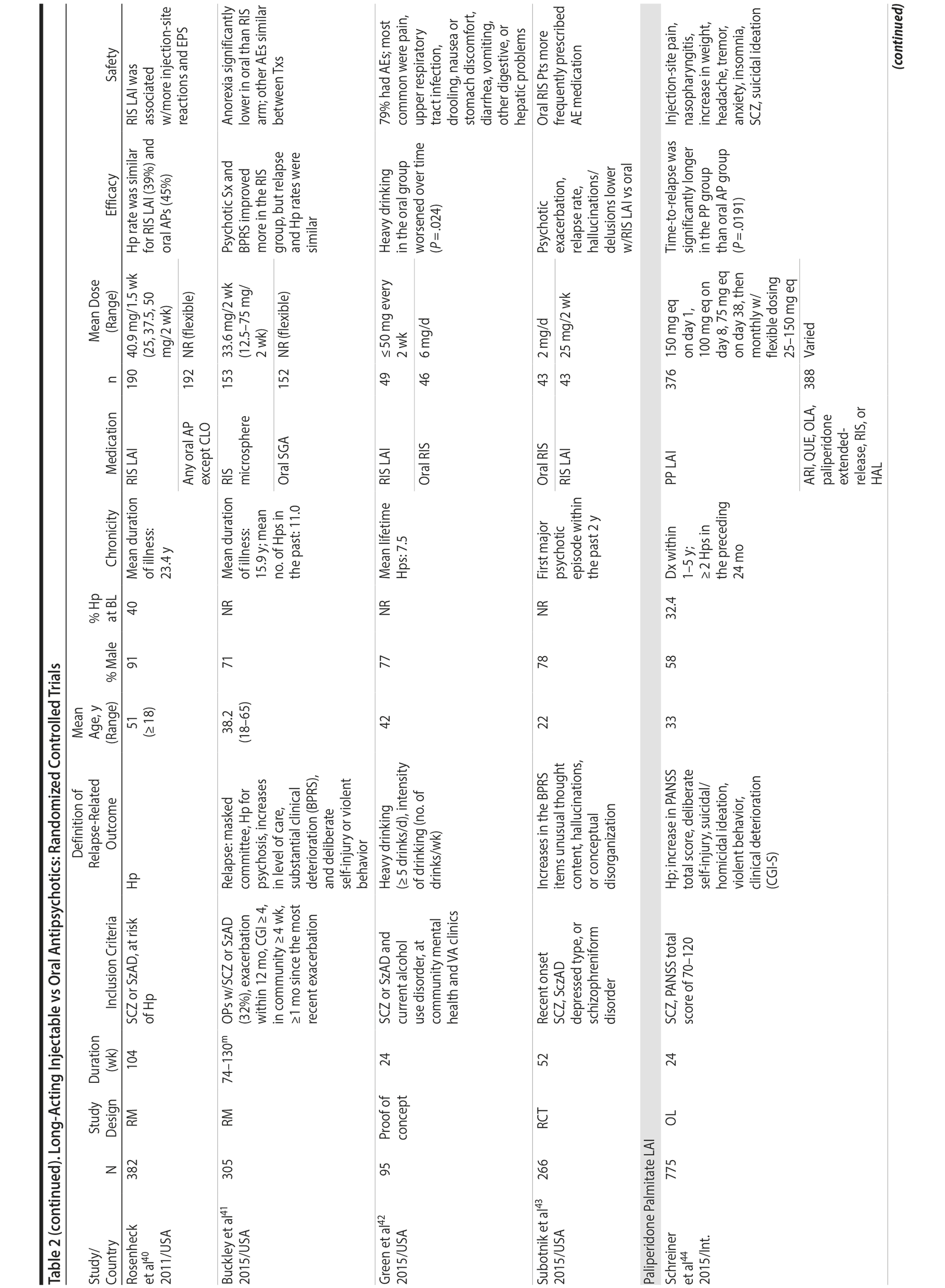
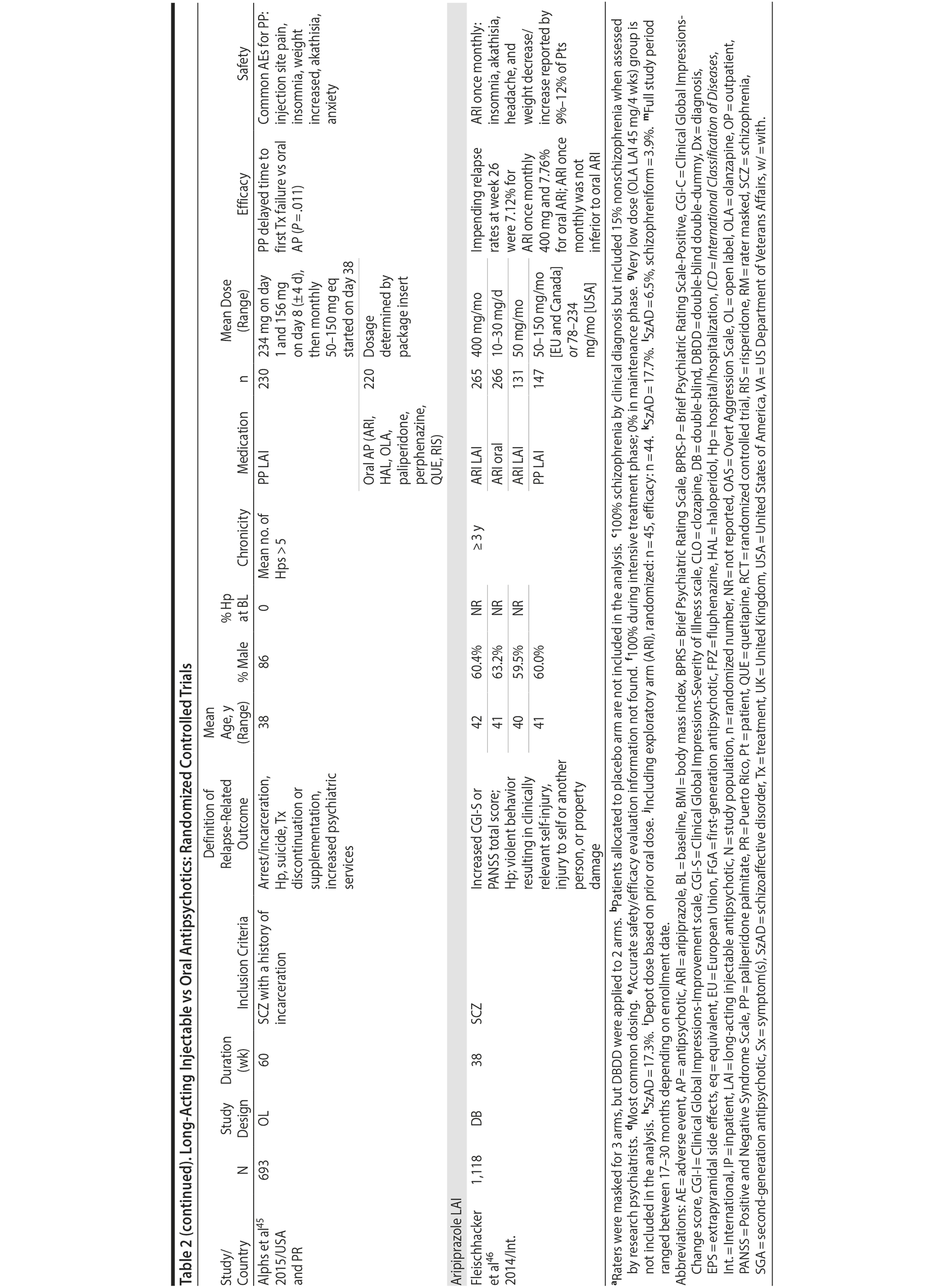
Strong evidence for the superiority of LAIs over oral antipsychotics is demonstrated by mirror-image studies, in which patients receive LAIs after an initial treatment with oral antipsychotics, with each patient serving as his or her own control. A meta-analysis of 25 mirror-image studies (N = 4,066) found that LAIs were superior to oral antipsychotics for preventing psychiatric hospitalization.47 The results of mirror-image studies that compared outcomes before and after switching to LAIs can be found in references 48 through 72 (see also Supplementary eTable 1 at PSYCHIATRIST.COM). Although mirror-image studies assess more representative patients than RCTs, they have methodological drawbacks, which are discussed later (see Implications of Study Design).73
LAIs may be especially useful in early-phase or first-episode patients, a population that is characterized by frequent nonadherence. Two recently published RCTs provided evidence for efficacy of LAIs in early-phase schizophrenia. In a 12-month study of 86 first-episode patients, relapse and/or exacerbation of psychosis was noted for 5% of patients randomized to an LAI antipsychotic versus 33% with oral risperidone (relative risk reduction, 84.7%; P < .001).43 In another study, recently diagnosed (≥ 1-5 years) patients with schizophrenia were randomized to up to 2 years of open-label, rater-blinded treatment with paliperidone palmitate (n = 352) versus investigator’s choice of oral antipsychotic (aripiprazole, olanzapine, quetiapine, paliperidone extended-release, risperidone, or haloperidol; n = 363).74 Time to relapse was significantly longer for patients randomized to paliperidone palmitate than to oral antipsychotics (P = .0191); relapse rates were also lower (14.8% vs 20.9%; P = .032). In a prospective, nationwide cohort study conducted in Finland (N = 2,588), depot antipsychotics (risperidone depot, zuclopenthixol decanoate, haloperidol decanoate, or perphenazine decanoate) were associated with a significantly reduced risk of rehospitalization compared with the same antipsychotics in oral form (hazard ratio, 0.36; P = .007).75 A very recent randomized open-label study showed no advantage for LAIs over oral antispsychotics (see Table 2).39
