Objective: To evaluate the efficacy, safety, and tolerability of vilazodone as an acute treatment for generalized anxiety disorder (GAD). Vilazodone is a selective serotonin reuptake inhibitor and 5-HT1A receptor partial agonist approved for the treatment of major depressive disorder in adults.
Methods: This was a randomized, placebo-controlled, parallel-group, multicenter, flexible-dose study conducted from May 2013-March 2014. Adult patients (18-70 years, inclusive) who met DSM-IV-TR criteria for GAD were randomized (1:1) to placebo or vilazodone 20-40 mg/d for 8 weeks of double-blind treatment. Primary and secondary efficacy parameters were change from baseline to week 8 in the Hamilton Anxiety Rating Scale (HARS) total score and in the Sheehan Disability Scale (SDS) total score, respectively, analyzed using a mixed-effects model for repeated measures approach on a modified intent-to-treat population. Safety outcomes were summarized descriptively.
Results: Efficacy analyses were based on 400 patients (placebo = 200, vilazodone = 200); 76% completed the study (placebo = 81%, vilazodone = 71%). The least squares mean difference (95% CI) in total score change from baseline to week 8 was statistically significant for vilazodone versus placebo on the HARS (−2.20 [−3.72 to −0.68]; P = .0048) and on the SDS (−1.89 [−3.52 to −0.26]; P = .0236). Treatment-emergent adverse events reported in ≥ 5% of vilazodone patients and at least twice the rate of placebo were nausea, diarrhea, dizziness, fatigue, delayed ejaculation, and erectile dysfunction.
Conclusion: Statistically significant differences in favor of vilazodone 20-40 mg/d versus placebo were seen on all measures of anxiety and functional impairment in patients with GAD. Vilazodone was generally well tolerated, and no new safety concerns were noted.
Trial Registration: ClinicalTrials.gov identifier: NCT01844115
Efficacy and Safety of Vilazodone in Patients With Generalized Anxiety Disorder:
A Randomized, Double-Blind, Placebo-Controlled, Flexible-Dose Trial

ABSTRACT
Objective: To evaluate the efficacy, safety, and tolerability of vilazodone as an acute treatment for generalized anxiety disorder (GAD). Vilazodone is a selective serotonin reuptake inhibitor and 5-HT1A receptor partial agonist approved for the treatment of major depressive disorder in adults.
Methods: This was a randomized, placebo-controlled, parallel-group, multicenter, flexible-dose study conducted from May 2013-March 2014. Adult patients (18-70 years, inclusive) who met DSM-IV-TR criteria for GAD were randomized (1:1) to placebo or vilazodone 20-40 mg/d for 8 weeks of double-blind treatment. Primary and secondary efficacy parameters were change from baseline to week 8 in the Hamilton Anxiety Rating Scale (HARS) total score and in the Sheehan Disability Scale (SDS) total score, respectively, analyzed using a mixed-effects model for repeated measures approach on a modified intent-to-treat population. Safety outcomes were summarized descriptively.
Results: Efficacy analyses were based on 400 patients (placebo = 200, vilazodone = 200); 76% completed the study (placebo = 81%, vilazodone = 71%). The least squares mean difference (95% CI) in total score change from baseline to week 8 was statistically significant for vilazodone versus placebo on the HARS (−2.20 [−3.72 to −0.68]; P = .0048) and on the SDS (−1.89 [−3.52 to −0.26]; P = .0236). Treatment-emergent adverse events reported in ≥ 5% of vilazodone patients and at least twice the rate of placebo were nausea, diarrhea, dizziness, fatigue, delayed ejaculation, and erectile dysfunction.
Conclusion: Statistically significant differences in favor of vilazodone 20-40 mg/d versus placebo were seen on all measures of anxiety and functional impairment in patients with GAD. Vilazodone was generally well tolerated, and no new safety concerns were noted.
Trial Registration: ClinicalTrials.gov identifier: NCT01844115
J Clin Psychiatry 2016;77(12):1687-1694
dx.doi.org/10.4088/JCP.15m09885
© Copyright 2016 Physicians Postgraduate Press, Inc.
aForest Research Institute, an affiliate of Actavis, Inc, Jersey City, New Jersey
bDistinguished University Health Professor Emeritus, University of South Florida College of Medicine, Tampa, Florida
*Corresponding author: Suresh Durgam, MD, Forest Research Institute, Harborside Financial Center, Jersey City, NJ 07311 ([email protected]).
Although worrying is a ubiquitous human experience, the worries that characterize generalized anxiety disorder (GAD) are so pervasive and excessive that they distort cognition and create a considerable burden of illness. The physical symptoms, psychiatric symptoms, and functional impairment associated with GAD collectively generate significant individual, societal, and economic costs.1 As such, GAD is an important mental health issue that affects patients, families, health care providers, payers, employers, and society at large.1,2
Vilazodone is a selective serotonin reuptake inhibitor (SSRI) and 5-HT1A receptor partial agonist that is approved by the US Food and Drug Administration (FDA) for treating major depressive disorder (MDD) in adults. SSRIs are first-line agents approved for treating GAD3; unlike other SSRIs, vilazodone also is a 5-HT1A receptor partial agonist. Based on clinical-trial evidence in MDD,4-7 the recommended daily dose of vilazodone is 20-40 mg.8 Since SSRI antidepressants used for the treatment of GAD have efficacy at the same dose levels that are effective in treating MDD,9 vilazodone 20-40 mg/d is being evaluated for GAD treatment.
A potential anxiolytic effect for vilazodone was supported by post hoc analysis of pooled data10 from patients with anxious depression in 2 MDD studies. In this analysis, a statistically significant difference in Hamilton Anxiety Rating Scale (HARS)11 total score change from baseline to week 8 was seen in favor of vilazodone 40 mg/d versus placebo (P < .001). Preliminary investigations into the effects of vilazodone in GAD have yielded positive results in a fixed-dose study of vilazodone 40 mg/d (20 mg/d did not separate from placebo)12 and a flexible-dose study of 20-40 mg/d.13 The present study (ClinicalTrials.gov NCT01844115) was designed to further evaluate the efficacy, safety, and tolerability of flexibly dosed vilazodone as an acute treatment for GAD in adult patients.
METHODS
The study was conducted at 36 US study centers between May 2013 and March 2014 in full compliance with FDA regulations relating to good clinical practices and the ethical principles of the Declaration of Helsinki. The protocol was approved by each site’s institutional review board, and all patients provided written informed consent before any study procedures were initiated.
