ABSTRACT
Objective: To compare the efficacy of vortioxetine and the serotonin-norepinephrine reuptake inhibitor (SNRI) desvenlafaxine in patients with major depressive disorder (MDD) experiencing partial response to initial treatment with a selective serotonin reuptake inhibitor (SSRI).
Methods: This randomized, double-blind, active-controlled, parallel-group, 8-week study of vortioxetine (10 or 20 mg/d; n = 309) versus desvenlafaxine (50 mg/d: n = 293) was conducted from June 2020 to February 2022 in adults with a DSM-5 diagnosis of MDD who experienced partial response to SSRI monotherapy. The primary endpoint was mean change from baseline to week 8 in Montgomery-Asberg Depression Rating Scale (MADRS) total score. Differences between groups were analyzed using mixed models for repeated measures.
Results: Non-inferiority of vortioxetine versus desvenlafaxine was established in terms of mean change from baseline to week 8 in MADRS total score; however, a numeric advantage was observed in favor of vortioxetine (difference, −0.47 MADRS points [95% CI, –1.61 to 0.67]; P = .420). At week 8, significantly more vortioxetine-treated than desvenlafaxine-treated patients had achieved symptomatic and functional remission (ie, Clinical Global Impressions–Severity of Illness scale [CGI-S] score ≤ 2) (32.5% vs 24.8%, respectively; odds ratio = 1.48 [95% CI, 1.03 to 2.15]; P = .034). Vortioxetine-treated patients also experienced significantly greater improvements in daily and social functioning assessed by the Functioning Assessment Short Test (P = .009 and .045 vs desvenlafaxine, respectively) and reported significantly greater satisfaction with their medication assessed by the Quality of Life Enjoyment and Satisfaction Questionnaire (P = .044). Treatment-emergent adverse events (TEAEs) were reported in 46.1% and 39.6% of patients in the vortioxetine and desvenlafaxine groups, respectively; these were mostly mild or moderate in intensity (> 98% of all TEAEs in each group).
Conclusions: Compared with the SNRI desvenlafaxine, vortioxetine was associated with significantly higher rates of CGI-S remission, better daily and social functioning, and greater treatment satisfaction in patients with MDD and partial response to SSRIs. These findings support the use of vortioxetine before SNRIs in the treatment algorithm in patients with MDD.
Trial Registration: ClinicalTrials.gov Identifier: NCT04448431
J Clin Psychiatry 2023;84(4):23m14780
To cite: McIntyre RS, Florea I, Pedersen MM, et al. Head-to-head comparison of vortioxetine versus desvenlafaxine in patients with major depressive disorder with partial response to SSRI therapy: results of the VIVRE study. J Clin Psychiatry. 2023;84(4):23m14780.
To share: https://doi.org/10.4088/JCP.23m14780
© 2023 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, University of Toronto, Toronto, Ontario, Canada
bBrain and Cognition Discovery Foundation, Toronto, Ontario, Canada
cH. Lundbeck A/S, Valby, Denmark
*Corresponding author: Michael Cronquist Christensen, H. Lundbeck A/S, Ottiliavej 9, 2500 Valby, Denmark ([email protected]).