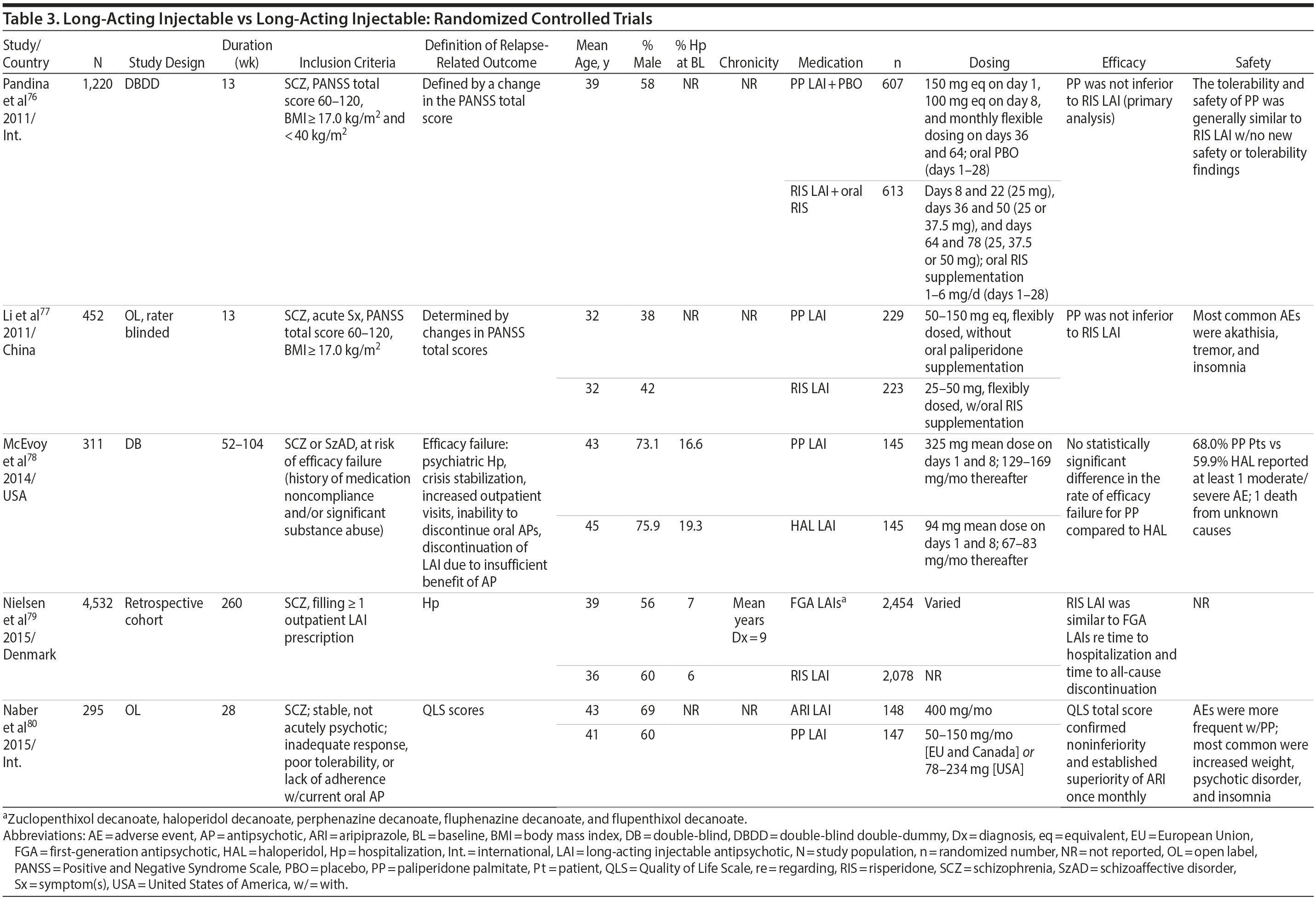
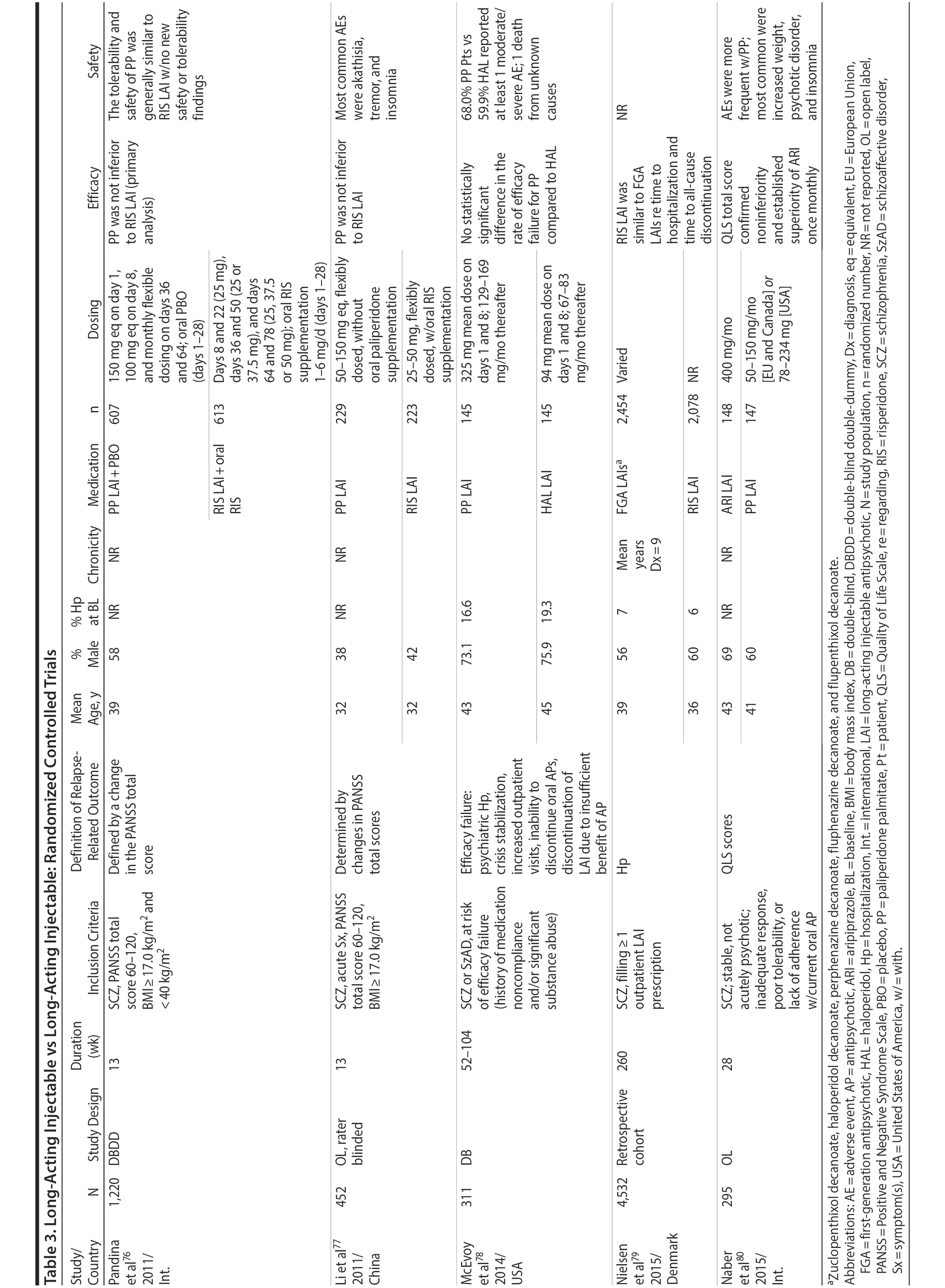
LAIs versus LAIs. There is little evidence for superior efficacy of one LAI versus another. The results of studies that compared one LAI versus another are summarized in Table 3.76-80 Although symptom improvement and relapse rates are generally similar with different LAIs, differences between LAIs have been reported for some other efficacy measures. For example, a recently published study (N = 295) compared aripiprazole once monthly 400 mg with paliperidone palmitate once monthly on a rating scale of quality of life and functioning in patients with schizophrenia.80 At the end of the 28-week study, the mean change from baseline on quality of life total score was significantly higher for patients treated with once monthly aripiprazole than paliperidone palmitate (P = .036), and the effect was driven by differences in younger patients (≤ 35 years).
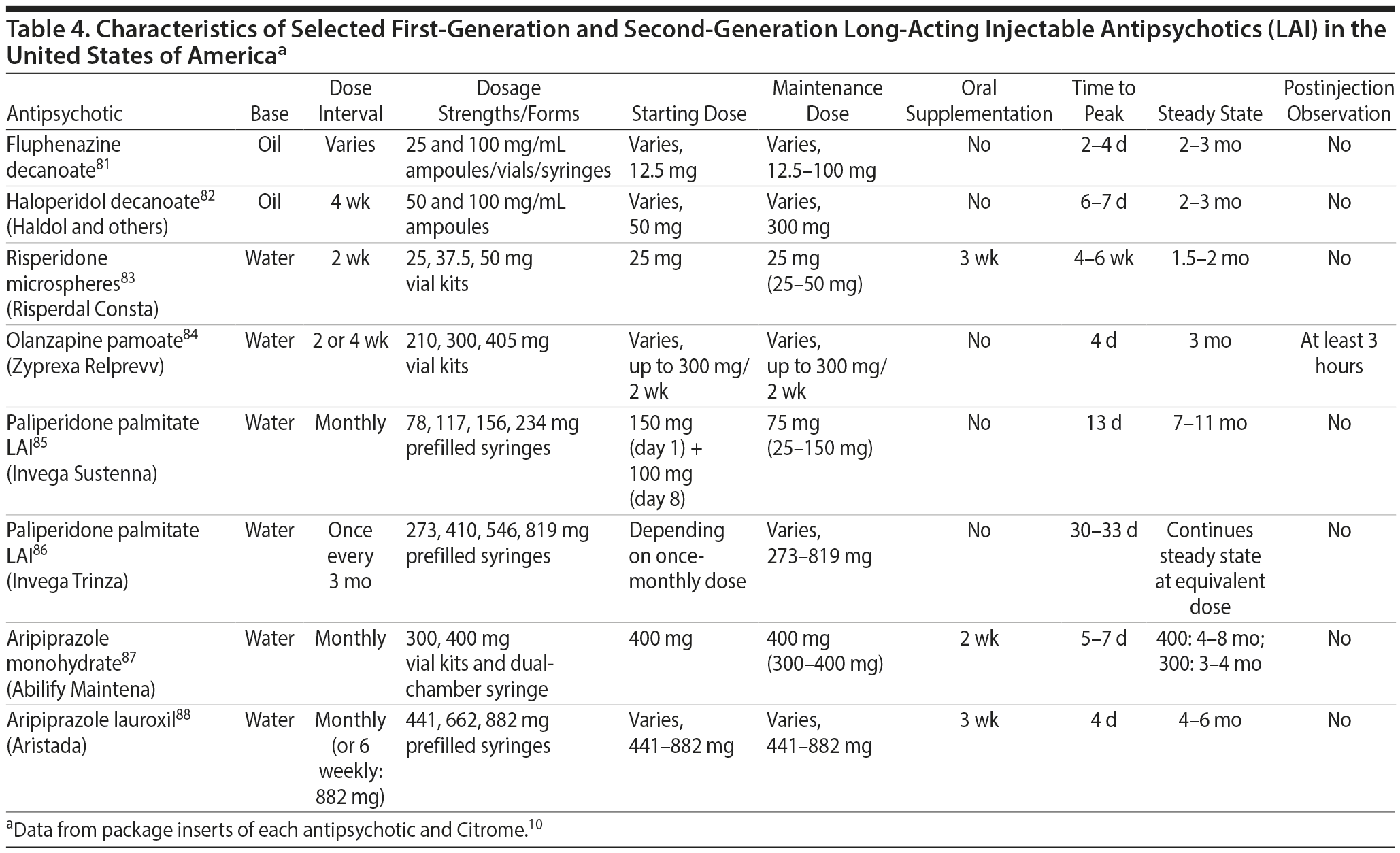
Characteristics of LAIs. Characteristics of selected FGA and SGA LAIs are summarized in Table 4.10,81-88 FGA LAIs (fluphenazine decanoate, haloperidol decanoate) are supplied in oil-based formulations, which may be more painful to inject than water-based formulations of SGA LAIs. Injection intervals vary from once every 2 to 4 weeks (or monthly) with most agents, to 6 weeks with aripiprazole lauroxil 882 mg, and once every 3 months with paliperidone palmitate LAI. Oral supplementation is required during the first few weeks of treatment with some medications, including risperidone LAI (3 weeks), aripiprazole LAI (2 weeks), and aripiprazole lauroxil (3 weeks). Time to reach steady-state plasma concentration varies considerably among the different agents. Olanzapine pamoate requires a 3-hour postinjection observation period due to potential postinjection delirium and sedation.89
Summary and conclusions. LAIs are superior to placebo for acute and maintenance treatment of schizophrenia. Superiority of LAIs over oral antipsychotics is most pronounced in mirror-image studies that arguably are more representative of usual care patients and practices. In general, LAIs appear to be similar to one another in terms of relapse prevention, although differences in other domains have been reported.
