Valproate and formulations thereof are approved for the management of seizure disorders, bipolar disorder, and migraine. Valproate is also used for many off-label indications. Unfortunately, data from observational studies indicate that valproate is perhaps the most teratogenic drug in the neuropsychiatric pharmacopeia. It is significantly more teratogenic than many other antiepileptic drugs (AEDs). In observational studies, gestational exposure to valproate is also associated with higher risks of cognitive, language, and psychomotor delay during early childhood and possibly with an increased risk of autism, as well. Although untreated epilepsy is itself associated with many adverse gestational and neonatal outcomes, confounding by indication is an unlikely explanation for the adverse outcomes observed with valproate. This is because of the range of the adverse outcomes, the magnitude of the risks, the dose-dependent nature of the risks, the consistency of findings across studies, the specificity of outcomes for valproate exposure, and the greater risks with valproate relative to other AEDs. Despite the existence of a large body of literature that documents these risks, valproate continues to be prescribed to a substantial proportion of women of childbearing potential. As a result, many regulatory bodies across the world have discouraged and even banned the use of valproate during pregnancy and in women of childbearing potential unless no satisfactory alternatives exist or unless a pregnancy prevention program is implemented.


ABSTRACT
Valproate and formulations thereof are approved for the management of seizure disorders, bipolar disorder, and migraine. Valproate is also used for many off-label indications. Unfortunately, data from observational studies indicate that valproate is perhaps the most teratogenic drug in the neuropsychiatric pharmacopeia. It is significantly more teratogenic than many other antiepileptic drugs (AEDs). In observational studies, gestational exposure to valproate is also associated with higher risks of cognitive, language, and psychomotor delay during early childhood and possibly with an increased risk of autism, as well. Although untreated epilepsy is itself associated with many adverse gestational and neonatal outcomes, confounding by indication is an unlikely explanation for the adverse outcomes observed with valproate. This is because of the range of the adverse outcomes, the magnitude of the risks, the dose-dependent nature of the risks, the consistency of findings across studies, the specificity of outcomes for valproate exposure, and the greater risks with valproate relative to other AEDs. Despite the existence of a large body of literature that documents these risks, valproate continues to be prescribed to a substantial proportion of women of childbearing potential. As a result, many regulatory bodies across the world have discouraged and even banned the use of valproate during pregnancy and in women of childbearing potential unless no satisfactory alternatives exist or unless a pregnancy prevention program is implemented.
J Clin Psychiatry 2018;79(3):18f12351
To cite: Andrade C. Valproate in pregnancy: recent research and regulatory responses. J Clin Psychiatry. 2018;79(3):18f12351.
To share: https://doi.org/10.4088/JCP.18f12351
© Copyright 2018 Physicians Postgraduate Press, Inc.
In most parts of the world, sodium valproate, valproic acid, and various formulations thereof are approved drugs for the treatment and/or prevention of seizure disorders, manic and mixed mood states, and migraine. Valproate is also used off-label and with inadequate evidence-based support to treat a variety of other conditions, including behavioral and psychological symptoms of dementia, aggression as a symptom, tardive dyskinesia, alcohol dependence, neuropathic pain, and cyclic vomiting.
Unfortunately, valproate is arguably one of the most teratogenic drugs in neuropsychiatry. Gestational exposure to valproate is also associated with a high risk of adverse childhood neuropsychological and neurodevelopmental outcomes.1-3 This article is not an exhaustive review of the consequences of valproate exposure in pregnancy; rather, it summarizes recently published data that illustrate the risks, as well as recent regulatory responses associated with the recognition of the risks.
Morphological Teratogenicity Associated With Gestational Exposure to Valproate
The teratogenicity of valproate has been recognized for decades.4 In a systematic review and meta-analysis of observational data on major congenital malformations in the offspring of mothers who received monotherapy with antiepileptic drugs (AEDs) to treat epilepsy during pregnancy, Weston et al1 showed that, in children with in utero exposure to valproate (N = 467), relative to children born to women without epilepsy (N = 1,936), there was a quintupled risk of congenital malformations (relative risk [RR] = 5.69; 95% confidence interval [CI], 3.33-9.73). This risk was higher than the risks associated with carbamazepine (RR = 2.01), phenobarbital (RR = 2.84), phenytoin (RR = 2.38), and topiramate (RR = 3.69).