Study Design
This was a multicenter, randomized, double-blind, placebo-controlled, parallel-group, flexible-dose study of vilazodone 20-40 mg/d in adult patients with GAD. The overall study was 10 weeks and comprised a 1-week screening period, 8week double-blind treatment, and a 1-week double-blind down-taper; all randomized patients were eligible to enter the down-taper period if the investigator considered it medically appropriate. Eligible patients (N = 400) were randomly assigned (1:1) to identically appearing placebo or vilazodone to be taken once daily with food.
Patients randomized to vilazodone received 10 mg/d for week 1 and 20 mg/d for week 2. At the end of week 2 or week 4, patients with inadequate response and no clinically significant tolerability issues could receive a dose increase to 40 mg/d; patients with adequate response continued taking 20 mg/d. No dose increases were allowed after the end of week 4.
Patients were randomized by computer-generated numbers and assigned to identically appearing treatment. Investigators and patients were blinded to the allocation of study drug throughout treatment and down-taper; patients were also blinded to dose increase. The blind was maintained via a secured randomization code list and was broken only in case of emergency; removing the blind for any reason disqualified a patient from further participation.

- Generalized anxiety disorder is characterized by pervasive worries, physical symptoms, psychiatric symptoms, and functional impairment that collectively generate significant individual, societal, and economic costs.
- At week 8, a statistically significant difference in mean change from baseline in Hamilton Anxiety Rating Scale total score was seen in favor of vilazodone 20-40 mg/d compared with placebo, suggesting a reduction in anxiety symptoms for vilazodone-treated patients.
- Statistically significant differences in change from baseline to week 8 on the Sheehan Disability Scale total score and its Work/School, Social Life, and Family Life individual items suggested that all domains of functional impairment decreased at the end of treatment.
Patients
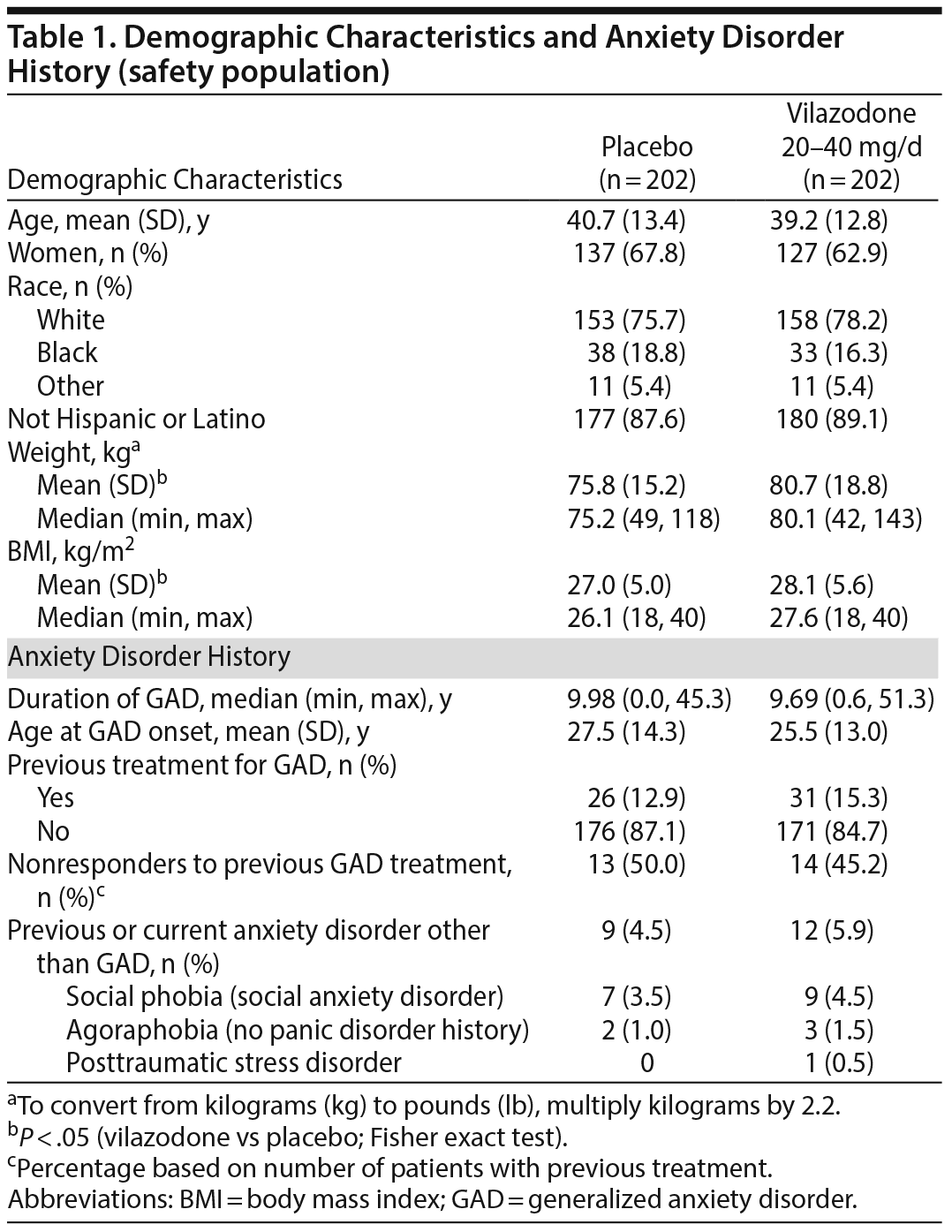
Male or female outpatients (18-70 years of age, inclusive) met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision (DSM-IV-TR) criteria14 for GAD and had the following: HARS total score ≥ 20, HARS items 1 (Anxious Mood) and 2 (Tension) scores ≥ 2, Clinical Global Impressions-Severity (CGI-S)15 score ≥ 4, and 17-item Hamilton Depression Rating Scale (HDRS17)16 total score ≤ 17. Patients had normal physical examination, clinical laboratory, and electrocardiogram (ECG) findings or abnormal results that were judged to be not clinically significant. Women of childbearing potential had a negative serum β-human chorionic gonadotropin pregnancy test.
Patients were excluded if they had a DSM-IV-TR-based Axis I diagnosis other than GAD within 6 months; secondary diagnoses of comorbid social anxiety disorder or specific phobias were allowed. Additional reasons for exclusion included a lifetime diagnostic history of various psychiatric disorders (eg, bipolar disorder, psychotic disorder, depressive episode with psychotic or catatonic features), suicide risk, substance abuse (within 6 months), nonresponse to adequate treatment with ≥ 2 SSRIs or serotonin-norepinephrine reuptake inhibitors (SNRIs) (≥ 8 weeks at the approved dose) for GAD treatment, and intolerance/hypersensitivity to vilazodone, SNRIs, or SSRIs. Medical conditions that could interfere with study conduct, confound the interpretation of results, or endanger patient well-being were exclusionary. Psychoactive drugs were prohibited. Patients requiring concomitant treatment with prohibited medications were excluded; eszopiclone, zopiclone, zaleplon, or zolpidem could be continued for insomnia.