Safety and Tolerability of LAIs Versus Placebo, Versus Oral Antipsychotics, and Among LAIs
Side effect liability is an important consideration when selecting antipsychotics. Antipsychotics differ in their propensity to cause a wide range of side effects including sedation, extrapyramidal symptoms (EPSs), weight gain, metabolic disturbance, and prolactin elevation.90 However, a simple division of FGAs and SGAs in terms of side effect profiles is today generally seen as simplistic and misleading, although in the past it was advocated. Adverse events (AEs) may contribute to poor treatment adherence and increased long-term morbidity, and they may limit the maximal level of functional recovery that patients can achieve.91,92 Importantly, patients and physicians may differ in their perceptions of the importance of AEs. The roundtable participants felt that patients are more likely to respond to the subjective distress produced by side effects, whereas clinicians typically focus more on the objective severity of the AE and how this affects patient safety and risk. All of these issues should be addressed through shared decision-making and psychoeducational approach.93
Comparison of adverse events associated with antipsychotic drugs. AEs associated with LAIs generally follow the known AE profiles of the oral molecule. In a large meta-analysis of 15 antipsychotics in schizophrenia, antipsychotics were ranked by 5 different AE domains (sedation, EPSs, weight gain, prolactin elevation, and QTc elevation). Results indicated small to large differences in adverse events among antipsychotics90 that should be taken into consideration also when choosing among LAIs. A more recent meta-analysis of 16 RCTs (n = 4,902) showed that of 119 reported adverse events, LAIs and oral antipsychotics did not differ significantly, aside from akinesia, low-density lipoprotein cholesterol change, anxiety (higher with LAIs), and prolactin change (lower with LAIs).93a
Differences in AE rates between antipsychotic drugs may be quantified using number needed to harm (NNH). NNH answers the question “How many patients would you need to treat with Intervention A instead of Intervention B before you would expect to encounter one additional outcome of interest that you would like to avoid?” In general, NNH values < 10 for medication versus placebo denote potentially common AEs that can be expected to be seen frequently in day-to-day clinical practice.94-96 As shown in Supplementary eTable 2, NNH versus placebo can help to determine how often we can expect to encounter important adverse outcomes such as weight gain ≥ 7%, somnolence, or akathisia with different atypical antipsychotics.97,98
Overall, treatment discontinuation rates have generally been similar for patients treated with LAI antipsychotics versus the same oral agent.99 The most common AEs with LAIs are summarized in Supplementary eTable 3.82-88
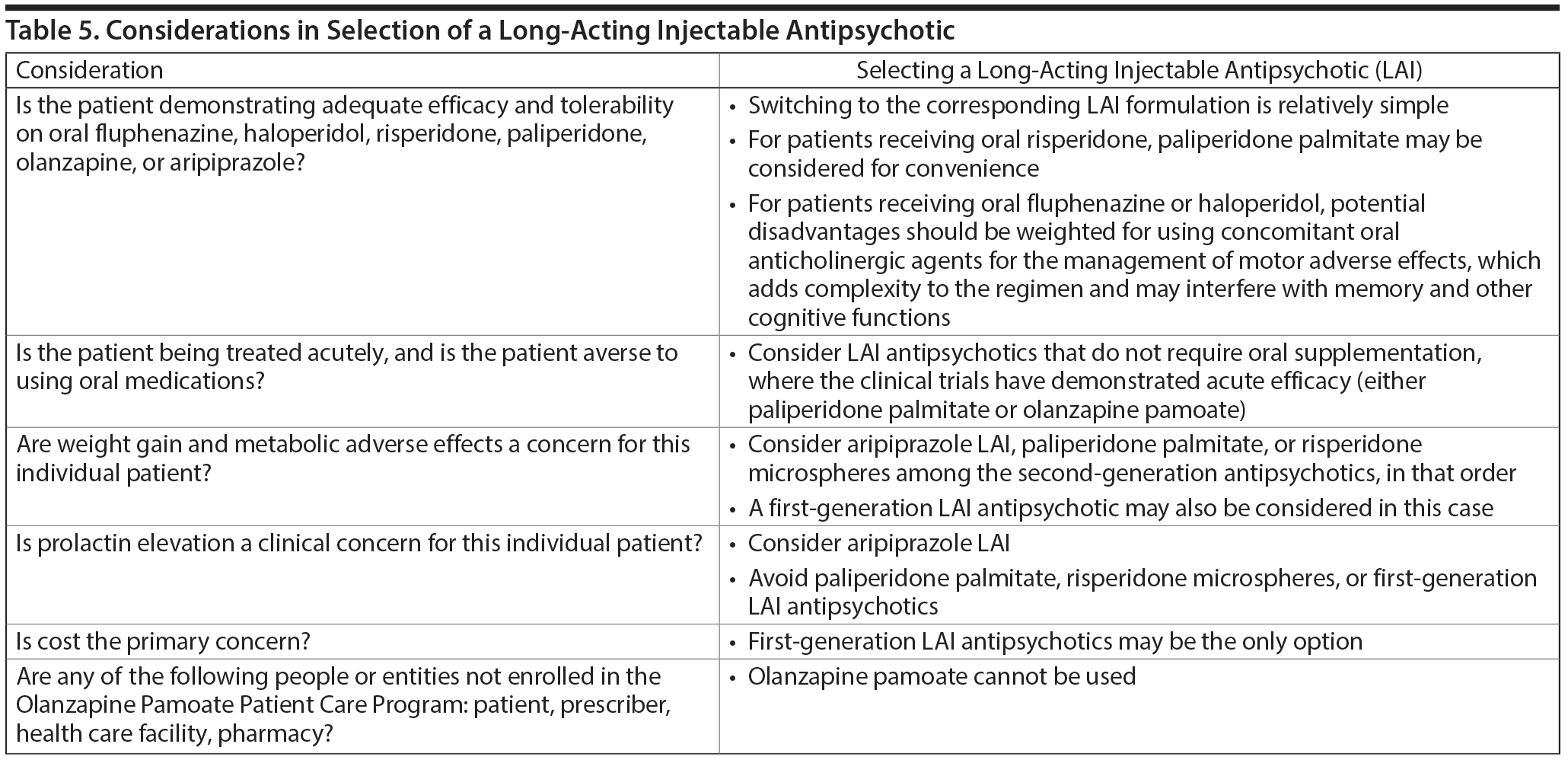
Considerations in choosing an LAI. Practical issues that can help in selecting among LAIs are summarized in Table 5.97
Summary and conclusions. Information about adverse event differences among LAIs comes largely from indirect comparisons and spontaneously reported AEs. The available data suggest that LAIs vary considerably in their propensity to cause certain adverse effects, including weight gain, EPSs, and prolactin elevation. This information can be used to help guide the selection of LAIs.
Implications of Study Design
LAIs have been examined using several study design strategies—RCTs, mirror-image studies, and cohort studies—each of which has strengths and limitations.18,73,77
RCTs are usually considered the “gold standard” for comparing the efficacy and safety of different treatments. However, explanatory RCTs are most likely not the optimal study design for the comparison of LAIs with oral antipsychotics. Strengths of RCTs include objective rating of patient outcomes and the elimination of biases on the part of investigators, including expectations about the different treatments. However, this approach may not be the best way to study interventions with potential adherence benefits, as patients enrolled in RCTs may differ from the general patient population in important ways, including higher levels of motivation or willingness to comply with instructions. RCT patients may also have less severe disease than many of those seen in typical practice. In addition, the trial itself, with reminders for appointments, more comprehensive assessments, payments for participation, free medication, and so on, can impact adherence rates.
Mirror-image studies examine patients who are switched from one medication to another, comparing pretreatment with posttreatment study periods. This design is more reflective of actual clinical practice than an RCT. Expectation bias is inherent in the design of a mirror-image study and may affect the main outcome. In mirror-image studies of LAIs, patients have been switched from oral antipsychotics to LAIs, but no studies have examined reversing this switching sequence. This design is also subject to potential time or cohort effects (eg, changing hospitalization practices over time).
In cohort studies, patient selection bias is reduced compared with other study types. However, the selection of medication in open studies may introduce bias by improving adherence. More importantly, patients selected for treatment with LAIs in cohort studies may be categorically different than those treated with oral antipsychotics, including having greater severity of illness and less illness insight or psychosocial support. Therefore, it is important to identify and adjust for confounding factors. One analysis of outcomes from LAI studies found that as study designs shift toward real-world patient populations, LAIs are associated with a larger magnitude of improvement on outcomes, such as relapses, hospitalizations, and all-cause discontinuation.100
Summary and conclusions. LAIs have been studied in RCTs, mirror-image studies, and cohort studies, each of which has its own strengths and limitations. Therefore, different methodological issues must be considered in the design and interpretation of clinical studies examining the effects of LAIs, creating a full picture only when viewed from these different angles.
Effect of LAIs on Adherence and Costs
Medication nonadherence in schizophrenia. Studies have demonstrated that approximately one-third of patients with schizophrenia are poorly adherent to oral medications at any time, whether this is evaluated using reports from patients, family members, other caregivers, or clinicians.101-104 More importantly, nonadherence is even higher when patients are followed over time. For example, in a study105 of more than 34,000 patients with schizophrenia in the Veterans Health Administration, approximately one-third were nonadherent in any one year, but more than 60% of patients were nonadherent at some point during the 4-year study, where nonadherence was defined as an entire year with a medication possession ratio < 0.8. Consistent nonadherence across all 4 years of the study was noted for 18% of the patients. This suggests that medication adherence is suboptimal but also varies over the long-term treatment course. Detection of nonadherence in clinical practice is often challenging, and adherence assessed by patient self-report or physician judgment may be markedly lower than adherence measured using quantitative techniques, such as pill counting, pharmacy records, or blood antipsychotic level tests.106 Potential clinical consequences of undetected medication nonadherence include unnecessary antipsychotic medication or dosage changes, addition of concomitant medications, and labeling of patients as “treatment resistant.”106,107 A 3-year, prospective study108 that examined functional outcomes associated with treatment adherence in patients with schizophrenia found that nonadherent patients had higher rates of several adverse outcomes, including psychiatric hospitalization (26.8% vs 14.1% for nonadherent vs adherent patients, respectively), emergency care (10% vs 6%), arrest (8.4% vs 3.5%), violent behaviors (10.8% vs 4.8%), being the victim of a crime (15.1% vs 7.8%), and substance misuse (31.1% vs 21.5%).108
Poor treatment adherence is usually considered the primary clinical indication for LAI use, yet studies have reported that fewer than 20% of patients with schizophrenia receive LAIs, even when there is evidence of recent poor treatment adherence.40,109
Health care costs. Studies have examined how the use of LAIs affects overall health care cost of patients with schizophrenia. One study110 compared treatment costs for patients with schizophrenia or schizoaffective disorder who were randomized to either risperidone LAI (n = 187) or the physician’s choice of an oral antipsychotic (n = 182). Overall, mean quarterly outpatient medication costs were higher for patients randomized to LAI ($3,028) than oral medication ($1,913; P = .003), although total treatment costs did not differ significantly between the two treatments ($14,916 vs $13,980; P = .73). Health care utilization and costs have also been compared among propensity score-matched adults with schizophrenia in the Veterans Health Administration system who initiated use of either LAI or oral antipsychotics. During the 12-month follow-up period, patients treated with LAI compared to oral antipsychotics had significantly lower average inpatient costs, higher average pharmacy costs, and similar total health care costs.111
A recent Medicaid health care utilization study112 in the United States compared health care utilization and treatment costs for hospitalized patients with schizophrenia who had been on short-duration LAI treatment (defined as 30-79 days; n = 2,856) versus longer-term LAI treatment (≥ 180 days; n = 2,838). The longer-term LAI patients had significantly lower levels of some health care utilization measures, including mean number of hospitalizations and mean length of hospital stay. Mean total hospital payments were 26% lower for patients in the long-term LAI group than those in the short-term LAI group, suggesting that the economic benefit of LAI therapy may increase over time. Lin and colleagues113 compared real-world health care costs and medication adherence between patients with schizophrenia who initiated LAI (n = 394) versus oral antipsychotics (n = 2,610) using medical claims data from commercially insured patients. Schizophrenia-related hospital costs decreased by a mean of $5,981 in the LAI group and increased by a mean of $758 for patients who received oral antipsychotics (P < .001). Mean outpatient cost increased by $134 versus $568 for the LAI and oral antipsychotic groups, respectively (P = .023). The mean drug cost was $4,132 with LAIs versus $2,562 with oral agents (P < .001). Similar outcomes were observed in patients with Medicare coverage.
The impact of LAI use on health care costs has also been examined in several international studies. A mirror-image study conducted in Taiwan48 examined treatment costs from medical claims data during 1 year before and after initiating LAI therapy. After a switch from oral to LAI antipsychotics, mean costs decreased for some outcomes (eg, inpatient services, other nonmedication services) but increased for others (eg, outpatient psychiatry, medication costs). A mirror-image study performed with patients treated in public hospitals in Hong Kong114 found that switching from oral to LAI therapy was associated with significantly lower total medical costs driven largely by lower hospitalization costs, although outpatient department and pharmacy costs significantly increased during the LAI treatment period. By contrast, a mirror-image study conducted in the United Kingdom,115 which included predominantly patients with schizophrenia, reported that in the year following LAI therapy initiation, total health care costs significantly increased along with inpatient bed days, although the number of inpatient admissions declined. These unexpected results may be partially explained by the high level of illness severity reflected in the large proportion of study patients who started LAI therapy as inpatients. In a study conducted in Sweden,116 investigators modeled per-patient costs associated with several sequences of LAI or oral antipsychotics, including total costs associated with medical care, institutional care, and indirect costs. Treatment strategies that used LAIs had lower total 1-year treatment costs than strategies that included oral antipsychotic therapy. A strategy of paliperidone palmitate LAI followed by olanzapine LAI for patients with relapses was considered not only cost-effective but also cost-saving for the health care system as a whole, compared with other antipsychotic strategies.