Weston et al1 also found that, in children with in utero exposure to valproate (N = 1,923), relative to children born to women with untreated epilepsy (N = 1,259), there was a more than trebled risk of malformation (RR = 3.13; 95% CI, 2.16-4.54). This risk was higher than the risks associated with carbamazepine (RR = 1.50) and phenytoin (RR = 2.40).
Finally, in direct comparisons, these authors1 found that valproate was associated with a significantly higher malformation risk than 9 other AEDs; that is, carbamazepine (RR = 2.44), gabapentin (RR = 6.21), levetiracetam (RR = 5.82), lamotrigine (RR= 3.56), topiramate (2.35), oxcarbazepine (RR = 3.71), phenobarbitone (RR = 1.59), phenytoin (RR = 2.00), and zonisamide (RR = 17.13).
In this study,1 relative to other AEDs, valproate was also associated with a significantly higher risk of neural tube, cardiac, orofacial/craniofacial, and skeletal and limb malformations. Finally, the risk of major malformations with valproate was dose-dependent. The absolute risk of major malformations with valproate was 10.93% (95% CI, 8.91-13.13).
Postnatal Developmental Risks Associated With Gestational Exposure to Valproate
Gestational exposure to valproate has effects that may only become evident during early childhood. In this context, in a systematic review and Bayesian network meta-analysis of 29 cohort studies with 5,100 children, Veroniki et al5 found that valproate was the only AED to be significantly associated with both cognitive delay (odds ratio [OR] = 7.40; 97% credible interval [CrI], 3.00-18.46) and language delay (OR = 7.95; 95% CrI, 1.50-49.13). Valproate was also associated with psychomotor delay (OR = 4.16; 95% CrI, 2.04-8.75) and autism/dyspraxia (OR = 17.29; 95% Cr, 2.40-217.60). AED polytherapies that included valproate were also associated with increase in certain developmental risks. No significant associations were found between AEDs and social impairment or attention-deficit/hyperactivity disorder.
Women who use valproate during pregnancy probably continue to use valproate during breastfeeding. However, neonatal exposure to valproate during breastfeeding is probably very small and of little clinical importance.6,7 Therefore, the neurodevelopmental effects associated with valproate are probably more relevant to gestational exposure than to exposure during breastfeeding.
Perinatal Outcomes in Women With Epilepsy
To what extent might the adverse outcomes associated with gestational exposure to valproate be a result of confounding by indication? To answer this question, it is first necessary to determine the extent to which epilepsy is associated with adverse gestational outcomes. This matter was studied by Razaz et al.8
These authors8 described a large population-based cohort study of singleton offspring born during 1997-2011 after at least 22 weeks of gestation. The data were drawn from various registries in Sweden. The sample included 1,424,279 pregnancies; data on AED exposure were available only for the subset of offspring born between July 1, 2005, and December 31, 2011.
Analyses were conducted on 869,947 women without epilepsy vs 3,585 women with epilepsy and on 1,015 women with epilepsy who were not receiving AEDs vs 926 women with epilepsy who were receiving AEDs. There were 5,373 births to the women with epilepsy; the mean age at first delivery in these women was 30.5 years. Poisson log-linear regression analyses were adjusted for potential confounds, including maternal age, education, height, early pregnancy body mass index, smoking, cohabitation with a partner, country of origin, year of delivery, pregestational diabetes, hypertension, and psychiatric disorders.
Important findings from the study8 are presented in Table 1. In summary, women with epilepsy were at an increased risk of a large number of pregnancy complications, including those related to adverse delivery outcomes and those related to adverse neonatal outcomes. In contrast, antiepileptic drug use did not appear to significantly raise most of the risks; the implication is that the underlying disorder, epilepsy, rather than treatment thereof with AEDs, was responsible for the adverse outcomes recorded. These results must be viewed with some caution:
- On the one hand, it is possible that the reassuring findings regarding AED exposures were because the AED analyses were underpowered. On the other hand, one would expect that women who continued AED medication during pregnancy would have had more severe epilepsy and would hence be found to be at greater risk of adverse outcomes. Either way, the finding that AED use was not associated with heightened risks is reassuring.
- Because this was not a randomized controlled trial, the findings are indicative but not confirmatory of the risks associated with untreated epilepsy during pregnancy and of the general safety of AEDs in pregnancy.