Efficacy and Safety Assessments
Efficacy was assessed at various weeks by the HARS11 (week −1 [screening]; week 0 [baseline]; weeks 1, 2, 4, 6, 8), Sheehan Disability Scale (SDS)17 (weeks 0, 2, 4, 6, 8), HDRS17 (weeks −1, 0, 8), CGI-S15 (all weeks), and CGI-Improvement (CGI-I)15 (weeks 1, 2, 4, 6, 8). Safety was assessed by adverse event (AE) reports, physical examination, clinical laboratory and vital sign measures, ECGs, the Columbia-Suicide Severity Rating Scale (C-SSRS)18 (all weeks and down-taper), and the Changes in Sexual Functioning Questionnaire (CSFQ)19 (weeks 0, 4, 8). Metabolic changes were assessed in post hoc analyses that evaluated the percentage of patients that made clinically relevant shifts from normal values at baseline to high values at any visit during double-blind treatment. Normal and high values were respectively defined as total cholesterol < 200 mg/dL and ≥ 240 mg/dL, low-density lipoprotein (LDL) cholesterol < 100 mg/dL and ≥ 160 mg/dL, and glucose < 100 mg/dL and ≥ 126 mg/dL.
Statistical Analyses
Safety analyses were based on the safety population (randomized patients who received ≥ 1 dose of double-blind study drug). Efficacy analyses were based on the modified intent-to-treat (m-ITT) population (patients in the safety population with a baseline and ≥ 1 postbaseline HARS assessment). All statistical tests were 2-sided hypothesis tests performed at the 5% significance level; all confidence intervals (CIs) were 2-sided 95% CIs.
The primary efficacy parameter was change in HARS total score from baseline to week 8. The primary analysis used a mixed-effects model for repeated measures (MMRM) with treatment group, pooled study center, visit, and treatment group-by-visit interaction as fixed effects, and the baseline value and baseline value-by-visit interaction as covariates. This analysis was based on observed cases without imputation of missing values. An unstructured covariance matrix was used to model the covariance of within-patient scores, and the Kenward-Roger approximation20 was used to estimate the denominator degrees of freedom. Two pre-specified sensitivity analyses were conducted on the primary parameter: a pattern-mixture model approach based on nonfuture dependent missing value restrictions,21 which tested for violations of the missing at random-missingness assumption, and a last observation carried forward (LOCF) approach, which is a more conservative imputation method than MMRM. Both approaches used an analysis of covariance model with treatment group and pooled study center as factors and baseline HARS total score as a covariate.
The secondary efficacy parameter was change from baseline to week 8 in SDS total score. Analysis was conducted using the MMRM approach on an m-ITT population consisting of patients with evaluable assessments on the 3 SDS domain items (Work/School, Social Life, Family Life); SDS total score was calculated as the sum of the domain items. A pre-specified LOCF sensitivity analysis was also performed. The fixed sequence testing procedure was applied to control the overall family-wise type I error rate for testing the primary and secondary efficacy parameters; analyses of the secondary efficacy parameter were carried out inferentially only if the null hypothesis for the primary efficacy parameter was rejected. Effect sizes were calculated for the primary and secondary efficacy outcomes using Cohen d.
Additional efficacy parameters included change from baseline to week 8 on the HARS Psychic Anxiety and Somatic Anxiety subscales,11 HARS items 1 and 2, SDS items (Work/School, Social Life, Family Life), HDRS17, and CGI-S; the CGI-I score at week 8; and the rates of response on the HARS (≥ 50% improvement from baseline) and CGI-I (score ≤ 2). Response rates were analyzed using a generalized linear mixed model with random intercept and fixed terms of treatment group, visit, treatment-by-visit interaction, and baseline score. Other additional efficacy parameters were analyzed using an MMRM approach; baseline CGI-S score was an explanatory variable for CGI-I analysis. Post hoc analyses estimated the rate of SDS remission (total score ≤ 6 with item scores ≤ 2)22 and the number needed to treat for HARS response, CGI-I response, and SDS remission. Safety analyses presented the number and percentage of patients with AEs, and descriptive statistics were used to evaluate change from baseline in laboratory values and vital signs.
RESULTS
Patient Disposition and Demographic Characteristics
Of the patients randomized to double-blind treatment, 11 did not receive study drug and were not included in the safety population (Figure 1). Double-blind treatment was completed by 76% of patients in the safety population; significantly more vilazodone- (29%) than placebo-treated (19%) patients prematurely discontinued the study (P < .05 [Fisher exact test]). AEs were the most common reason for discontinuation in the vilazodone group (P = .0004 vs placebo).