A prospective observational study117 that recruited and followed adults with schizophrenia from 10 European countries provides additional support for health care savings related to LAI therapy. Among outpatients who were previously medication nonadherent, those who initiated FGA LAIs were significantly more likely to be medication adherent (55%) than those who initiated FGA oral agents (39%) during the 18-month follow-up period. The total schizophrenia-related treatment costs of the patients treated with LAIs were only one-half of those incurred by the patients treated with oral antipsychotics. Finally, a study conducted in Canada118 compared health care resource use during 1 year before and after initiation of LAI treatment in 1,992 patients with schizophrenia or schizoaffective disorder. Overall 1-year costs associated with health care utilization were significantly lower after a switch to LAI therapy ($27,234 vs $16,987 for the preinitiation vs the postinitiation year; P < .001).
Summary and conclusions. Nonadherence in patients with schizophrenia is common and difficult to detect. Although LAIs may provide one method to help improve treatment adherence, only a minority of medication nonadherent patients receive them. Significant reductions in health care utilization or costs associated with schizophrenia have been demonstrated in some studies of LAI antipsychotics, although other studies have not demonstrated these effects and showed cost-neutrality or even greater cost.
Part 2: Practical Considerations and Recommendations Regarding LAI Use
When to Consider LAI Treatment: Patient Eligibility and Selection
Guidelines for LAI use. Several schizophrenia management guidelines, including those by the American Psychiatric Association, recognize LAIs as a treatment option but usually only when nonadherence to oral medication has resulted in repeated schizophrenia relapses or when LAI is preferred by the patient.119-122 However, even when guidelines recommend LAIs as an option if preferred by the patient, many patients may not be aware that LAIs are available.
Patient and mental health provider perceptions of LAIs. The use of LAIs in clinical practice may depend to a large degree on provider and patient attitudes, which are closely related to previous and current experiences. In a survey of attitudes about LAIs among patients with schizophrenia shortly before hospital discharge, acceptance of LAI therapy was 73% among patients who were current users of LAIs (n = 60), 45% among past users of LAIs (n = 95), and 23% among LAI-naive patients (n = 145).123 These data show that patients are more likely to favor their current treatment. Similar findings were reported in a study of outpatients with schizophrenia or schizoaffective disorder in the United Kingdom.124 Perceptions about LAI use may also differ among health care providers. In a survey in the United Kingdom,125 most psychiatrists (91%) felt that LAIs were as efficacious as oral medications and improve patient adherence (81%) and prevent relapse (94%); however, despite this, 48% felt that depot medications are stigmatizing, and 69% believed LAI antipsychotics are less acceptable to patients. However, psychiatrists’ knowledge about LAIs was positively associated with more favorable attitudes (r = 0.39, P < .001). When these data were compared to data from a survey of nurses in the United Kingdom, the nurses were significantly more likely than the psychiatrists to characterize LAIs as coercive, compromising of patient autonomy, or more bothersome to prescribe and monitor than oral medication.126
Why are psychiatrists reluctant to use LAIs? Despite the high rate of nonadherence and the consequences of poor adherence among patients with schizophrenia, many psychiatrists remain reluctant to use LAI antipsychotics. Most psychiatrists say that they are interested in using LAI antipsychotics only if they can be clearly shown to be superior to oral agents. For example, in a survey of 106 German psychiatrists, most favored an LAI only if it was associated with an absolute decrease in relapse rate of 10% compared with oral therapy.127
In addition, many clinicians lack knowledge about practical issues in the use of LAIs, including dose selection, pharmacokinetics, and what to do when a patient is late for an injection or has persistent symptoms after starting therapy. Younger staff members may have little or no experience with FGA LAIs. Many clinicians mistakenly believe that LAIs are associated with a greater side effect burden than oral agents, among other misconceptions about LAI treatment. Clinician attitudes may also be a barrier to LAI use. Physicians often overestimate the treatment adherence of their own patients, and they may have concerns about suggesting LAIs to their patients because of beliefs about stigmatization or coercion.125,128
Although several studies suggested possible benefits of LAIs in first-episode schizophrenia,43,74,75 surveys of clinicians have shown that many psychiatrists regard LAIs as inappropriate for first-episode patients.128,129 In a study conducted in the United Kingdom, approximately one-third of psychiatrists thought that LAIs were always inappropriate for first-episode patients, whereas in a German study approximately 70% of psychiatrists thought that LAIs were inappropriate for a first episode.128,129 In many cases, physician beliefs and perceptions about LAIs may prevent patients from learning that LAIs are a potential option.130,131 For example, in a study of communication patterns in the offer of LAIs made by psychiatrists to patients with schizophrenia at 10 health clinics, psychiatrists generally presented LAIs in a negative light, resulting in only 11 of 33 LAI recommendations (33%) being accepted by patients.132 However, during a postvisit interview, during which LAIs were presented in a more positive light and with more information, 27 of the 28 patients (96%) who declined the initial recommendation changed their mind, stating that they actually would be willing to try LAI treatment.
Other obstacles to LAI use include service barriers (eg, lack of community nurses to administer injections, failure to consider partnering with primary care providers to administer maintenance LAI treatment) and financial barriers (eg, higher acquisition costs, payer reluctance to cover LAIs unless there is clear documentation of nonadherence, clinician and payer failure to consider the total costs associated with treating the illness).123,125
What patient and illness factors should influence LAI use? Several factors may favor the use of LAI therapy in patients with schizophrenia:
- Willingness by clinicians to consider LAI treatment
- Early-phase or first-episode schizophrenia, as these patients usually have the most to gain by remaining in remission and the most to lose through relapse (eg, in terms of education or employment)
- A history of nonadherence with oral medication
- Risk factors that are associated with increased risk of poor adherence, such as younger age, comorbid substance misuse, or lack of insight
- Factors that suggest a high risk of relapse and that relapse is associated with significant clinical risk, such as a history of psychosis associated with vulnerability, self-harm, or aggression or a history of violence
- Preference of LAI by the patient
Conversely, LAIs may be less suitable for some patients, including those who demonstrate intolerance to or inefficacy with the parent compound, establish good adherence with oral therapy, have a strong preference for oral therapy, or require chronic anticoagulation therapy. Furthermore, cost considerations may limit access to LAIs in certain states or settings.
What factors should influence LAI selection? As the efficacy of different LAIs is generally similar, side effect profiles are often a key consideration when selecting an LAI. However, few studies have directly compared side effect profiles of different LAIs in the treatment of schizophrenia. Rubio and colleagues133 compared risperidone LAI and zuclopenthixol decanoate in patients with schizophrenia and substance misuse. Over 6 months of follow-up, risperidone LAI was associated with fewer EPSs as well as more improvement on the Positive and Negative Syndrome Scale and better adherence to a substance use management plan. McEvoy and colleagues78 compared haloperidol LAI and paliperidone LAI in patients with schizophrenia, reporting greater weight gain and prolactin elevation but less akathisia with paliperidone LAI, although changes in glucose and lipid parameters and in the overall rate of EPS were similar between the two treatment groups. Finally, Naber and colleagues80 examined use of aripiprazole LAI and paliperidone LAI in patients with schizophrenia, reporting that aripiprazole LAI was associated with numerically fewer adverse events and treatment discontinuations and significantly greater improvement in interviewer-rated quality of life scores.
In the absence of an extensive body of research comparing the safety profiles of different LAIs, it is reasonable to extrapolate from the oral formulation of the same drug. Meta-analysis has quantified the relative risk of a range of side effects using data from RCTs of oral antipsychotics.90 Cost is often an important consideration in treatment selection, with acquisition costs of SGA LAIs higher than those of FGA LAIs. Finally, the patient’s current oral regimen is also an important consideration. If the patient is well stabilized on one oral medication, switching to a different medication in a LAI formulation might be associated with a risk of relapse or new adverse effects. In such cases, there would be an argument for using the same antipsychotic in LAI form, assuming this was available.
Summary and conclusions. Schizophrenia treatment guidelines generally emphasize nonadherence and relapse with oral antipsychotic agents as the most important reasons for LAI use. Barriers to LAI use in current practice include clinicians’ lack of knowledge and negative attitudes about LAIs, resource issues, and cost. Those who might benefit from LAIs include first-episode patients and patients early in the course of psychosis as well as patients with known poor adherence, high risk of nonadherence, lack of insight, and the potential for significant consequences associated with relapse.
Best Practices to Maximize LAI Acceptability and Experience for Stakeholders
The appropriate use of antipsychotics is a concern not only for patients and physicians but also for many additional stakeholder groups, including family and friends, employers, court-appointed guardians, law enforcement and the judiciary, and society as a whole. In some cases, stakeholder groups may have differing interests and concerns. For example, in a survey of perceptions about medication use, patients with schizophrenia were less likely to agree that the good things about medication outweigh the bad (61% of patients) than were psychiatrists (81%) or family members (80%).130 Stakeholder groups may also differ in their attitudes toward other issues, such as medication cost, access to care, and reimbursement.
Patient-centered medicine. The concept of patient-centered medicine provides one approach that can help optimize LAI treatment and find a balance between the concerns and considerations of patients and physicians. Patient-centered medicine seeks to focus attention first on the needs and concerns of the patient, rather than the physician, and to consider social and economic factors.134 Several steps may help to maximize alignment of treatment goals between patient and provider. The clinician should take a thorough history and listen carefully to the patient’s account and beliefs. The patient should be given time to make his or her views known and to ask questions. Consideration of the patient’s past positive and negative treatment experiences is critical to developing a successful treatment plan. The clinician should be flexible, adjusting treatment when appropriate to make sure the patient has a voice in his or her care. However, the clinician should not agree to a treatment plan that is not clinically indicated or that could result in patient harm. Patient-centered care relies heavily on collaboration between the provider, the patient, the patient’s family members, and other caregivers as well as a broader support network, such as close friends or clergy. Patients should be encouraged to be involved in all aspects of planning, delivery, and evaluation of their health services, with particular emphasis on empowering patients and family members to make effective decisions.135
Education. Psychoeducation for patients about schizophrenia and the benefits and risks of its treatment is clearly a critical part of this process and may be especially important in making decisions about the use of LAIs. Psychoeducation should also reinforce the concept that the patient is “an expert” by experience and that the patient should be involved in the development of the treatment plan. Education should also include a plan to improve adherence, crisis management, and prevention of relapses and suicide.
Likewise, education about the potential benefits of LAIs should also be provided to clinicians and the health care team.136 Health care providers should be able to anticipate and address issues that patients have about LAIs. The first step in this educational process is to acknowledge concerns about using LAIs. An overview of the benefits and limitations of LAIs should be followed by detailed education about LAI therapy and its most appropriate uses. Team members are often the first line of contact to identify patients for whom LAIs may improve outcomes and to educate those patients and their families about this treatment option.
There is also a need to educate clinicians regarding practical issues associated with the dosing and switching of LAIs and what to do should adverse effects emerge. The LAIs currently available each have specific and unique characteristics. The clinician needs to understand how each LAI should be initiated (eg, test dosing using the oral counterpart, the need for loading doses or necessary oral coverage). Selecting the appropriate dose can be informed by previous experiences with the oral version; dosage may need to be titrated and adjusted based on clinical presentation. There is also some flexibility in dosing intervals, and each LAI has recommendations for what to do if the patient misses his or her injection appointment.
Finally, payers also need to be educated and encouraged to look beyond the short-term financial decisions around drug cost and consider the potential long-term benefits of reduced hospitalizations, relapses, and quality of life. Research recommendations to help make this case as well as financial analyses within health systems will be helpful in this regard. Furthermore, payers and industry should be encouraged to take a “value-based” approach to payment for and pricing of drugs so that access is more broadly assured.