- The results were pooled across AEDs, and so risks associated with specific AEDs would have been difficult to estimate. However, it is important to note that, in this study, valproate exposure was associated with a 10.6% rate of major congenital malformations.
Confounding by Indication
Valproate has broad spectrum antiepileptic action and so may be used in more severely ill, difficult-to-diagnose, or otherwise problematic patients who are at greater risk of adverse gestational outcomes because of the nature of the seizure disorder for which the drug is prescribed. Women who have epilepsy for which valproate is more likely to be prescribed may have lower IQ, may be less likely to use folic acid before and during pregnancy, and may have other risk factors for adverse gestational and child development outcomes. In theory, these sources of confounding by indication are indeed possible. Or, it is possible that valproate is causal for only some and not all adverse gestational and neurodevelopmental outcomes.
How is the situation with valproate different from, for example, SSRI exposure during pregnancy and the risk of autism spectrum disorders, where it has been suggested that confounding rather than drug is responsible for the adverse outcomes9-11? For one, the range and magnitude of the findings with valproate are large; for another, the risk is dose-dependent; next, and importantly, the findings have been repeatedly replicated with almost no disagreement across studies; additionally, the adverse findings among AEDs are relatively specific to valproate; and finally, the risks with valproate are significantly greater than those with other AEDs.
Valproate Use in Women
Is the growing concern about the use of valproate in women of childbearing potential evident from prescribing behavior? In a study of 40,526 individuals with active prescriptions for non-antipsychotic mood stabilizers, extracted from New York State Medicaid data, Wisner et al12 found that valproate was the most commonly prescribed drug among young women (23.4%). Citing US data for 2012, the US Food and Drug Administration13 observed that as many as (approximately) 22% of prescriptions for valproic acid and derivative products were for women of reproductive potential (age 13-45 years). In Finland, the use of valproate among women aged 15-44 years decreased only marginally, from 50 per 10,000 to 40 per 10,000 between 2012 and 2016.14
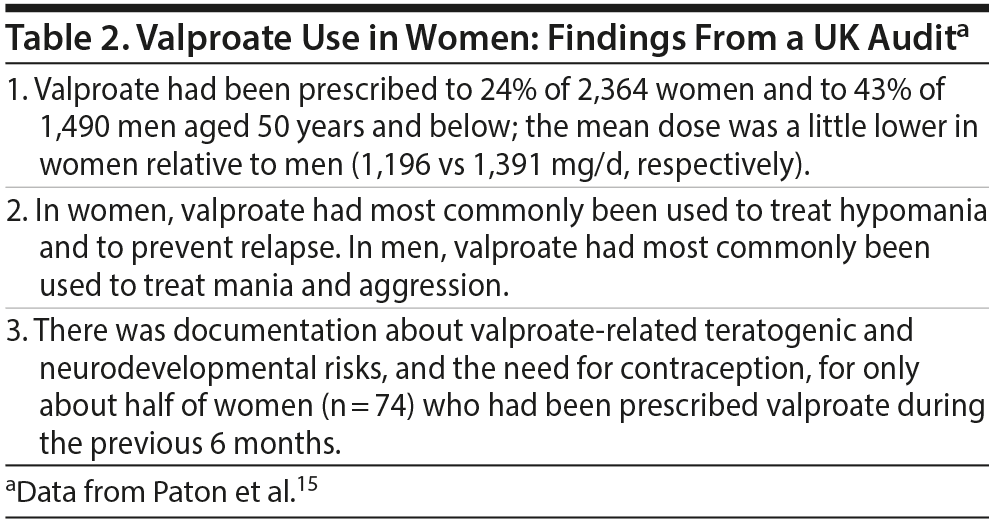
Paton et al15 described an audit of 648 clinical teams from 55 mental health trusts in the United Kingdom. The audit sample comprised 6,705 patients with bipolar disorder, 3,854 of whom were aged 50 years and below. The time period that the audit covered was not specified by the authors. Important findings from the audit are presented in Table 2. In summary, a quarter of potentially fertile women with bipolar disorder were prescribed valproate; in only half of these women was there documentation about the provision of education about valproate-related risks and the need for contraception during valproate therapy. The authors noted that the lower dosing of valproate in women, relative to men, may not have been related to concerns about dose-dependent gestational risks; rather, the lower dosing may have been related to the less challenging target of treatment in women. Clearly, audits need to be conducted in other parts of the world, as well, to determine the magnitude of exposure of bipolar women to valproate.