Demographics and baseline physical characteristics were similar between groups except for weight and body mass index (Table 1). Overall, mean age was 39.9 years, 65% of patients were women, and 77% were white. Baseline efficacy scores were generally similar between treatment groups. The mean baseline score was higher on the HARS Psychic Anxiety subscale (approximately 14) than on the Somatic Anxiety subscale (approximately 10). Mean HDRS17 baseline scores were 13 in both groups, suggesting a nondepressed/mildly depressed patient population.23
Analysis of Efficacy
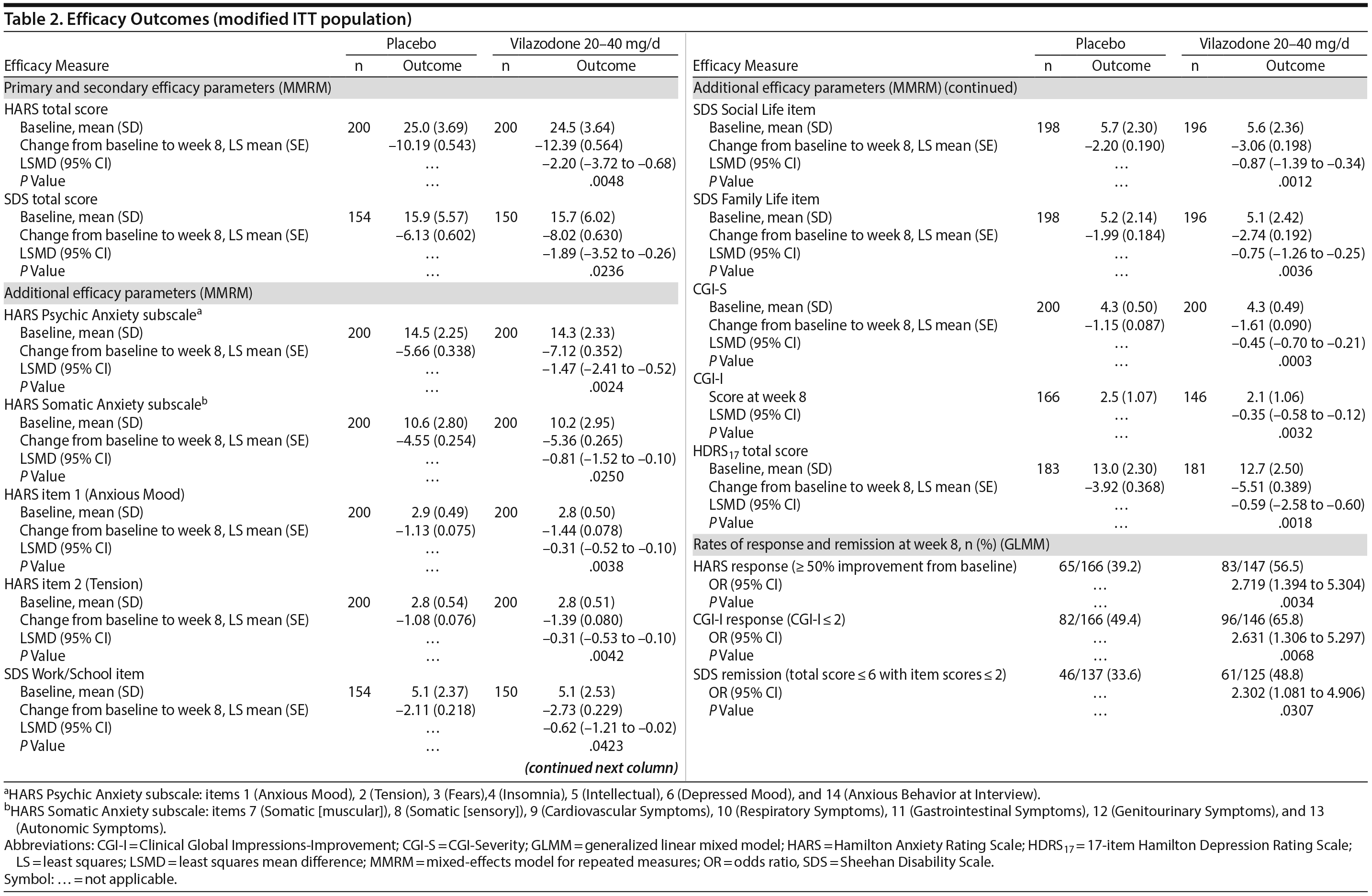
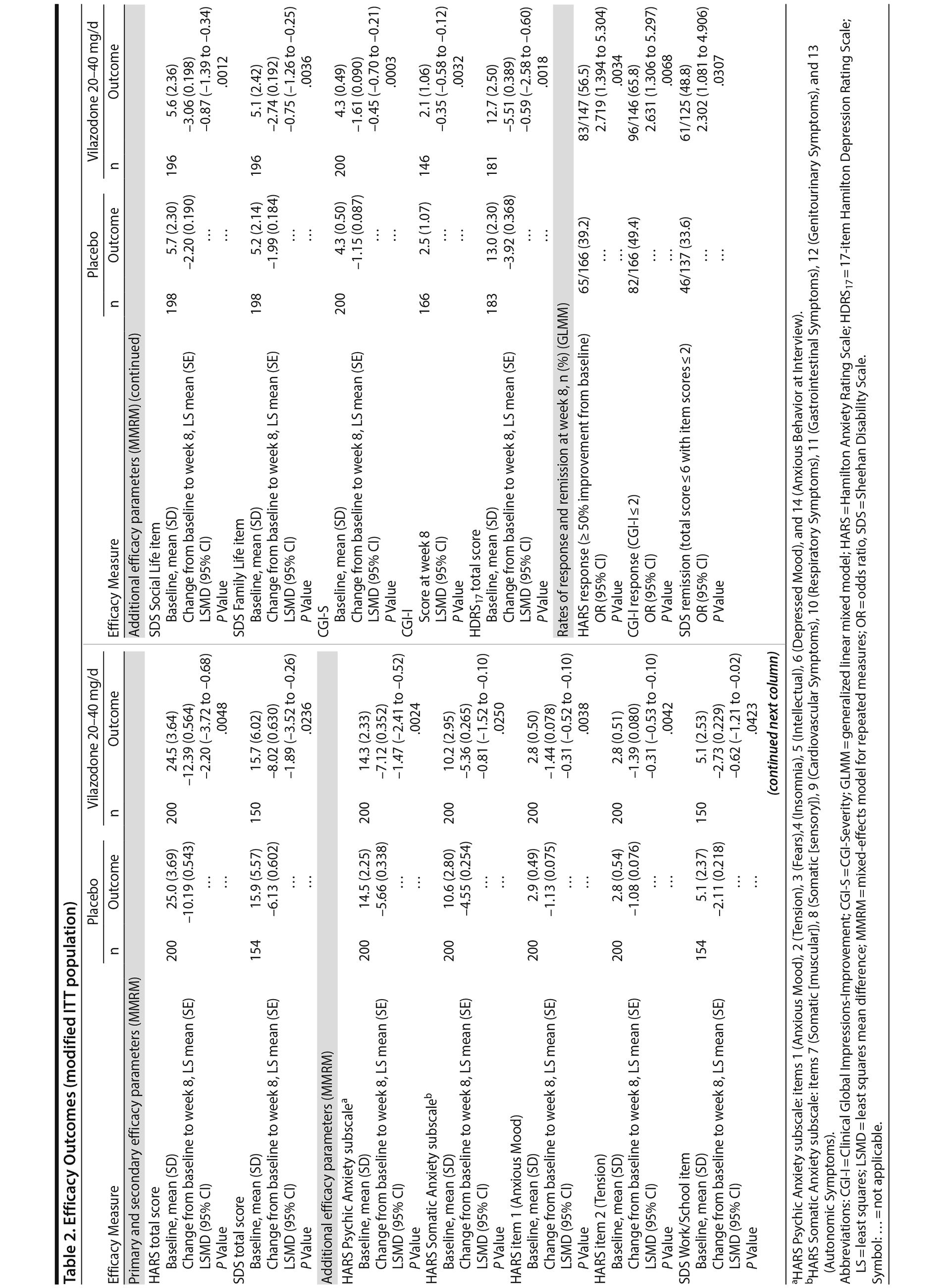
The least squares mean difference in change from baseline to week 8 in HARS total score was statistically significant in favor of vilazodone 20-40 mg/d versus placebo using the primary MMRM approach (Table 2), with an estimated effect size of 0.31. The difference between vilazodone and placebo was statistically significant beginning at week 4, and it remained so through week 8 (Figure 2). In pre-specified sensitivity analyses (data not shown), pattern-mixture model analysis supported the primary results, and the between-group difference using the LOCF approach was not statistically significant.