Head-to-head studies of antidepressants in patients with major depressive disorder (MDD) are rare, particularly in those experiencing partial or no response to prior therapy. Selective serotonin reuptake inhibitors (SSRIs) are generally used as initial treatment in patients with MDD.1–5 However, in approximately 50% of patients, symptoms do not improve or show only partial response to initial SSRI therapy.6 Residual symptoms, such as emotional blunting, are associated with a more severe course of depression and poorer patient outcomes.7–9 Partial response is also associated with decreased treatment satisfaction10; this can lead to non-adherence to medication,11,12 which increases the risk of worsening symptoms and relapse.13
Clinical guidelines recommend switching to an agent from a different pharmacologic class in patients with suboptimal response to initial antidepressant therapy.1–5 Serotonin-norepinephrine reuptake inhibitors (SNRIs) are typically used as second-line therapy in patients with partial response or non-response to SSRIs. However, this may not be the most appropriate option given the overlapping mechanisms of action and adverse event profiles of these drug classes.14 Switching to an antidepressant with a mechanism of action that is more distinct from that of SSRIs may be a more appropriate strategy in partial responders.15
Vortioxetine is a multimodal antidepressant that mediates its effects through modulation of the activity of several serotonin (5-HT) receptor types (specifically, 5-HT1A, 5-HT1B, 5-HT1D, 5-HT3, and 5-HT7 receptors) in addition to inhibition of the 5-HT transporter (the principal target of SSRIs and SNRIs).16,17 Vortioxetine thereby modulates not only the activity of the serotoninergic system, but also that of other neurotransmitter systems relevant to the neurobiology of depression.18,19 Vortioxetine has demonstrated efficacy across the spectrum of symptoms experienced by patients with MDD, including depressive, cognitive, and physical symptoms, as well as anxiety and functional impairment.20–27 Vortioxetine has also been shown to improve functional and occupational outcomes in working patients with MDD.28–30 This is particularly relevant―and warrants further investigation―as most adult patients with MDD remain in work despite their disease and the resulting functional impairment.31
In previous comparative studies versus SNRIs in patients with MDD, vortioxetine demonstrated superior efficacy versus venlafaxine extended-release (XR) in terms of depressive symptom reduction32,33 and versus duloxetine in terms of improvement on a novel dual-outcome composite measure of depressive symptoms and functional capacity.34,35 It is therefore appropriate to investigate whether vortioxetine should be used earlier in the treatment algorithm in patients with MDD—specifically, before SNRIs in patients with partial response or no response to initial SSRI therapy.
The VIVRE study was an international, active-controlled, double-blind phase IV study undertaken to compare the efficacy of vortioxetine versus desvenlafaxine on depressive symptoms, overall functioning, and health-related quality of life in patients with MDD experiencing only partial response to initial SSRI therapy. Efficacy was also evaluated in the large subgroup of working patients participating in this study. Desvenlafaxine was chosen as the active comparator in this study as it was the most recently approved SNRI in most countries worldwide.
METHODS
Study Design and Participants
This phase IV, multicenter, randomized, double-blind, active-controlled, parallel-group study was conducted at 77 sites across 12 countries (Argentina, Belgium, Bulgaria, Czech Republic, Estonia, Latvia, Mexico, Russia, Slovakia, Spain, Sweden, and Ukraine) from June 2020 to February 2022. Due to the COVID-19 pandemic, a crisis management plan was implemented that allowed remote assessments, except for the screening, baseline, and primary outcome visits.
Participants were outpatients aged 18–65 years with a primary diagnosis of MDD (Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [DSM-5] criteria, confirmed by the Mini-International Neuropsychiatric Interview36), who were experiencing partial response to SSRI monotherapy (ie, escitalopram, sertraline, paroxetine, or citalopram at the approved dose for ≥ 6 weeks) and were, in the investigator’s opinion, suitable candidates for switching to an alternative antidepressant. Other inclusion criteria were duration of current major depressive episode of ≥ 3 and < 12 months and baseline Montgomery-Asberg Depression Rating Scale (MADRS) total score of ≥ 24 points (ie, moderate-to-severe depression). Exclusion criteria included any other current DSM-5 psychiatric or Axis I disorder, treatment-resistant depression (ie, inadequate response to at least 2 antidepressants for the current depressive episode), baseline Digit Symbol Substitution Test37 score ≥ 70, history of alcohol/substance use within the past 6 months, and clinically significant risk for suicide.