Summary and conclusions. Patient-centered medicine involves aligning the priorities of the patient and the provider to arrive at a shared vision of care. For patients with schizophrenia, this shared vision might include agreement about the goals of therapy, a discussion of whether LAI use is helpful for meeting these goals, and offering an LAI if indicated. Patient and provider should also arrive at a flexible arrangement for administration of the LAI, which might include administration at the physician’s office or clinic, in the pharmacy, at a health care home, or possibly even in the patient’s own home.
Additional LAI Considerations
Beyond efficacy and safety data for LAIs, the panel discussed a number of additional factors that should be considered when weighing the use of LAI therapy. These factors impact multiple different stakeholders, including patients and their families, providers, and payers.
Nonadherence. Although efficacy of LAIs and oral antipsychotics has been generally similar in RCTs, some studies have demonstrated benefits of LAIs in first-episode patients, a population that is associated with a high rate of nonadherence.44,75 First-episode patients, or patients early in the course of psychosis, may be especially likely to discontinue treatment because they do not accept the chronic nature of the illness. In addition, nonadherence is frequently unrecognized, and current medication adherence should not preclude the use of LAIs, since an important goal of therapy should be to prevent future nonadherence, which is always a possibility for patients with schizophrenia. Although often used in research studies, the medication possession ratio alone is not a sufficient indicator of treatment adherence. Patients may fill a prescription but then fail to take the medication. Together, these observations suggest that LAIs should not be viewed simply as a treatment for patients with poor adherence.
Currently, patients who are adherent to antipsychotic therapy may not meet criteria for insurance reimbursement of LAIs. The roundtable experts recommended that patients should not have to first prove that they have relapsed on oral medications before becoming eligible for LAIs. Relapses produce detrimental effects at many levels, including societal, individual, biological, and psychosocial. Preventing the likelihood of future relapses should be an important goal of therapy, even for those who do not yet have a history of relapse.
Relapses. Patients may relapse because of lack of drug effectiveness but also because of changes in the use of other drugs, home care, or other environmental factors. In many cases, the reason for a relapse is unknown, and scant research has examined why patients relapse on medication. Clinicians may be unsure of what to do when a patient relapses on an LAI or how to switch from LAI to another antipsychotic (oral or another LAI), and more research is needed to better define the optimal approach to patients with relapsing schizophrenia while on LAI therapy. Blood antipsychotic levels may help in understanding whether relapses occur because patients are not receiving an adequate medication dose, although these levels are not always obtained in routine clinical practice.137
One of the most important issues in schizophrenia care in the United States is the gap between in-hospital treatment for an acute episode and the transition to outpatient care after discharge. The observations that LAIs reduce relapse risk in acutely ill patients suggest that these agents provide an important strategy to help bridge this gap and ensure that patients leave the hospital with effective antipsychotic coverage.
Severity of episode or relapse. The patient’s type and history of relapse may also be important considerations when weighing the potential benefits of LAI treatment. For example, continuous coverage with LAI treatment may be considered especially desirable when there is a history of relapse associated with violence, self-harm, or self-neglect.
Very little research has focused on the treatment of patients with LAIs beginning in the emergency room, which could provide more data regarding relapse. A clinical trial could investigate randomization at the time of initial contact with the emergency department or as soon as possible afterward to treatment with an LAI versus home or in-hospital treatment with oral antipsychotics. However, accessing the patient’s treatment history while the patient is in the emergency department may be difficult at some facilities, and the informed consent process might delay randomization.
Cognitive and memory problems. Patients with cognitive impairment or memory problems may have difficulty remembering to take medication as prescribed, and they may also overestimate their own adherence to treatment when asked. LAIs are a good choice to reduce the impact of forgetting on antipsychotic adherence.
Ease of use. Aside from issues of comparative efficacy or safety of LAI versus oral formulations, LAIs offer many patients the benefit of improved convenience. Some patients may simply prefer to receive a single injection every 2, 4, 6, or even 12 weeks rather than being required to take oral medication every day. LAIs that offer longer intervals between administrations may be even more convenient for patients. Patients and families should understand that longer injection intervals do not mean that the patient will not be seen by the physician or other mental health team members; they should know that the patient will continue to have regular follow-up visits and that the injection schedule is distinct from the schedule of follow-up appointments.
For providers, there may be differences in ease of use among the available LAIs. For example, risperidone microspheres and paliperidone palmitate require specialized injection needles with a modified internal bore, which is larger than that of a conventional needle. With aripiprazole lauroxil, there is an opportunity to administer the second injection as early as 14 days after the first, according to the product label.88 This LAI also provides longer coverage against a late injection (up to a month on the 662 mg or 882 mg doses) without needing to restart oral supplementation.
Substance misuse. Substance misuse is one of the most common reasons for poor adherence to antipsychotics,138 yet patients with substance misuse are often excluded from RCTs. Data from the Paliperidone Palmitate Research in Demonstrating Effectiveness (PRIDE) study, which included patients with substance misuse problems among other patient groups who are often excluded from clinical studies, suggest that these patients are more likely to benefit from an LAI compared to an oral antipsychotic.45
Access and cost. Issues of access and cost may be viewed from many perspectives, including those of the patient, the provider, insurers, and the health care system as a whole. In fact, many of these issues will vary by state or geographic region.
An important barrier to LAI access is the fact that many patients have copayments or deductibles. In addition, many regions are underserved by health care professionals, especially those with experience in mental health. It might be possible to improve patient access to care through the use of 2-way video or other telemedicine approaches. There may also be significant differences in access to LAI therapy from state to state.
The cost of LAIs presents a barrier to their use. However, from the health system perspective, although LAI products are associated with higher drug acquisition costs, they may save money across the entire continuum of care due to reduced relapses or hospitalizations. A review of 28 studies that examined health care costs associated with LAIs or oral antipsychotics found that most studies demonstrated lower overall treatment costs with LAIs.139 In studies in the United States, cost-effectiveness analyses are largely influenced by number of hospital days, and even substantially greater medicine acquisition costs can be offset by small changes in the required days of hospitalization. Potential cost savings were shown on a system-wide basis in Canada118 and Sweden.116 There is a clear need for a large-scale cost study in the United States. Although 30-day hospital readmission rate is often used as a measure of care quality, this time period may be too short to adequately evaluate the effects of LAIs on quality and service utilization, and post hoc analyses have demonstrated that the benefits of LAIs are greater for patients who have been on treatment for longer periods of time.140,141
Stigma. LAIs are most frequently recommended for patients with schizophrenia who have a history of poor adherence to medication. Patients or families may view the physician’s recommendation of LAI therapy as a form of punishment or as a sign that the patient is very ill or has recently worsened.124 However, it should be recognized that there are many different types of nonadherence. Some patients exhibit what might be referred to as “distracted nonadherence,” which results from issues such as cognitive impairment or disorganization. Other patients exhibit insight-based nonadherence (ie, the patient does not believe that he or she has an illness or that the illness requires medication) or accessibility-based nonadherence (eg, lack of insurance coverage). Patients with distracted or insight-based nonadherence may respond favorably to the suggestion of LAI use as a lifestyle benefit that reduces the need to take daily medication. This may be especially true for patients who work night shifts or otherwise experience many daily stressors. In this way, the physician is not recommending an LAI because the patient is at fault for not taking medication, but simply as a way to make the patient’s life simpler. This approach may help to reduce some of the stigma associated with nonadherence. When talking to patients, it may also help to note that long-acting treatment strategies are increasingly common in other areas of medicine, such as rheumatoid arthritis or infusion pumps for patients with diabetes. It might also be preferable to present LAIs as a tool that might be used for a period of time rather than as something that the patient will remain on indefinitely. In some regions, patients may receive LAI injections directly from a pharmacist, without needing to come to the physician’s office. Because other people in the pharmacy might be receiving injections for any number of other conditions, including standard vaccinations, patients may experience less stigma when obtaining the injection. Laws about pharmacist-administered injections and access to this type of program vary from state to state.
Stigma about the use of LAI antipsychotics is not limited to patients and their families. Many health care professionals also have negative attitudes toward the use of these agents and may be unwilling to inform patients about the availability of this treatment option.125,126,129 It is critical to the care process that all health care providers are knowledgeable about the use of LAIs, including physicians, nursing staff, pharmacists, social workers, and others who are charged with patient care. Nurses in particular may play an important role in patient attitudes toward LAIs.120 Nurses spend the most time with patients and family and may have a significant influence on perception of LAI therapy. There is an urgent need for training of mental health professionals, including social workers, nurses, and peer counselors. Moreover, it is critical to identify the key aspects of LAI use that should be communicated to professionals in different disciplines. Exposure to using LAIs in a residency setting may reduce the perceived stigma associated with LAIs and improve confidence among providers regarding their use.
Finally, it is also important to emphasize the need to destigmatize nonadherence. It is sufficiently common across all of medicine to view nonadherence as an aspect of human nature, not “bad behavior.”
Level of social support. Poor adherence affects families as well as patients, and family members often help patients to ensure that they are adherent to medications. Family members, therefore, may have a key role in acting as advocates to help patients to use LAIs when appropriate. In addition, it can be difficult for patients with schizophrenia to advocate for themselves. Including families in the discussion can help to ensure that patients have an advocate who is educated about the role of LAIs in schizophrenia treatment. In addition, family members are often those who suffer the immediate and disturbing consequences of relapse. They are the ones who might find themselves needing to call the police, for example, when a loved one relapses and loses insight.
Conflict over and fear of nonadherence. Medication adherence can become a source of ongoing conflict between patients and providers. One potential advantage of LAIs is the removal of this source of conflict by changing how LAIs are approached and discussed, possibly strengthening the patient-provider relationship.
Autonomy. There is sometimes a concern among patients, family members, and even clinicians that LAIs diminish patient autonomy. Some may perceive an injection of LAI as similar to injecting a sedative for a patient with acute agitation. However, it should be stressed that in the case of LAIs, the patient voluntarily agrees to receive each injection. Agreeing to LAI therapy does not involve a surrender of autonomy on the part of the patient. The goal is to control the illness, not the patient.
Wish to control treatment and dosing. Because LAI treatment is administered only once every few weeks or months, some patients or providers may believe that it is difficult to adjust or control the medication dose. As LAIs are used as a maintenance treatment, there is likely to be less need to adjust the dose than would be the case during acute treatment. In addition, many LAIs are available in a range of doses, enabling dose adjustment. Finally, if symptoms of relapse occur during LAI treatment, then symptom control can often be most rapidly achieved by coprescribing an oral antipsychotic and simultaneously increasing the dose of the LAI. If the patient’s symptoms respond, then the oral supplementation can subsequently be withdrawn once sufficient time has elapsed for the LAI to reach a new steady state. Clinical experience suggests that it is also possible to adjust LAI dosing through the use of calendars and scheduling injection visits at a specific interval to attain higher or lower plasma drug concentrations. For example, if the label recommends injections every 26 to 28 days, an individual patient who is believed by his or her physician to be just below a therapeutic plasma drug concentration may be treated at a somewhat shorter dosing interval (eg, 25 days). Conversely, a patient who is thought to be at a higher-than-optimal plasma drug concentration might be treated once every 29 or 30 days.
Fear of needles and pain. Some patients are especially concerned about the potential for pain or discomfort with LAIs. Clinical experience suggests that the older depot antipsychotics that used oil-based vehicles were associated with more discomfort than the aqueous solution used with newer LAIs, although no head-to-head studies have investigated this. Selecting agents with smaller injection volumes or choosing agents with longer injection intervals may also reduce the discomfort associated with LAIs.
Potential for loss of functioning. LAIs may offer an important option when the potential for loss of normal functioning associated with a relapse could have important consequences for the patient. Examples include college students living away from home who have recently experienced a first psychotic episode and need to take treatment while away from their usual source of care/support, or individuals with employment that could be jeopardized by a relapse of schizophrenia.
Recommending LAI Therapy for Patients With Schizophrenia
Table 6 summarizes the opinions of participants regarding the importance of several clinical factors in the selection of LAIs and how these factors might influence decision-making by patients, health care professionals, family members, and payers.