The difference in mean change from baseline to week 8 in SDS total score was statistically significant for vilazodone versus placebo using the MMRM approach (Table 2), with an estimated effect size of 0.29. The between-group difference on the pre-specified LOCF analysis was not statistically significant (data not shown).
Statistically significant differences in score change from baseline to week 8 were noted on all additional efficacy parameters in favor of vilazodone versus placebo (MMRM); the difference in CGI-I score at week 8 was also statistically significant (Table 2). Differences in the rate of HARS and CGI-I response and SDS remission were statistically significant for vilazodone versus placebo; the number needed to treat for HARS and CGI-I response was 6, and for SDS remission it was 7.
Safety and Tolerability
Extent of exposure. The mean duration of treatment was 46.7 days in the vilazodone group and 50.1 days in the placebo group. Patient-years of exposure ([total treatment duration in days]/365.25) were 27.7 and 25.8 for placebo and vilazodone, respectively. The majority of vilazodone patients (63.9%) were titrated to 40 mg/d, and the mean (SD) daily dose was 26.2 (8.1) mg/d.
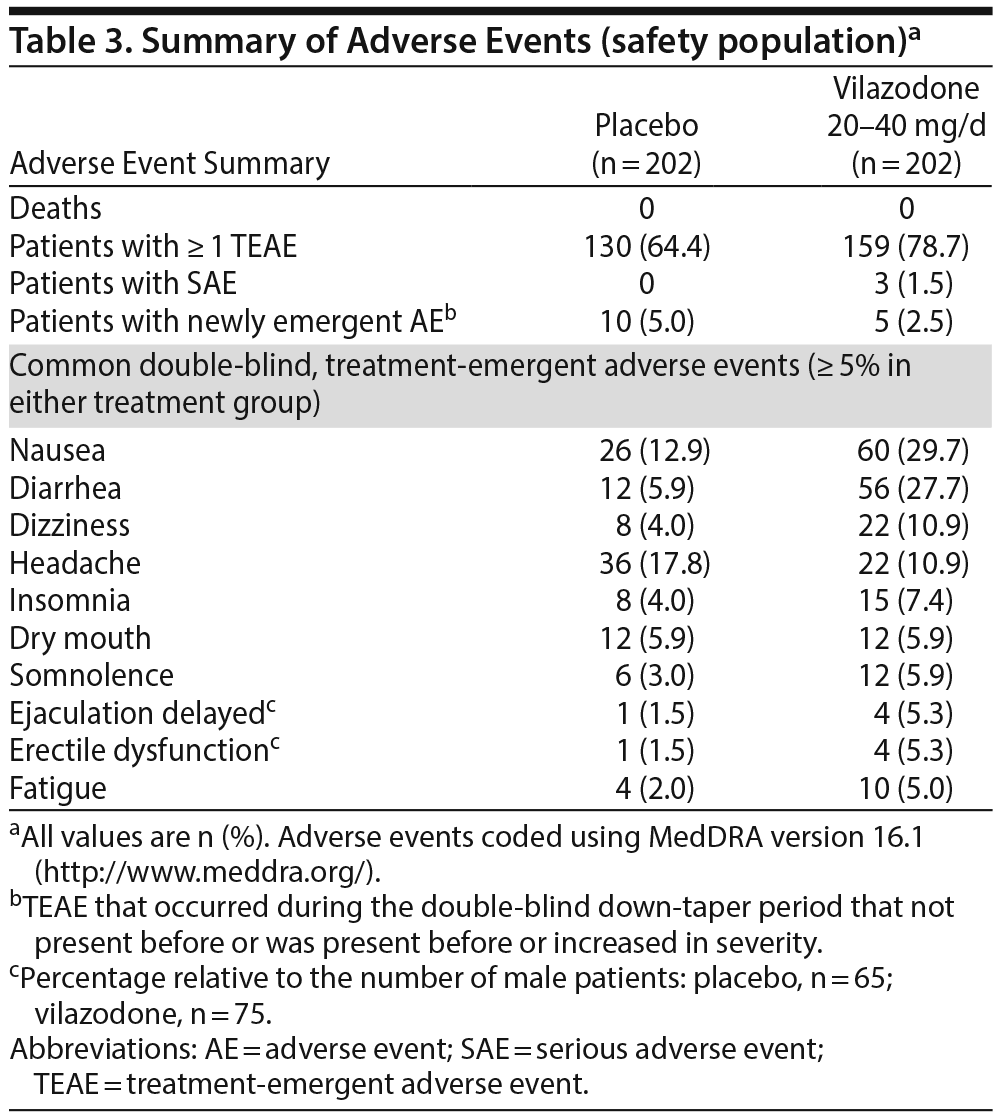
Adverse events. An overall summary of AEs is presented in Table 3. Treatment-emergent AEs (TEAEs) were reported for 64% of placebo- and 79% of vilazodone-treated patients. AEs leading to study discontinuation were more frequent in the vilazodone-treatment group than in the placebo-treatment group (P < .001); the only AEs that resulted in discontinuation of ≥ 2 vilazodone patients were nausea (n = 6), dizziness (n = 5), diarrhea (n = 4), and headache (n = 2).
TEAEs reported in at least 5% of vilazodone patients and at least twice the rate of placebo were nausea, diarrhea, dizziness, fatigue, delayed ejaculation, and erectile dysfunction. The majority of TEAEs in both groups were considered mild (about 60%) or moderate (about 36%) in severity and related to the double-blind treatment (placebo = 63%, vilazodone = 77%). Only 3 serious AEs were reported (laceration/stab wound, urinary tract infection, and impaired gastric emptying [1 vilazodone patient each]); none were considered related to study drug or resulted in study discontinuation.
Clinical laboratory, vital sign, electrocardiogram evaluation. Mean changes from baseline to end of double-blind treatment in most laboratory parameters, vital signs, and liver enzyme parameters were small and similar between groups. No patient met Hy’s law criteria (alanine aminotransferase [ALT] or aspartate aminotransferase [AST] elevation ≥ 3 ×— upper limit of normal [ULN], total bilirubin elevation > 2 ×— ULN, and alkaline phosphatase < 2 ×— ULN). Clinically relevant shifts (normal to high) were similar for vilazodone- versus placebo-treated patients, respectively, for total cholesterol (1.0% vs 2.0%), LDL cholesterol (0% vs 1.3%), and glucose (2.6% vs 1.3%) levels. The most frequently reported potentially clinically significant postbaseline values in metabolic parameters (> 1.1 ×— ULN) for vilazodone and placebo, respectively, were for total cholesterol (16% and 12%) and triglycerides (11% and 10%).