After a 2-week screening period, eligible patients were randomized (1:1) to treatment with vortioxetine (10 or 20 mg/d) or desvenlafaxine (50 mg/d) for 8 weeks, followed by a 4-week safety follow-up period. Study drugs were administered in accordance with local prescribing information. Vortioxetine was initiated at the recommended starting dose of 10 mg/d, with up-titration to 20 mg/d in all patients after 1 week. The vortioxetine dose could then be adjusted (10 or 20 mg/d) at scheduled or unscheduled visits based on investigator judgment until week 4; after week 4, no further dose adjustments were permitted. Patients in the desvenlafaxine group received the recommended dose of 50 mg/d. To maintain the blind, desvenlafaxine dose adjustment could be requested up to week 4 based on patient response and the investigator’s clinical judgment. However, as highlighted in the prescribing information for desvenlafaxine, there is no evidence that doses > 50 mg/d confer any additional clinical benefit, and adverse events and withdrawals are more frequent at higher doses.38,39 Consequently, even if desvenlafaxine dose adjustment was requested, the dosage was not changed and patients continued to receive 50 mg/d throughout the study period. Prior SSRI monotherapy was discontinued before the baseline visit, with dose-tapering if required.
The study (ClinicalTrials.gov identifier: NCT04448431) was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the local ethics committee at each study site. Patients provided written informed consent before participation.
Study Assessments
All assessment scales and questionnaires were administered in the local language. Severity of core depressive symptoms was assessed by MADRS total score.40 Overall disease severity and its impact on global patient functioning was assessed using the Clinical Global Impressions–Severity of Illness scale (CGI-S) and CGI-Improvement scale (CGI-I).41 Patient functioning was also assessed using the Functioning Assessment Short Test (FAST). This clinician-rated scale assesses functioning over the past 14 days across 6 domains: autonomy (ie, daily functioning), occupational functioning, cognitive functioning, financial issues, interpersonal relationships (ie, social functioning), and leisure time.42 FAST total score ranges from 0 to 72 points; higher scores indicate greater impairment in functioning, with scores of 12–20, 21–40, and > 40 indicating mild, moderate, and severe functional impairment, respectively.43
Health-related quality of life was assessed using the long form of the Quality of Life Enjoyment and Satisfaction Questionnaire (Q-LES-Q).44 This provides a comprehensive patient-rated assessment of health-related quality of life across 10 domains: physical health, subjective feelings, work, household duties, school/course work, leisure time activities, social relationships, general activities, satisfaction with medication, and overall satisfaction and contentment. Q-LES-Q domain scores are expressed as a percentage of the maximum score possible; higher scores signify better health-related quality of life.
Safety was evaluated by the incidence of treatment-emergent adverse events (TEAEs) and using the Columbia-Suicide Severity Rating Scale.45
Statistical Analysis
Planned randomization was 600 patients (300 in each group); with this sample size and an expected withdrawal rate of 10%, an assumed standard deviation of 9.6 points for the change from baseline in MADRS total score at week 8 (primary study endpoint), and a priori no difference between treatments, power of at least 85% for concluding non-inferiority was expected. Efficacy was analyzed in all randomized patients who received at least one dose of study medication and had a valid baseline and at least one post-baseline assessment for MADRS total score (full analysis set). Safety was analyzed in all enrolled patients who received at least one dose of study medication (all-patients-treated set).
The primary study endpoint (ie, change from baseline to week 8 in MADRS total score) was analyzed using a mixed model for repeated measures to estimate treatment difference between vortioxetine and desvenlafaxine at week 8 and the associated 95% confidence interval (CI). The model included country and treatment (vortioxetine or desvenlafaxine) as fixed factors, baseline MADRS total score as a continuous covariate, treatment-by-week interaction, and an interaction between week and baseline MADRS total score, and an unstructured covariance matrix was applied. Non-inferiority was declared if the upper bound of the 95% CI did not exceed 2.5 MADRS points. If non-inferiority was established, then superiority of vortioxetine over desvenlafaxine was investigated (2-sided test at 5% significance level).
Pre-specified secondary endpoints included changes from baseline to week 8 in CGI-S score, FAST total and domain scores, and Q-LES-Q domain scores, and the CGI-I score at week 8. Rates of symptomatic and functional remission (ie, CGI-S score ≤ 2) and response (ie, CGI-I score ≤ 2), and of MADRS response (ie, ≥ 50% reduction in MADRS total score from baseline) and MADRS remission (ie, MADRS total score ≤ 10), were also evaluated at week 8. FAST total and domain scores, and CGI-S remission and CGI-I response rates, were assessed for the overall study population and in the subgroup of working patients (ie, those in paid employment or self-employed at baseline).