Concluding Recommendations
Concluding recommendations from the panel are summarized in Table 7.
Drug names: aripiprazole (Abilify), aripiprazole lauroxil (Aristada), aripiprazole monohydrate (Abilify Maintena), clozapine (Clozaril, FazaClo, and others), haloperidol (Haldol and others), olanzapine (Zyprexa and others), olanzapine pamoate (Zyprexa Relprevv), paliperidone palmitate (Invega Sustenna, Invega Trinza), pimozide (Orap), quetiapine (Seroquel and others), risperidone (Risperdal and others), risperidone microspheres (Risperdal Consta).
Supplementary material: Available at PSYCHIATRIST.COM.
To obtain CME credit, go to http://programs.rmei.com/SZ/Assessment/posttest.html and complete the posttest and evaluation.
© Copyright 2016 by Physicians Postgraduate Press, Inc.
REFERENCES
1. Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216-226. PubMed doi:10.1002/wps.20060
2. Carbon M, Correll CU. Clinical predictors of therapeutic response to antipsychotics in schizophrenia. Dialogues Clin Neurosci. 2014;16(4):505-524. PubMed
3. Novick D, Haro JM, Suarez D, et al. Predictors and clinical consequences of non-adherence with antipsychotic medication in the outpatient treatment of schizophrenia. Psychiatry Res. 2010;176(2-3):109-113. PubMed doi:10.1016/j.psychres.2009.05.004
4. Heres S. Long-acting injectable antipsychotics: an underutilized treatment option. J Clin Psychiatry. 2014;75(11):1263-1265. PubMed doi:10.4088/JCP.14com09541
5. Robinson D, Woerner MG, Alvir JM, et al. Predictors of relapse following response from a first episode of schizophrenia or schizoaffective disorder. Arch Gen Psychiatry. 1999;56(3):241-247. PubMed doi:10.1001/archpsyc.56.3.241
6. Brissos S, Veguilla MR, Taylor D, et al. The role of long-acting injectable antipsychotics in schizophrenia: a critical appraisal. Ther Adv Psychopharmacol. 2014;4(5):198-219. PubMed doi:10.1177/2045125314540297
7. Pandina GJ, Lindenmayer JP, Lull J, et al. A randomized, placebo-controlled study to assess the efficacy and safety of 3 doses of paliperidone palmitate in adults with acutely exacerbated schizophrenia. J Clin Psychopharmacol. 2010;30(3):235-244. PubMed doi:10.1097/JCP.0b013e3181dd3103
8. Kane JM, Peters-Strickland T, Baker RA, et al. Aripiprazole once-monthly in the acute treatment of schizophrenia: findings from a 12-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(11):1254-1260. PubMed doi:10.4088/JCP.14m09168
9. Meltzer HY, Risinger R, Nasrallah HA, et al. A randomized, double-blind, placebo-controlled trial of aripiprazole lauroxil in acute exacerbation of schizophrenia. J Clin Psychiatry. 2015;76(8):1085-1090. PubMed doi:10.4088/JCP.14m09741
10. Citrome L. New second-generation long-acting injectable antipsychotics for the treatment of schizophrenia. Expert Rev Neurother. 2013;13(7):767-783. PubMed doi:10.1586/14737175.2013.811984
11. Berwaerts J, Liu Y, Gopal S, et al. Efficacy and safety of the 3-month formulation of paliperidone palmitate vs placebo for relapse prevention of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):830-839. PubMed doi:10.1001/jamapsychiatry.2015.0241
12. Kane JM, Eerdekens M, Lindenmayer JP, et al. Long-acting injectable risperidone: efficacy and safety of the first long-acting atypical antipsychotic. Am J Psychiatry. 2003;160(6):1125-1132. PubMed doi:10.1176/appi.ajp.160.6.1125
13. Lauriello J, Lambert T, Andersen S, et al. An 8-week, double-blind, randomized, placebo-controlled study of olanzapine long-acting injection in acutely ill patients with schizophrenia. J Clin Psychiatry. 2008;69(5):790-799. PubMed doi:10.4088/JCP.v69n0512
14. Hough D, Gopal S, Vijapurkar U, et al. Paliperidone palmitate maintenance treatment in delaying the time-to-relapse in patients with schizophrenia: a randomized, double-blind, placebo-controlled study. Schizophr Res. 2010;116(2-3):107-117. PubMed doi:10.1016/j.schres.2009.10.026
15. Kramer M, Litman R, Hough D, et al. Paliperidone palmitate, a potential long-acting treatment for patients with schizophrenia: results of a randomized, double-blind, placebo-controlled efficacy and safety study. Int J Neuropsychopharmacol. 2010;13(5):635-647. PubMed doi:10.1017/S1461145709990988
16. Nasrallah HA, Gopal S, Gassmann-Mayer C, et al. A controlled, evidence-based trial of paliperidone palmitate, a long-acting injectable antipsychotic, in schizophrenia. Neuropsychopharmacology. 2010;35(10):2072-2082. PubMed doi:10.1038/npp.2010.79
17. Kane JM, Sanchez R, Perry PP, et al. Aripiprazole intramuscular depot as maintenance treatment in patients with schizophrenia: a 52-week, multicenter, randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2012;73(5):617-624. PubMed doi:10.4088/JCP.11m07530
18. Kishimoto T, Robenzadeh A, Leucht C, et al. Long-acting injectable vs oral antipsychotics for relapse prevention in schizophrenia: a meta-analysis of randomized trials. Schizophr Bull. 2014;40(1):192-213. PubMed doi:10.1093/schbul/sbs150
19. Kane JM, Kishimoto T, Correll CU. Assessing the comparative effectiveness of long-acting injectable vs oral antipsychotic medications in the prevention of relapse provides a case study in comparative effectiveness research in psychiatry. J Clin Epidemiol. 2013;66(suppl):S37-S41. PubMed doi:10.1016/j.jclinepi.2013.01.012
20. Crawford R, Forrest A. Controlled trial of depot fluphenazine in out-patient schizophrenics. Br J Psychiatry. 1974;124(0):385-391. PubMed doi:10.1192/bjp.124.4.385
21. del Giudice J, Clark WG, Gocka EF. Prevention of recidivism of schizophrenics treated with fluphenazine enanthate. Psychosomatics. 1975;16(1):32-36. PubMed doi:10.1016/S0033-3182(75)71232-X
22. Rifkin A, Quitkin F, Rabiner CJ, et al. Fluphenazine decanoate, fluphenazine hydrochloride given orally, and placebo in remitted schizophrenics, I: relapse rates after one year. Arch Gen Psychiatry. 1977;34(1):43-47. PubMed doi:10.1001/archpsyc.1977.01770130045004
23. Falloon I, Watt DC, Shepherd M. A comparative controlled trial of pimozide and fluphenazine decanoate in the continuation therapy of schizophrenia. Psychol Med. 1978;8(1):59-70. PubMed doi:10.1017/S0033291700006632
24. Hogarty GE, Schooler NR, Ulrich R, et al. Fluphenazine and social therapy in the aftercare of schizophrenic patients: relapse analyses of a two-year controlled study of fluphenazine decanoate and fluphenazine hydrochloride. Arch Gen Psychiatry. 1979;36(12):1283-1294. PubMed doi:10.1001/archpsyc.1979.01780120013001
25. Schooler NR, Levine J, Severe JB, et al. Prevention of relapse in schizophrenia: an evaluation of fluphenazine decanoate. Arch Gen Psychiatry. 1980;37(1):16-24. PubMed doi:10.1001/archpsyc.1980.01780140018002
26. Barnes TR, Milavic G, Curson DA, et al. Use of the Social Behaviour Assessment Schedule (SBAS) in a trial of maintenance antipsychotic therapy in schizophrenic outpatients: pimozide versus fluphenazine. Soc Psychiatry. 1983;18(4):193-199. PubMed doi:10.1007/BF00583530
27. Kaneno S, Ohkuma T, Yamashita I, et al. A double blind comparative study on the efficacy and safety of fluphenazine decanoate (SQ10, 733) and oral haloperidol in the treatment of schizophrenic patients. Rinsho Hyoka (clinical evaluation). 1991;19:15-45.
28. Glick ID, Marder SR. Long-term maintenance therapy with quetiapine versus haloperidol decanoate in patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2005;66(5):638-641. PubMed doi:10.4088/JCP.v66n0515
29. Arango C, Bomb×n I, González-Salvador T, et al. Randomised clinical trial comparing oral versus depot formulations of zuclopenthixol in patients with schizophrenia and previous violence. Eur Psychiatry. 2006;21(1):34-40. PubMed doi:10.1016/j.eurpsy.2005.07.006
30. Kane JM, Detke HC, Naber D, et al. Olanzapine long-acting injection: a 24-week, randomized, double-blind trial of maintenance treatment in patients with schizophrenia. Am J Psychiatry. 2010;167(2):181-189. PubMed doi:10.1176/appi.ajp.2009.07081221
31. Detke HC, Weiden PJ, Llorca P-M, et al. Open-label comparison of olanzapine long-acting injection and oral olanzapine: a 2-year, randomized study in outpatients with schizophrenia. Poster presented at the NCDEU 51st Annual Meeting; June 13-16, 2011; Boca Raton, FL.
32. Chue P, Eerdekens M, Augustyns I, et al. Comparative efficacy and safety of long-acting risperidone and risperidone oral tablets. Eur Neuropsychopharmacol. 2005;15(1):111-117. PubMed doi:10.1016/j.euroneuro.2004.07.003
33. Keks NA, Ingham M, Khan A, et al. Long-acting injectable risperidone v. olanzapine tablets for schizophrenia or schizoaffective disorder: randomised, controlled, open-label study. Br J Psychiatry. 2007;191(2):131-139. PubMed doi:10.1192/bjp.bp.105.017020
34. Bai YM, Ting Chen T, Chen JY, et al. Equivalent switching dose from oral risperidone to risperidone long-acting injection: a 48-week randomized, prospective, single-blind pharmacokinetic study. J Clin Psychiatry. 2007;68(8):1218-1225. PubMed doi:10.4088/JCP.v68n0808
35. Potapov A, Tsukarzi E, Mosolov S. Response, remission and relapse during the long-term treatment of schizophrenia patients with long-acting injectable risperidone versus olanzapine. Int J Neuropsychopharmacol. 2008;11:158.
36. Kamijima K, Ishigooka J, Komada Y. Comparison study between risperidone long-acting injectable and risperidone tablets in patients with schizophrenia. Jpn J Clin Psychopharmacol. 2009;12:1199-1222.