The incidence of orthostatic hypotension (≥ 20 mm Hg systolic blood pressure reduction or ≥ 10 mm Hg diastolic blood pressure reduction while changing position from supine to standing) was similar for vilazodone and placebo (7%). Increases in mean body weight were small in the vilazodone (0.39 kg [0.86 lb]) and placebo (0.30 kg [0.66 lb]) groups. No patients had an ECG finding that was considered clinically significant, and no patient had a QTc Bazett (QTcB) or QTc Fridericia (QTcF) interval increase to > 500 ms.
Suicidality and suicide-related adverse events. CSSRS-rated suicidal ideation was reported in 16 (8%) vilazodone- and 11 (5%) placebo-treated patients; no suicidal behavior was reported in either group. TEAEs of suicidal ideation were reported in 1 patient in each treatment group; both TEAEs resolved the same day they were reported, were not considered related to study drug, and did not result in discontinuation.
Sexual functioning. At the end of double-blind treatment, mean CSFQ total score changes from baseline were +0.9 and +0.5 in the placebo- and vilazodone-treatment groups, respectively (increase indicates improvement on this scale). Vilazodone-treated men had a small mean decrease (−0.3) in score, while placebo-treated men (+0.5) and women in both the vilazodone (+1.0) and placebo (+1.1) groups had small mean increases. Sexual function TEAEs occurred in 17 patients (8%) in the vilazodone group and in 4 (2%) patients in the placebo group; 1 vilazodone patient discontinued due to sexual function TEAEs. The only sexual function TEAEs that occurred in more than 2 vilazodone patients were delayed ejaculation, erectile dysfunction (4 [5% male only] patients each), and decreased libido (5 [2%] patients).
DISCUSSION
In this positive clinical study of patients with GAD, the difference in mean change from baseline to week 8 in HARS total score was statistically significant for vilazodone 20-40 mg/d compared with placebo using the primary MMRM approach. The effect size for vilazodone was 0.31, which is comparable to what has been observed for second-generation antidepressants in the treatment of GAD (0.32; 95% CI, 0.25-0.39).24
Broad anxiolytic efficacy was suggested by significant differences in favor of vilazodone on all other anxiety measures. Improvement in functional impairment was demonstrated by statistically significant differences for vilazodone versus placebo in mean change from baseline in SDS total score and each item score (MMRM). Further indications of symptomatic and overall improvement for patients treated with vilazodone were suggested by statistically significant differences versus placebo in rates of HARS and CGI-I response, CGI-I score at week 8, and mean change from baseline on the CGI-S. The difference in mean change on the HDRS17 was also statistically significant, but this result may not be clinically meaningful given the low level of depressive symptoms in this patient population.
Although numerous pharmaceutical agents are approved for the treatment of GAD, as many as 50% of patients have inadequate response,25 and considerable unmet medical and social needs persist. GAD and MDD frequently present as comorbid conditions26 and are associated with comparable degrees of impairment.27,28 Similar to MDD, GAD more likely generates considerable public health and economic ramifications.1 For example, patients with anxiety disorders overutilize medical resources, which accounts for substantial direct and indirect health care costs.29-32 Despite high medical resource use, however, GAD is frequently unrecognized or untreated in primary care,33,34 suggesting that inadequate or inappropriate medical intervention contributes to overall costs. Anxiety disorders are also associated with excess workplace costs due to lost productivity and absenteeism,32 which adds to the economic and social burdens of GAD.
Evidence suggests that recovery from psychiatric disorders is associated with improved functional impairment.35-37 In studies that use mean change from baseline as an outcome, minimal clinically important differences are used as benchmarks to suggest the point at which patients are likely to recognize the benefits of treatment. On the SDS, the suggested minimal clinically important differences are approximately 4 for total score and 1-2 on each item.22 These thresholds suggest that the magnitude of SDS mean change in the vilazodone group in our study (total score: −8.02 Work: −2.73, Social Life: −3.06, Family Life: −2.74) may have been sufficient for patients to perceive improvement in functional impairment. Additionally, the difference in the rate of SDS remission was statistically significant in favor of vilazodone- versus placebo-treated patients. This remission result is relevant to recovery as shown by a prior GAD investigation38 in which significantly more patients who returned to normative levels on functional assessments achieved symptomatic remission compared with patients who did not return to normative values.
Safety findings in this population of patients with GAD were similar to what has been observed in vilazodone studies in patients with MDD. Gastrointestinal effects, especially nausea and diarrhea, occurred more frequently in vilazodone-treated patients than placebo patients but resulted in few study discontinuations for vilazodone patients (nausea = 6; diarrhea = 4). Overall, mean changes in clinical laboratory values, metabolic parameters, vital signs, and ECG findings were low and similar between treatment groups. Although epidemiologic data suggest that GAD may be associated with suicidality,1,39 no suicidal behavior was reported during the study; only 2 suicidal ideation TEAEs (1 patient each group), which resolved the same day, were reported.
Sexual dysfunction, a known effect of SSRI treatment, is considered by patients to be one of the most unacceptable side effects.40 Mean increases in CSFQ scores were seen in the placebo (+0.9) and vilazodone-treatment groups (+0.5). The incidence of sexual function TEAEs was higher in vilazodone- than placebo-treated patients, but only 1 patient discontinued as a result.
Limitations of the study include its short duration and lack of an active comparator. Since GAD typically presents with comorbid anxiety and mood disorders, exclusion of patients with significant depressive symptoms and most anxiety comorbidities limits the ability to generalize these results to patients with a broader symptom profile or comorbid MDD. Additionally, some analyses were performed post hoc and should be interpreted accordingly.
In this positive study, mean differences in change from baseline to week 8 on HARS total score, SDS total score, and SDS individual items were statistically significant in favor of vilazodone versus placebo, suggesting a reduction in anxiety symptoms and a decrease in functional impairment for vilazodone-treated patients. These results indicate that vilazodone may be a treatment option for GAD, a disorder that is associated with considerable psychiatric and functional impairment, as well as pronounced individual, economic, and societal burdens.
Submitted: February 12, 2015; accepted August 18, 2015.
Online first: May 24, 2016.
Drug names: eszopiclone (Lunesta); vilazodone (Viibryd); zaleplon (Sonata and others); zolpidem (Ambien, Edluar, and others).