Continuous secondary endpoints were analyzed using mixed models for repeated measures similar to that used for the primary endpoint or using an analysis of covariance, observed-cases model. For the analysis of CGI-I scores, respective baseline CGI-S scores were included as a covariate. The analysis of covariance model was used for endpoints with only one post-baseline assessment and as a sensitivity analysis for other endpoints. Rates of response and remission were analyzed using logistic regression. No adjustment for multiplicity was made.
Safety endpoints were summarized using descriptive statistics. All analyses were performed using SAS statistical software (version 9.4; SAS Institute Inc.; Cary, North Carolina).
RESULTS
Study Population
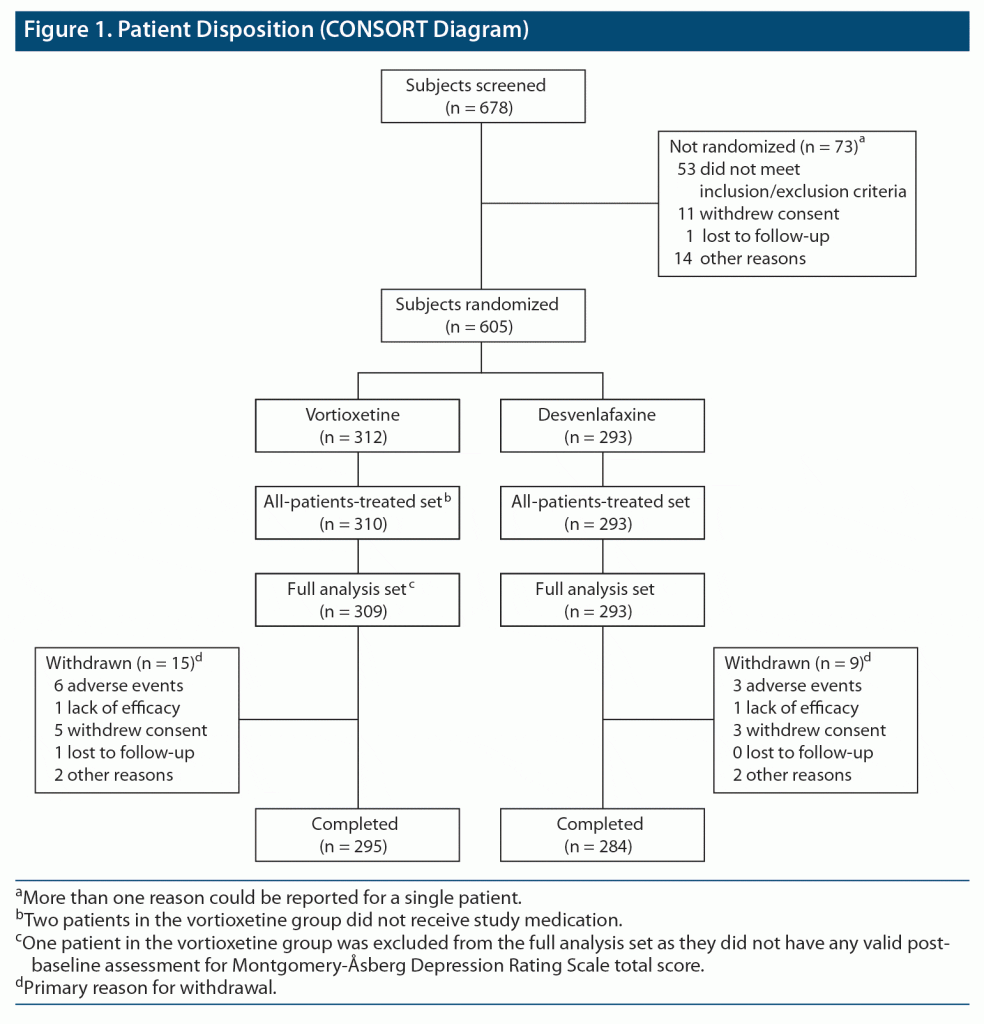
Of the 678 patients screened, 603 were randomized and received at least one dose of study medication (310 and 293 in the vortioxetine and desvenlafaxine groups, respectively) (Figure 1). Patient disposition by country is shown in Supplementary Table 1.