Recent Regulatory Responses
The risks associated with gestational exposure to valproate have resulted in largely similar regulatory responses in many parts of the world. These responses reflect the concern that the state is responsible for the rights of the unborn child, potentially overriding choices that women and their physicians may make. For example, on July 6, 2017, the French National Agency for the Safety of Medicines and Health Products banned the use of valproate by girls and women who are diagnosed with bipolar disorder and who are either pregnant or of childbearing age and are not using an efficient form of contraception. The ban does not apply to the use of valproate for epilepsy.16 Guidance, but not a ban, already exists to discourage the use of valproate in girls and women who are of reproductive age and who are diagnosed with epilepsy, unless the use of the drug is unavoidable.16
Restrictions have been placed on the use of valproate by women in the United Kingdom, as well.17 The Medicines and Healthcare Products Regulatory Agency in the United Kingdom has prohibited the use of valproate in all women of childbearing potential who are not enrolled in a pregnancy prevention program. Girls and women who are already taking valproate are required to have their treatment reviewed. This prohibition will be further supported by smaller valproate pack sizes that encourage monthly prescribing, by a pictogram or warning image on the medication labeling, and by medical professional computer alerts. The National Institute for Health and Care Excellence guidelines are also amended to reflect the regulatory change.17
On March 23, 2018, the European Medicines Agency18 announced that the Coordination group for Mutual recognition and Decentralized procedures—human (CMDh), European Medicines Agency, had endorsed the recommendations of the Pharmacovigilance Risk Assessment Committee regarding the use of valproate by pregnant women and women of childbearing age. The recommendations ban the use of valproate for migraine or bipolar disorder during pregnancy, as well as ban the use of valproate to treat epilepsy during pregnancy unless no other effective treatment is available. The recommendations also prohibit the use of valproate in females of childbearing potential unless the conditions of a pregnancy prevention program are met. The recommendations are being sent to the European Commission for the final, legally binding decision that will be applied across the European Union.
Earlier, the US FDA13 issued a drug safety communication that contraindicated the use of valproate for the prevention of migraine in pregnant women. The safety communication added that valproate should be used in pregnant women with epilepsy or bipolar disorder only if other treatments did not provide adequate symptom control or were otherwise unacceptable and that valproate should not be administered to a woman of childbearing age unless the medication is essential for the management of her medical condition. The safety communication also noted that women who are planning a pregnancy should be counseled regarding the risks and benefits of valproate use during pregnancy and that alternative therapeutic options should be considered for such women. Other regulatory responses were summarized by Angus-Leppan and Liu.19
The concerns about valproate have not been adequately addressed in recent treatment guidelines. For example, the Canadian Network for Mood and Anxiety Treatments guidelines20 include divalproex among the first-line treatments for a manic episode and for the maintenance treatment of bipolar I disorder; the guidelines merely state in table footnotes that divalproex and carbamazepine should be used with caution in women of childbearing age. However, in a section on pregnancy as a special situation, the guidelines discourage the use of divalproex during pregnancy. The guidelines cite Health Canada recommendations that valproate should not be used in female children and adolescents or in women of childbearing potential unless alternate treatments are ineffective or not tolerated and that women of childbearing potential should use effective contraception during treatment and be educated about the risks associated with gestational exposure to valproate. Finally, Health Canada recommends that, in valproate-treated women planning to become pregnant, effort should be made to switch to other treatments before conception.20
Conclusions
Gestational exposure to valproate is associated with high teratogenic and neurodevelopmental risks. This indictment is based on observational data obtained from cohort, case-control, and registry studies, most of which were conducted in patients with epilepsy. Observational data can never confirm causality. Nevertheless, the association between gestational exposure to valproate and adverse outcomes is so strong as to make it prudent to advise against the use of valproate in women of childbearing age, and especially during pregnancy, unless the need is substantial and compelling. In all cases, women should be fully educated about the risks, and decision-making should be shared between client and physician. In all cases, local clinical and regulatory guidance should be followed.
Published online: May 29, 2018.
 Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Each month in his online column, Dr Andrade considers theoretical and practical ideas in clinical psychopharmacology with a view to update the knowledge and skills of medical practitioners who treat patients with psychiatric conditions.