Potential conflicts of interest: Dr Durgam and Mr Gommoll acknowledge a potential conflict of interest as employees of Forest Research Institute, an affiliate of Actavis, Inc; Drs Mathews, Nunez, and Tang and Ms Forero acknowledge a potential conflict of interest as employees of Forest Research Institute, an affiliate of Actavis, Inc, at the time of the study. Drs Durgam and Tang, Mr Gommoll, and Ms Forero are Actavis stock shareholders and Mr Gommoll is a Forest stock shareholder. Dr Mathews has participated in Forest and Actavis speaker/advisory boards. Dr Sheehan has received grant/research support from, has been affiliated with, is a stock holder in, or has received honoraria and travel expenses related to lectures/presentations/royalties or consultant activities from the following organizations: Abbott Laboratories; Actavis; Ad Hoc Committee, Treatment Drug & Assessment Research Review Committee (RRC) of NIMH on Anxiety and Phobic Disorders Projects; Alexa; Alza Pharmaceuticals, Palo Alto, CA; American Medical Association; American Psychiatric Association Task Force on Benzodiazepine Dependency; American Psychiatric Association Task Force on Treatments of Psychiatric Disorders; American Psychiatric Association Working Group to Revise DSM III Anxiety Disorders Section; Anclote Foundation; Anxiety Disorders Resource Center; Anxiety Drug Efficacy Case, US Food and Drug Administration; Applied Health Outcomes/Xcenda; Apsen Pharma; AstraZeneca; Avera Pharmaceuticals; BioMarin; Bionomics; Boehringer Ingelheim; Boots Pharmaceuticals; Bristol-Myers Squibb; Burroughs Wellcome; Cephalon; Charter Hospitals; Ciba Geigy; Connecticut and Ohio Academies of Family Physicians; Cortex Pharmaceutical; Council on Anxiety Disorders; Cpc Coliseum Medical Center; Cypress Bioscience; Daiichi Sankyo Pharma Development; Daiichi Sahnkyo/MMS Holdisngs Inc; Daiichi Sumitomo; Dista Products Company; Division of Drugs and Technology, American Medical Association; Eisai; Eli Lilly; Excerpta Medica Asia; Faxmed, Inc; Forest Laboratories, an affiliate of Actavis, Inc; Glaxo Pharmaceuticals; GlaxoSmithKline; Glaxo-Wellcome; Hikma Pharmaceuticals; Hospital Corporation of America; Humana; ICI; INC Research; International Clinical Research (ICR); International Society for CNS Drug Development (ISCDD); Janssen Pharmaceutica; Jazz Pharmaceuticals; Kali-Duphar; Labopharm-Angellini; Layton Bioscience; Lilly Research Laboratories; Lundbeck, Denmark; Marion Merrill Dow; McNeil Pharmaceuticals; Mead Johnson; Macmillan; MAPI; Medical Outcome Systems; MediciNova; Merck Sharp & Dohme; National Anxiety Awareness Program; National Anxiety Foundation; National Depressive and Manic Depressive Association; National Institute on Drug Abuse; National Institutes of Health; Neuronetics; NovaDel; Novartis Pharmaceuticals; Novo Nordisk; Organon; Orion Pharma; Otsuka; Parexel International; Parke-Davis; Pfizer; Pharmacia; Pharmacia & Upjohn; PharmaNeuroBoost; Philadelphia College of Pharmacy and Science; Pierre Fabre, France; Quintiles; ProPhase; Rhone Laboratories; Rhone-Poulenc Rorer Pharmaceuticals; Roche; Roerig; Sagene; Sandoz Pharmaceuticals; Sanofi-Aventis; Sanofi-Synthelabo Recherche/Sanofi Aventis; Schering; Sepracor; Shire Laboratories; Simon and Schuster; SmithKlineBeecham; Solvay Pharmaceuticals; Sunovion; Takeda Pharmaceuticals; Tampa General Hospital (TGH); University of South Florida Psychiatry Center; University of South Florida (USF) College of Medicine; TAP Pharmaceuticals; Targacept; TGH-University Psychiatry Center; Tikvah Therapeutics; Titan Pharmaceuticals; Tonix Pharmaceuticals; United Bioscience; The Upjohn Company; US Congress-House of Representatives Committee; USF Friends of Research in Psychiatry, Board of Trustees; Warner Chilcott; World Health Organization; Worldwide Clinical Trials; Wyeth-Ayerst; ZARS; and Zeneca Pharmaceuticals.
Funding/support: Supported by funding from Forest Laboratories, LLC, an affiliate of Actavis, Inc, Jersey City, New Jersey.
Role of the sponsor: Forest Laboratories, LLC, was involved in the study design, collection (via contracted clinical investigator sites), analysis and interpretation of data, and the decision to present these results.
Previous presentation: Poster presented at the 168th Annual Meeting of the American Psychiatric Association; May 16-20, 2015; Toronto, Ontario, Canada.
Acknowledgments: Writing assistance and editorial support for the preparation of this manuscript were provided by Carol Brown, MS, of Prescott Medical Communications Group, Chicago, Illinois, a contractor of Forest Research Institute, an affiliate of Actavis, Inc.
REFERENCES
1. Revicki DA, Travers K, Wyrwich KW, et al. Humanistic and economic burden of generalized anxiety disorder in North America and Europe. J Affect Disord. 2012;140(2):103-112. PubMed doi:10.1016/j.jad.2011.11.014
2. Wittchen HU. Generalized anxiety disorder: prevalence, burden, and cost to society. Depress Anxiety. 2002;16(4):162-171. PubMed doi:10.1002/da.10065
3. Huh J, Goebert D, Takeshita J, et al. Treatment of generalized anxiety disorder: a comprehensive review of the literature for psychopharmacologic alternatives to newer antidepressants and benzodiazepines. Prim Care Companion CNS Disord. 2011;13(2):doi:10.4088/PCC.08r00709blu. PubMed doi:10.4088/PCC.08r00709
4. Khan A, Cutler AJ, Kajdasz DK, et al. A randomized, double-blind, placebo-controlled, 8-week study of vilazodone, a serotonergic agent for the treatment of major depressive disorder. J Clin Psychiatry. 2011;72(4):441-447. PubMed doi:10.4088/JCP.10m06596
5. Rickels K, Athanasiou M, Robinson DS, et al. Evidence for efficacy and tolerability of vilazodone in the treatment of major depressive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(3):326-333. PubMed doi:10.4088/JCP.08m04637
6. Croft HA, Pomara N, Gommoll C, et al. Efficacy and safety of vilazodone in major depressive disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2014;75(11):e1291-e1298. PubMed doi:10.4088/JCP.14m08992
7. Mathews M, Gommoll C, Chen D, et al. Efficacy and safety of vilazodone 20 and 40 mg in major depressive disorder: a randomized, double-blind, placebo-controlled trial. Int Clin Psychopharmacol. 2015;30(2):67-74. PubMed doi:10.1097/YIC.0000000000000057