37. Gaebel W, Schreiner A, Bergmans P, et al. Relapse prevention in schizophrenia and schizoaffective disorder with risperidone long-acting injectable vs quetiapine: results of a long-term, open-label, randomized clinical trial. Neuropsychopharmacology. 2010;35(12):2367-2377. PubMed doi:10.1038/npp.2010.111
38. Macfadden W, Ma YW, Thomas Haskins J, et al. A prospective study comparing the long-term effectiveness of injectable risperidone long-acting therapy and oral aripiprazole in patients with schizophrenia. Psychiatry (Edgmont). 2010;7(11):23-31. PubMed
39. Malla A, Chue P, Jordan G, et al. An exploratory, open-label, randomized trial comparing risperidone long-acting injectable with oral antipsychotic medication in the treatment of early psychosis. Clin Schizophr Relat Psychoses. 2016;9(4):198-208. PubMed doi:10.3371/CSRP.MACH.061213
40. Rosenheck RA, Krystal JH, Lew R, et al; CSP555 Research Group. Long-acting risperidone and oral antipsychotics in unstable schizophrenia. N Engl J Med. 2011;364(9):842-851. PubMed doi:10.1056/NEJMoa1005987
41. Buckley PF, Schooler NR, Goff DC, et al; PROACTIVE Study. Comparison of SGA oral medications and a long-acting injectable SGA: the PROACTIVE study. Schizophr Bull. 2015;41(2):449-459. PubMed doi:10.1093/schbul/sbu067
42. Green AI, Brunette MF, Dawson R, et al. Long-acting injectable vs oral risperidone for schizophrenia and co-occurring alcohol use disorder: a randomized trial. J Clin Psychiatry. 2015;76(10):1359-1365. PubMed doi:10.4088/JCP.13m08838
43. Subotnik KL, Casaus LR, Ventura J, et al. Long-acting injectable risperidone for relapse prevention and control of breakthrough symptoms after a recent first episode of schizophrenia: a randomized clinical trial. JAMA Psychiatry. 2015;72(8):822-829. PubMed doi:10.1001/jamapsychiatry.2015.0270
44. Schreiner A, Aadamsoo K, Altamura AC, et al. Paliperidone palmitate versus oral antipsychotics in recently diagnosed schizophrenia. Schizophr Res. 2015;169(1-3):393-399. PubMed doi:10.1016/j.schres.2015.08.015
45. Alphs L, Benson C, Cheshire-Kinney K, et al. Real-world outcomes of paliperidone palmitate compared to daily oral antipsychotic therapy in schizophrenia: a randomized, open-label, review board-blinded 15-month study. J Clin Psychiatry. 2015;76(5):554-561. PubMed doi:10.4088/JCP.14m09584
46. Fleischhacker WW, Sanchez R, Perry PP, et al. Aripiprazole once-monthly for treatment of schizophrenia: double-blind, randomised, non-inferiority study. Br J Psychiatry. 2014;205(2):135-144. PubMed doi:10.1192/bjp.bp.113.134213
47. Kishimoto T, Nitta M, Borenstein M, et al. Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry. 2013;74(10):957-965. PubMed doi:10.4088/JCP.13r08440
48. Chang HC, Tang CH, Huang ST, et al. A cost-consequence analysis of long-acting injectable risperidone in schizophrenia: a one-year mirror-image study with national claim-based database in Taiwan. J Psychiatr Res. 2012;46(6):751-756. PubMed doi:10.1016/j.jpsychires.2012.02.019
49. Rosa F, Schreiner A, Thomas P, et al. Switching patients with stable schizophrenia or schizoaffective disorder from olanzapine to risperidone long-acting injectable. Clin Drug Investig. 2012;32(4):267-279. PubMed doi:10.2165/11599080-000000000-00000
50. Crivera C, DeSouza C, Kozma CM, et al. Resource utilization in patients with schizophrenia who initiated risperidone long-acting therapy: results from the Schizophrenia Outcomes Utilization Relapse and Clinical Evaluation (SOURCE). BMC Psychiatry. 2011;11(1):168. PubMed doi:10.1186/1471-244x-11-168
51. Ren XS, Crivera C, Sikirica M, et al. Evaluation of health services use following the initiation of risperidone long-acting therapy among schizophrenia patients in the Veterans Health Administration. J Clin Pharm Ther. 2011;36(3):383-389. PubMed doi:10.1111/j.1365-2710.2010.01211.x
52. Peng X, Ascher-Svanum H, Faries D, et al. Decline in hospitalization risk and health care cost after initiation of depot antipsychotics in the treatment of schizophrenia. Clinicoecon Outcomes Res. 2011;3:9-14. PubMed
53. Carswell C, Wheeler A, Vanderpyl J, et al. Comparative effectiveness of long-acting risperidone in New Zealand: a report of resource utilization and costs in a 12-month mirror-image analysis. Clin Drug Investig. 2010;30(11):777-787. PubMed doi:10.2165/11537680-000000000-00000
54. Girardi P, Serafini G, Pompili M, et al. Prospective, open study of long-acting injected risperidone versus oral antipsychotics in 88 chronically psychotic patients. Pharmacopsychiatry. 2010;43(2):66-72. PubMed doi:10.1055/s-0029-1239541
55. Su KP, Chang HC, Tsai SJ, et al. Relapse and long-acting injectable risperidone: a 1-year mirror image study with a national claims database in Taiwan. Value Health. 2009;12(suppl 3):S118-S121. PubMed doi:10.1111/j.1524-4733.2009.00643.x
56. Lam A, Chue P, Ligate L, et al. P.3.c.068 efficacy outcomes of risperidone long acting injection in patients previously on oral atypicals versus conventional depots. Dur Neuropsychopharmacol. 2009;19:S549-S550. doi:10.1016/S0924-977X(09)70875-2
57. Fuller M, Shermock K, Russo P, et al. Hospitalisation and resource utilisation in patients with schizophrenia following initiation of risperidone long-acting therapy in the Veterans Affairs Healthcare System. J Med Econ. 2009;12(4):317-324. PubMed doi:10.3111/13696990903303902
58. Beauclair L, Lam A, McCormick J, et al. Impact of risperidone long-acting injectable on hospitalization and medication use in patients with schizophrenia. Value Health. 2005;8(6):A202-A203. doi:10.1016/S1098-3015(10)67769-8
59. Bourin M, Jolliet P, Hery P, et al. Is rehospitalization a measure of the efficacy of neuroleptics in the treatment of schizophrenia? Int J Psychiatry Clin Pract. 1998;2(4):275-278. PubMed doi:10.3109/13651509809115373
60. Svestka J, Náhunek K, Cesková E, et al. A 1-year experience with the administration of clopenthixol decanoate in schizophrenic psychoses [in Czech]. Cesk Psychiatr. 1984;80(3):146-154. PubMed
61. Waldmann KD, Neumann J. Clinical experience with depot neuroleptic treatment [in German]. Z Arztl Fortbild (Jena). 1984;78(20):853-856. PubMed
62. Michel G, Vásquez R, Basso L. Follow-up study of patients treated with depot phenothiazines in Valparaiso, Chile [in Spanish]. Bol Oficina Sanit Panam. 1981;91(5):418-427. PubMed
63. Tan CT, Ong TC, Chee KT. The use of fluphenazine decanoate (Modecate)depot therapy in outpatient schizophrenics-a retrospective study. Singapore Med J. 1981;22(4):214-218. PubMed
64. Arató M, Erdós A. Experience with depot neuroleptics [in Hungarian]. Orv Hetil. 1979;120(17):1015-1021. PubMed
65. Devito RA, Brink L, Sloan C, et al. Fluphenazine decanoate vs oral antipsychotics: a comparison of their effectiveness in the treatment of schizophrenia as measured by a reduction in hospital readmissions. J Clin Psychiatry. 1978;39(1):26-34. PubMed
66. Polonowita A, James NM. Fluphenazine decanoate maintenance in schizophrenia: a retrospective study. N Z Med J. 1976;83(563):316-318. PubMed
67. Lindholm H. The consumption of inpatient psychiatric resources prior to and during treatment with a depot neuroleptic, perphenazine enanthate: a mirror study. Nord J Psychiatry. 1975;29(6):513-520.
68. Gottfries CG, Green L. Flupenthixol decanoate—in treatment of out-patients. Acta Psychiatr Scand. 1974;50(s255):15-24. doi:10.1111/j.1600-0447.1974.tb08890.x
69. Morritt C. Long-acting phenothiazines and schizophrenia. Nurs Mirror Midwives J. 1974;138(4):57-59. PubMed
70. Johnson DA, Freeman H. Long-acting tranquillizers. Practitioner. 1972;208(245):395-400. PubMed
71. Denham J, Adamson L. The contribution of fluphenazine enanthate and decanoate in the prevention of readmission of schizophrenic patients. Acta Psychiatr Scand. 1971;47(4):420-430. PubMed doi:10.1111/j.1600-0447.1971.tb03699.x
72. Malm U. Fluphenazine depot: the usefulness of neuroleptics in perspective [in Swedish]. Nord Psykiatr Tidsskr. 1971;25(4):309-314. PubMed doi:10.3109/08039487109094675
73. Haddad PM, Kishimoto T, Correll CU, et al. Ambiguous findings concerning potential advantages of depot antipsychotics: in search of clinical relevance. Curr Opin Psychiatry. 2015;28(3):216-221. PubMed doi:10.1097/YCO.0000000000000160
74. Schreiner A, Aadamsoo K, Altamura AC, et al. A randomized, active-controlled rater-blinded 2-year study of paliperidone palmitate versus investigators’ choice of oral antipsychotic monotherapy in patients with schizophrenia (prosipal). Poster LP-01-013. Poster presented at the 29th International College of Neuropsychopharmacology (CINP) World Congress of Neuropsychopharmacology; June 22-26, 2014; Vancouver, British Columbia, Canada.
75. Tiihonen J, Haukka J, Taylor M, et al. A nationwide cohort study of oral and depot antipsychotics after first hospitalization for schizophrenia. Am J Psychiatry. 2011;168(6):603-609. PubMed doi:10.1176/appi.ajp.2011.10081224
76. Pandina G, Lane R, Gopal S, et al. A double-blind study of paliperidone palmitate and risperidone long-acting injectable in adults with schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):218-226. PubMed doi:10.1016/j.pnpbp.2010.11.008
77. Li H, Rui Q, Ning X, et al. A comparative study of paliperidone palmitate and risperidone long-acting injectable therapy in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):1002-1008. PubMed doi:10.1016/j.pnpbp.2011.02.001
78. McEvoy JP, Byerly M, Hamer RM, et al. Effectiveness of paliperidone palmitate vs haloperidol decanoate for maintenance treatment of schizophrenia: a randomized clinical trial. JAMA. 2014;311(19):1978-1987. PubMed doi:10.1001/jama.2014.4310
79. Nielsen J, Jensen SO, Friis RB, et al. Comparative effectiveness of risperidone long-acting injectable vs first-generation antipsychotic long-acting injectables in schizophrenia: results from a nationwide, retrospective inception cohort study. Schizophr Bull. 2015;41(3):627-636. PubMed doi:10.1093/schbul/sbu128
80. Naber D, Hansen K, Forray C, et al. Qualify: a randomized head-to-head study of aripiprazole once-monthly and paliperidone palmitate in the treatment of schizophrenia. Schizophr Res. 2015;168(1-2):498-504. PubMed doi:10.1016/j.schres.2015.07.007
81. Fluphenazine decanoate [package insert]. Schaumburg, IL: APP Pharmaceuticals; 2010.
82. Haldol decanoate 50; Haldol decanoate 100 [package insert]. Beerse, Belgium: Janssen Pharmaceutica; 2011.
83. Risperdal Consta [package insert]. Wallingstown, Little Island, County Cork, Ireland: Janssen Pharmaceutical; 2015.
84. Zyprexa Relprevv [package insert]. Indianapolis, IN: Eli Lilly and Company; 2015.
85. Invega Sustenna [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2015.
86. Invega Trinza [package insert]. Titusville, NJ: Janssen Pharmaceuticals; 2015.
87. Abilify Maintena [package insert]. Tokyo, Japan: Otsuka Pharmaceutical Company; 2015.
88. Aristada [package insert]. Waltham, MA: Alkermes; 2015.
89. Luedecke D, Schöttle D, Karow A, et al. Post-injection delirium/sedation syndrome in patients treated with olanzapine pamoate: mechanism, incidence, and management. CNS Drugs. 2015;29(1):41-46. PubMed doi:10.1007/s40263-014-0216-9
90. Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951-962. PubMed doi:10.1016/S0140-6736(13)60733-3
91. Tandon R. Safety and tolerability: how do newer generation “atypical” antipsychotics compare? Psychiatr Q. 2002;73(4):297-311. PubMed doi:10.1023/A:1020464017021
92. Velligan DI, Weiden PF, Sajatovic M, et al; Expert Consensus Panel on Adherence Problems in Serious and Persistent Mental Illness. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry. 2009;70(suppl 4):1-46, quiz 47-48. PubMed doi:10.4088/jcp.7090su1cj
93. Weiden PJ, Buckley PF. Reducing the burden of side effects during long-term antipsychotic therapy: the role of “switching” medications. J Clin Psychiatry. 2007;68(suppl 6):14-23.