Department of Clinical Psychopharmacology and Neurotoxicology, National Institute of Mental Health and Neurosciences, Bangalore, India ([email protected]).
Financial disclosure and more about Dr Andrade.
REFERENCES
1. Weston J, Bromley R, Jackson CF, et al. Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database Syst Rev. 2016;11:CD010224. PubMed
2. Gentile S. Risks of neurobehavioral teratogenicity associated with prenatal exposure to valproate monotherapy: a systematic review with regulatory repercussions. CNS Spectr. 2014;19(4):305-315. PubMed CrossRef
3. Bromley RL, Calderbank R, Cheyne CP, et al; UK Epilepsy and Pregnancy Register. Cognition in school-age children exposed to levetiracetam, topiramate, or sodium valproate. Neurology. 2016;87(18):1943-1953. PubMed CrossRef
4. Dalens B, Raynaud EJ, Gaulme J. Teratogenicity of valproic acid. J Pediatr. 1980;97(2):332-333. PubMed CrossRef
5. Veroniki AA, Rios P, Cogo E, et al. Comparative safety of antiepileptic drugs for neurological development in children exposed during pregnancy and breast feeding: a systematic review and network meta-analysis. BMJ Open. 2017;7(7):e017248. PubMed CrossRef
6. Chaudron LH, Jefferson JW. Mood stabilizers during breastfeeding: a review. J Clin Psychiatry. 2000;61(2):79-90. PubMed CrossRef
7. Harden CL, Pennell PB, Koppel BS, et al; American Epilepsy Society. Management issues for women with epilepsy—focus on pregnancy (an evidence-based review), III: vitamin K, folic acid, blood levels, and breast-feeding: Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia. 2009;50(5):1247-1255. PubMed CrossRef
8. Razaz N, Tomson T, Wikström AK, et al. Association between pregnancy and perinatal outcomes among women with epilepsy. JAMA Neurol. 2017;74(8):983-991. PubMed CrossRef
9. Andrade C. Antidepressant use in pregnancy and risk of autism spectrum disorders: a critical examination of the evidence. J Clin Psychiatry. 2013;74(9):940-941. PubMed CrossRef
10. Andrade C. Antidepressant exposure during pregnancy and risk of autism in the offspring, 1: meta-review of meta-analyses. J Clin Psychiatry. 2017;78(8):e1047-e1051. PubMed CrossRef
11. Andrade C. Antidepressant exposure during pregnancy and risk of autism in the offspring, 2: do the new studies add anything new? J Clin Psychiatry. 2017;78(8):e1052-e1056. PubMed CrossRef
12. Wisner KL, Leckman-Westin E, Finnerty M, et al. Valproate prescription prevalence among women of childbearing age. Psychiatr Serv. 2011;62(2):218-220. PubMed CrossRef
13. US FDA. FDA Drug Safety Communication: valproate anti-seizure products contraindicated for migraine prevention in pregnant women due to decreased IQ scores in exposed children. FDA website. https://www.fda.gov/ForConsumers/ConsumerUpdates/ucm350684.htm. 2013. Accessed May 13, 2018.
14. Virta LJ, Kפlviפinen R, Villikka K, et al. Declining trend in valproate use in Finland among females of childbearing age in 2012-2016: a nationwide registry-based outpatient study [published online ahead of print March 6, 2018]. Eur J Neurol. PubMed CrossRef
15. Paton C, Cookson J, Ferrier IN, et al. A UK clinical audit addressing the quality of prescribing of sodium valproate for bipolar disorder in women of childbearing age. BMJ Open. 2018;8(4):e020450. PubMed CrossRef
16. Casassus B. France bans sodium valproate use in case of pregnancy. Lancet. 2017;390(10091):217. PubMed CrossRef
17. Iacobucci G. MHRA bans valproate prescribing for women not in pregnancy prevention programme. BMJ. 2018;361:k1823. PubMed CrossRef
18. Valproate and related substances. European Medicines Agency website. http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/
Valproate_and_related_substances/human_referral_prac_000066.jsp&mid=WC0b01ac05805c516f. 2018. Accessed May 13, 2018.
19. Angus-Leppan H, Liu RSN. Weighing the risks of valproate in women who could become pregnant. BMJ. 2018;361:k1596. PubMed CrossRef
20. Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97-170. PubMed CrossRef
Save
Cite
Advertisement
GAM ID: sidebar-top