8. Viibryd [package insert]. St. Louis, MO: Forest Pharmaceuticals, Inc; 2014.
9. Davidson JR, Zhang W, Connor KM, et al. A psychopharmacological treatment algorithm for generalised anxiety disorder (GAD). J Psychopharmacol. 2010;24(1):3-26. PubMed doi:10.1177/0269881108096505
10. Thase ME, Chen D, Edwards J, et al. Efficacy of vilazodone on anxiety symptoms in patients with major depressive disorder. Int Clin Psychopharmacol. 2014;29(6):351-356. PubMed doi:10.1097/YIC.0000000000000045
11. Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32(1):50-55. PubMed doi:10.1111/j.2044-8341.1959.tb00467.x
12. Gommoll C, Durgam S, Mathews M, et al. A double-blind, randomized, placebo-controlled, fixed-dose phase III study of vilazodone in patients with generalized anxiety disorder. Depress Anxiety. 2015;32(6):451-459. PubMed doi:10.1002/da.22365
13. Sambunaris A, Forero G, Mathews M, et al. Vilazodone in patients with generalized anxiety disorder: a double-blind, randomized, placebo-controlled, flexible-dose study. Poster presented at 168th Annual Meeting of the American Psychiatric Association. May 16-20, 2015; Toronto, Canada.
14. American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
15. Guy W. ECDEU Assessment Manual for Psychopharmacology. Revised Edition. Washington, DC: US Department of Health, Education, and Welfare; 1976.
16. Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23(1):56-62. PubMed doi:10.1136/jnnp.23.1.56
17. Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996;11(suppl 3):89-95. PubMed doi:10.1097/00004850-199606003-00015
18. Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266-1277. PubMed doi:10.1176/appi.ajp.2011.10111704
19. Clayton AH, McGarvey EL, Clavet GJ, et al. Comparison of sexual functioning in clinical and nonclinical populations using the Changes in Sexual Functioning Questionnaire (CSFQ). Psychopharmacol Bull. 1997;33(4):747-753. PubMed
20. Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53(3):983-997. PubMed doi:10.2307/2533558
21. Kenward MG, Molenberghs G, Thijs H. Pattern-mixture models with proper time dependence. Biometrika. 2003;90(1):53-71. doi:10.1093/biomet/90.1.53
22. Sheehan KH, Sheehan DV. Assessing treatment effects in clinical trials with the discan metric of the Sheehan Disability Scale. Int Clin Psychopharmacol. 2008;23(2):70-83. PubMed doi:10.1097/YIC.0b013e3282f2b4d6
23. Zimmerman M, Martinez JH, Young D, et al. Severity classification on the Hamilton Depression Rating Scale. J Affect Disord. 2013;150(2):384-388. PubMed doi:10.1016/j.jad.2013.04.028
24. Roest AM, de Jonge P, Williams CD, et al. Reporting bias in clinical trials investigating the efficacy of second-generation antidepressants in the treatment of anxiety disorders: a report of 2 meta-analyses. JAMA Psychiatry. 2015;72(5):500-510. PubMed doi:10.1001/jamapsychiatry.2015.15
25. Buoli M, Caldiroli A, Caletti E, et al. New approaches to the pharmacological management of generalized anxiety disorder. Expert Opin Pharmacother. 2013;14(2):175-184. PubMed doi:10.1517/14656566.2013.759559
26. Kessler RC, Gruber M, Hettema JM, et al. Co-morbid major depression and generalized anxiety disorders in the National Comorbidity Survey follow-up. Psychol Med. 2008;38(3):365-374. PubMed
27. Kessler RC, DuPont RL, Berglund P, et al. Impairment in pure and comorbid generalized anxiety disorder and major depression at 12 months in two national surveys. Am J Psychiatry. 1999;156(12):1915-1923. PubMed
28. Wittchen HU, Carter RM, Pfister H, et al. Disabilities and quality of life in pure and comorbid generalized anxiety disorder and major depression in a national survey. Int Clin Psychopharmacol. 2000;15(6):319-328. PubMed doi:10.1097/00004850-200015060-00002
29. Issakidis C, Sanderson K, Corry J, et al. Modelling the population cost-effectiveness of current and evidence-based optimal treatment for anxiety disorders. Psychol Med. 2004;34(1):19-35. PubMed doi:10.1017/S003329170300881X
30. Marciniak MD, Lage MJ, Dunayevich E, et al. The cost of treating anxiety: the medical and demographic correlates that impact total medical costs. Depress Anxiety. 2005;21(4):178-184. PubMed doi:10.1002/da.20074
31. Olfson M, Gameroff MJ. Generalized anxiety disorder, somatic pain and health care costs. Gen Hosp Psychiatry. 2007;29(4):310-316. PubMed doi:10.1016/j.genhosppsych.2007.04.004
32. Greenberg PE, Sisitsky T, Kessler RC, et al. The economic burden of anxiety disorders in the 1990s. J Clin Psychiatry. 1999;60(7):427-435. PubMed doi:10.4088/JCP.v60n0702
33. Culpepper L. Generalized anxiety disorder in primary care: emerging issues in management and treatment. J Clin Psychiatry. 2002;63(suppl 8):35-42. PubMed
34. Wittchen HU, Kessler RC, Beesdo K, et al. Generalized anxiety and depression in primary care: prevalence, recognition, and management. J Clin Psychiatry. 2002;63(suppl 8):24-34. PubMed
35. Katzelnick DJ, Simon GE, Pearson SD, et al. Randomized trial of a depression management program in high utilizers of medical care. Arch Fam Med. 2000;9(4):345-351. PubMed doi:10.1001/archfami.9.4.345
36. Mintz J, Mintz LI, Arruda MJ, et al. Treatments of depression and the functional capacity to work. Arch Gen Psychiatry. 1992;49(10):761-768. PubMed doi:10.1001/archpsyc.1992.01820100005001
37. Schulberg HC, Block MR, Madonia MJ, et al. Treating major depression in primary care practice: eight-month clinical outcomes. Arch Gen Psychiatry. 1996;53(10):913-919. PubMed doi:10.1001/archpsyc.1996.01830100061008
38. Pollack MH, Endicott J, Liebowitz M, et al. Examining quality of life in patients with generalized anxiety disorder: clinical relevance and response to duloxetine treatment. J Psychiatr Res. 2008;42(12):1042-1049. PubMed doi:10.1016/j.jpsychires.2007.11.006
39. Weisberg RB. Overview of generalized anxiety disorder: epidemiology, presentation, and course. J Clin Psychiatry. 2009;70(suppl 2):4-9. PubMed doi:10.4088/JCP.s.7002.01
40. Hu XH, Bull SA, Hunkeler EM, et al. Incidence and duration of side effects and those rated as bothersome with selective serotonin reuptake inhibitor treatment for depression: patient report versus physician estimate. J Clin Psychiatry. 2004;65(7):959-965. PubMed doi:10.4088/JCP.v65n0712
Save
Cite
Advertisement
GAM ID: sidebar-top