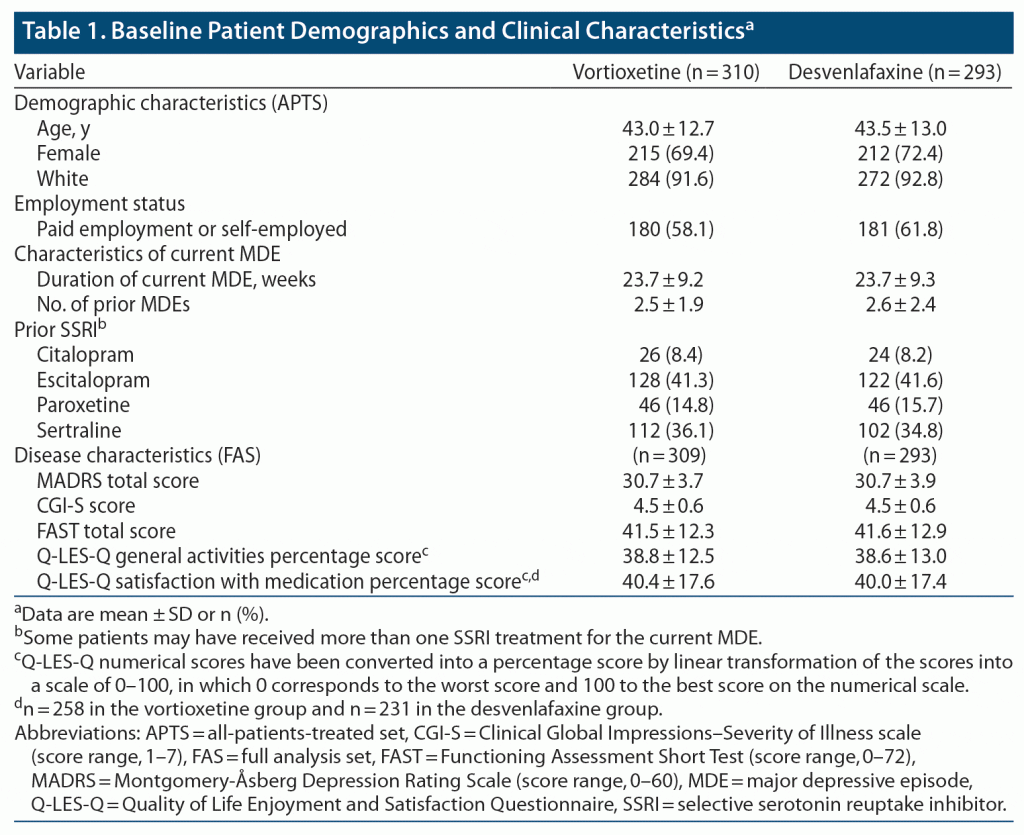
Treatment groups were well matched in terms of baseline demographic and clinical characteristics (Table 1 and Supplementary Table 2). Mean age was 43.3 years, 70.8% of participants were female, and most (92.2%) were White. Escitalopram and sertraline were the most frequently used prior SSRIs (received by 41.5% and 35.5% of patients, respectively). Concomitant antidepressants were used during the study by 3 patients in the vortioxetine group (sertraline [n = 2] and escitalopram [n = 1]) and 2 patients in the desvenlafaxine group (sertraline and vortioxetine [both n = 1]). Benzodiazepines were continued or started after the first dose of study medication by 41 patients (13.2%) and 34 patients (11.6%) in the two groups, respectively.