93a. Misawa F, Kishimoto T, Hagi K, et al. Safety and tolerability of long-acting injectable versus oral antipsychotics: a meta-analysis of randomized controlled studies comparing the same antipsychotics [Epub ahead of print August 4, 2016]. Schizophr Res. P doi:10.1016/j.schres.2016.07.018
94. Citrome L. Quantifying clinical relevance. Innov Clin Neurosci. 2014;11(5-6):26-30. PubMed
95. Citrome L. Relative vs absolute measures of benefit and risk: what’s the difference? Acta Psychiatr Scand. 2010;121(2):94-102. PubMed doi:10.1111/j.1600-0447.2009.01449.x
96. Citrome L, Ketter TA. When does a difference make a difference? interpretation of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Int J Clin Pract. 2013;67(5):407-411. PubMed doi:10.1111/ijcp.12142
97. Citrome L. A review of the pharmacology, efficacy and tolerability of recently approved and upcoming oral antipsychotics: an evidence-based medicine approach. CNS Drugs. 2013;27(11):879-911. PubMed doi:10.1007/s40263-013-0105-7
98. Citrome L. Brexpiprazole for schizophrenia and as adjunct for major depressive disorder: a systematic review of the efficacy and safety profile for this newly approved antipsychotic—what is the number needed to treat, number needed to harm and likelihood to be helped or harmed? Int J Clin Pract. 2015;69(9):978-997. PubMed doi:10.1111/ijcp.12714
99. Leucht C, Heres S, Kane JM, et al. Oral versus depot antipsychotic drugs for schizophrenia—a critical systematic review and meta-analysis of randomised long-term trials. Schizophr Res. 2011;127(1-3):83-92. PubMed doi:10.1016/j.schres.2010.11.020
100. Kirson NY, Weiden PJ, Yermakov S, et al. Efficacy and effectiveness of depot versus oral antipsychotics in schizophrenia: synthesizing results across different research designs. J Clin Psychiatry. 2013;74(6):568-575. PubMed doi:10.4088/JCP.12r08167
101. Baloush-Kleinman V, Levine SZ, Roe D, et al. Adherence to antipsychotic drug treatment in early-episode schizophrenia: a six-month naturalistic follow-up study. Schizophr Res. 2011;130(1-3):176-181. PubMed doi:10.1016/j.schres.2011.04.030
102. Huang XY, Hung BJ, Sun FK, et al. The experiences of carers in Taiwanese culture who have long-term schizophrenia in their families: a phenomenological study. J Psychiatr Ment Health Nurs. 2009;16(10):874-883. PubMed doi:10.1111/j.1365-2850.2009.01468.x
103. Klingberg S, Schneider S, Wittorf A, et al. Collaboration in outpatient antipsychotic drug treatment: analysis of potentially influencing factors. Psychiatry Res. 2008;161(2):225-234. PubMed doi:10.1016/j.psychres.2007.07.027
104. Meier J, Becker T, Patel A, et al. Effect of medication-related factors on adherence in people with schizophrenia: a European multi-centre study. Epidemiol Psichiatr Soc. 2010;19(3):251-259. PubMed doi:10.1017/S1121189X00001184
105. Valenstein M, Ganoczy D, McCarthy JF, et al. Antipsychotic adherence over time among patients receiving treatment for schizophrenia: a retrospective review. J Clin Psychiatry. 2006;67(10):1542-1550. PubMed doi:10.4088/JCP.v67n1008
106. Velligan DI, Wang M, Diamond P, et al. Relationships among subjective and objective measures of adherence to oral antipsychotic medications. Psychiatr Serv. 2007;58(9):1187-1192. PubMed doi:10.1176/ps.2007.58.9.1187
107. Velligan DI, Lam YW, Glahn DC, et al. Defining and assessing adherence to oral antipsychotics: a review of the literature. Schizophr Bull. 2006;32(4):724-742. PubMed doi:10.1093/schbul/sbj075
108. Ascher-Svanum H, Faries DE, Zhu B, et al. Medication adherence and long-term functional outcomes in the treatment of schizophrenia in usual care. J Clin Psychiatry. 2006;67(3):453-460. PubMed doi:10.4088/JCP.v67n0317
109. Mond J, Morice R, Owen C, et al. Use of antipsychotic medications in Australia between July 1995 and December 2001. Aust N Z J Psychiatry. 2003;37(1):55-61. PubMed doi:10.1046/j.1440-1614.2003.01110.x
110. Barnett PG, Scott JY, Krystal JH, et al; CSP 555 Research Group. Cost and cost-effectiveness in a randomized trial of long-acting risperidone for schizophrenia. J Clin Psychiatry. 2012;73(5):696-702. PubMed doi:10.4088/JCP.11m07070
111. Baser O, Xie L, Pesa J, et al. Healthcare utilization and costs of Veterans Health Administration patients with schizophrenia treated with paliperidone palmitate long-acting injection or oral atypical antipsychotics. J Med Econ. 2015;18(5):357-365. PubMed doi:10.3111/13696998.2014.1001514
112. Bera R, Offord S, Zubek D, et al. Hospitalization resource utilization and costs among Medicaid insured patients with schizophrenia with different treatment durations of long-acting injectable antipsychotic therapy. J Clin Psychopharmacol. 2014;34(1):30-35. PubMed doi:10.1097/JCP.0b013e3182a6082a
113. Lin J, Wong B, Offord S, et al. Healthcare cost reductions associated with the use of LAI formulations of antipsychotic medications versus oral among patients with schizophrenia. J Behav Health Serv Res. 2013;40(3):355-366. PubMed doi:10.1007/s11414-013-9329-z
114. Bin-Chia D, Le EHM, Chung WS, et al. Cost analysis of risperidone long-acting injection in the treatment of schizophrenia and schizoaffective disorder in Hong Kong: an approach using generalized estimating equations. Psychol Res. 2013;210:745-750.
115. Young CL, Taylor DM. Health resource utilization associated with switching to risperidone long-acting injection. Acta Psychiatr Scand. 2006;114(1):14-20. PubMed doi:10.1111/j.1600-0447.2006.00766.x
116. Einarson TR, Vicente C, Zilbershtein R, et al. Pharmacoeconomics of depot antipsychotics for treating chronic schizophrenia in Sweden. Nord J Psychiatry. 2014;68(6):416-427. PubMed doi:10.3109/08039488.2013.852243
117. Hong J, Novick D, Brugnoli R, et al. Changes in adherence and treatment costs following initiation of oral or depot typical antipsychotics among previously non-adherent patients with schizophrenia. Hum Psychopharmacol. 2013;28(5):438-446. PubMed doi:10.1002/hup.2328
118. Lachaine J, Lapierre ME, Abdalla N, et al. Impact of switching to long-acting injectable antipsychotics on health services use in the treatment of schizophrenia. Can J Psychiatry. 2015;60(suppl 2):S40-S47. PubMed
119. Lehman AF, Lieberman JA, Dixon LB, et al; Steering Committee on Practice Guidelines. Practice guideline for the treatment of patients with schizophrenia, second edition. Am J Psychiatry. 2004;161(2 suppl):1-56.
120. Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for the Treatment of Schizophrenia and Related Disorders. Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for the treatment of schizophrenia and related disorders. Aust N Z J Psychiatry. 2005;39(1-2):1-30. PubMed doi:10.1111/j.1440-1614.2005.01516.x
121. Lehman AF, Kreyenbuhl J, Buchanan RW, et al. The Schizophrenia Patient Outcomes Research Team (PORT): updated treatment recommendations 2003. Schizophr Bull. 2004;30(2):193-217. PubMed
122. Psychosis and schizophrenia in adults: prevention and management. NICE Guidelines CG178. National Institute for Health and Care Excellence (NICE) Web site. https://www.nice.org.uk/guidance/cg178?unlid=3595877802015121882226. Updated 2014.
123. Heres S, Schmitz FS, Leucht S, et al. The attitude of patients towards antipsychotic depot treatment. Int Clin Psychopharmacol. 2007;22(5):275-282. PubMed doi:10.1097/YIC.0b013e3280c28424
124. Patel MX, De Zoysa N, Bernadt M, et al. Depot and oral antipsychotics: patient p and attitudes are not the same thing. J Psychopharmacol. 2009;23(7):789-796. PubMed doi:10.1177/0269881108092124
125. Patel MX, Nikolaou V, David AS. Psychiatrists’ attitudes to maintenance medication for patients with schizophrenia. Psychol Med. 2003;33(1):83-89. PubMed doi:10.1017/S0033291702006797
126. Patel MX, DE Zoysa N, Baker D, et al. Antipsychotic depot medication and attitudes of community psychiatric nurses. J Psychiatr Ment Health Nurs. 2005;12(2):237-244. PubMed doi:10.1111/j.1365-2850.2004.00826.x
127. Hamann J, Mendel R, Heres S, et al. How much more effective do depot antipsychotics have to be compared to oral antipsychotics before they are prescribed? Eur Neuropsychopharmacol. 2010;20(4):276-279. PubMed doi:10.1016/j.euroneuro.2010.01.001
128. Heres S, Hamann J, Kissling W, et al. Attitudes of psychiatrists toward antipsychotic depot medication. J Clin Psychiatry. 2006;67(12):1948-1953. PubMed doi:10.4088/JCP.v67n1216
129. Patel MX, Haddad PM, Chaudhry IB, et al. Psychiatrists’ use, knowledge and attitudes to first- and second-generation antipsychotic long-acting injections: comparisons over 5 years. J Psychopharmacol. 2010;24(10):1473-1482. PubMed doi:10.1177/0269881109104882
130. Jaeger M, Rossler W. Attitudes towards long-acting depot antipsychotics: a survey of patients, relatives and psychiatrists. Psychiatry Res. 2010;175(1-2):58-62. PubMed doi:10.1016/j.psychres.2008.11.003
131. Das AK, Malik A, Haddad PM. A qualitative study of the attitudes of patients in an early intervention service towards antipsychotic long-acting injections. Ther Adv Psychopharmacol. 2014;4(5):179-185. PubMed doi:10.1177/2045125314542098
132. Weiden PJ, Roma RS, Velligan DI, et al. The challenge of offering long-acting antipsychotic therapies: a preliminary discourse analysis of psychiatrist recommendations for injectable therapy to patients with schizophrenia. J Clin Psychiatry. 2015;76(6):684-690. PubMed doi:10.4088/JCP.13m08946
133. Rubio G, Mart×nez I, Ponce G, et al. Long-acting injectable risperidone compared with zuclopenthixol in the treatment of schizophrenia with substance abuse comorbidity. Can J Psychiatry. 2006;51(8):531-539. PubMed
134. Bardes CL. Defining “patient-centered medicine”. N Engl J Med. 2012;366(9):782-783. PubMed doi:10.1056/NEJMp1200070
135. Tellis-Nayak V. A person-centered workplace: the foundation for person-centered caregiving in long-term care. J Am Med Dir Assoc. 2007;8(1):46-54. PubMed doi:10.1016/j.jamda.2006.09.009
136. Correll CU. The role of the extended health care team in successful LAI therapy: education to overcome barriers. J Clin Psychiatry. 2014;75(9):e25. doi:10.4088/jcp.13024tx4c
137. Lopez LV, Kane JM. Recommendations for the monitoring of serum concentrations of antipsychotic drugs in the treatment of schizophrenia. J Clin Psychiatry. 2015;76(9):1249-1250. PubMed doi:10.4088/JCP.15ac10243
138. Hudson TJ, Owen RR, Thrush CR, et al. A pilot study of barriers to medication adherence in schizophrenia. J Clin Psychiatry. 2004;65(2):211-216. PubMed doi:10.4088/JCP.v65n0211
139. Achilla E, McCrone P. The cost effectiveness of long-acting/extended-release antipsychotics for the treatment of schizophrenia: a systematic review of economic evaluations. Appl Health Econ Health Policy. 2013;11(2):95-106. PubMed doi:10.1007/s40258-013-0016-2
140. Detke HC, Correll CU, Liu C, et al. Comparison of outcomes in patients with early phase versus later phase schizophrenia. Poster presented at the New Clinical Drug Evaluation Unit (NCDEU) 52nd Annual Meeting; May 29-June 1, 2012; Phoenix, AZ.
141. Hough D, Nasrallah H, Turkoz I, et al. Which schizophrenia patients relapse despite adherence to long-acting antipsychotic therapy? Poster presented at the New Clinical Drug Evaluation Unit (NCDEU) 52nd Annual Meeting; May 29-June 1, 2012; Phoenix, AZ.
Save
Cite
Advertisement
GAM ID: sidebar-top