Mean MADRS total score at baseline was 30.7, indicating a population with moderate-to-severe depression. Mean baseline CGI-S and FAST scores were 4.5 and 41.5, respectively, indicating moderate-to-severe illness and severely impaired patient functioning. Patients reported poor health-related quality of life at baseline (mean Q-LES-Q domain scores ranging from 28.7% to 42.6%) and low satisfaction with prior SSRI therapy (mean Q-LES-Q satisfaction with medication score, 40.3%) (Supplementary Table 2).
Dosing
Vortioxetine dose was increased to 20 mg/d after 1 week of treatment in all but 1 patient (99.7%). Of the 309 patients in whom vortioxetine dose was increased, 275 (89.0%) received vortioxetine 20 mg/d from week 1 to week 8. Vortioxetine dose was reduced to 10 mg/d between weeks 1 and 4 in 30 patients (9.7%); further dose information was unavailable for the remaining 4 patients (1.3%). Dose reduction was requested for 7.0% of patients in the desvenlafaxine group; however, dose remained unchanged in these patients.
Efficacy
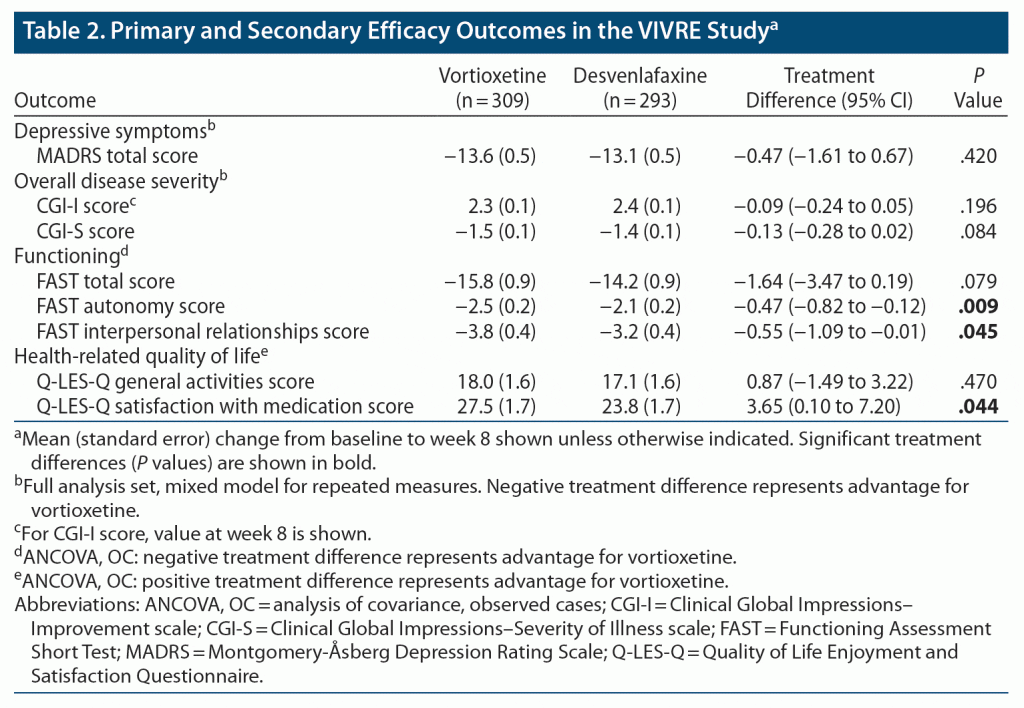
Comparable effect was observed in terms of depressive symptom reduction from baseline to week 8 (ie, mean change in MADRS total score at week 8, the primary endpoint) for vortioxetine and desvenlafaxine (Table 2), with a numeric difference in favor of vortioxetine of −0.47 points (95% CI, −1.61 to 0.67; P = .420). Significantly more vortioxetine-treated patients achieved symptomatic and functional remission (ie, CGI-S score ≤ 2) (32.5% vs 24.8% in the desvenlafaxine group; odds ratio [OR] = 1.48 [95% CI, 1.03 to 2.15]; P = .034). The proportions of patients achieving response assessed by CGI-I and MADRS criteria and remission assessed by MADRS criteria were similar in the two groups (Supplementary Table 3).
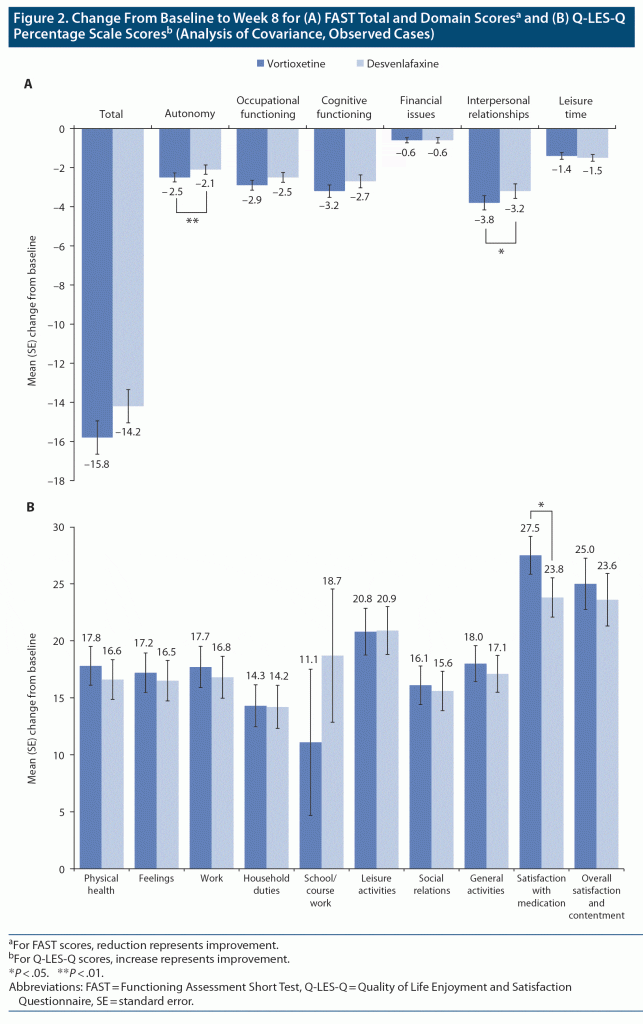
Improvement in functioning assessed by the FAST was seen in both groups over the 8 weeks of treatment (Figure 2A), with numerically greater improvement in FAST total score and significantly greater improvements in FAST autonomy (daily functioning) and interpersonal relationships (social functioning) domain scores in vortioxetine-treated patients (pre-specified comparisons; P = .009 and .045 vs desvenlafaxine, respectively). In terms of health-related quality of life assessed by the Q-LES-Q, mean improvements from baseline were generally numerically greater in vortioxetine-treated patients across all Q-LES-Q domains (Figure 2B). There was a significant difference in favor of vortioxetine in mean change in the Q-LES-Q satisfaction with medication domain score from baseline to week 8 (pre-specified comparison, 27.5% vs 23.8% in the desvenlafaxine group; P = .044).
Working Patients Subgroup
Overall, 361 patients were in paid employment or self-employed at baseline (ie, 59.9% of the all-patients-treated set, evenly distributed between treatment groups). In working patients, mean FAST total score at baseline was 40.0 in the vortioxetine group and 40.3 in the desvenlafaxine group, indicating severe functional impairment (Supplementary Table 4). A significantly greater change from baseline to week 8 in FAST total score was seen in vortioxetine-treated patients (−16.6 vs −14.1 points in desvenlafaxine-treated patients; difference, −2.52 [95% CI, −4.88 to −0.15]; P = .037). Significantly greater improvements in daily and social functioning were also seen with vortioxetine (P = .012 and .046 vs desvenlafaxine for the autonomy and interpersonal relationship domains, respectively) (Supplementary Figure 1). At week 8, the proportion of working patients achieving symptomatic and functional remission (ie, CGI-S score ≤ 2) was 36.2% (63/174) with vortioxetine versus 25.1% (44/175) with desvenlafaxine (OR = 1.72 [95% CI, 1.08 to 2.75; P = .023).
Safety
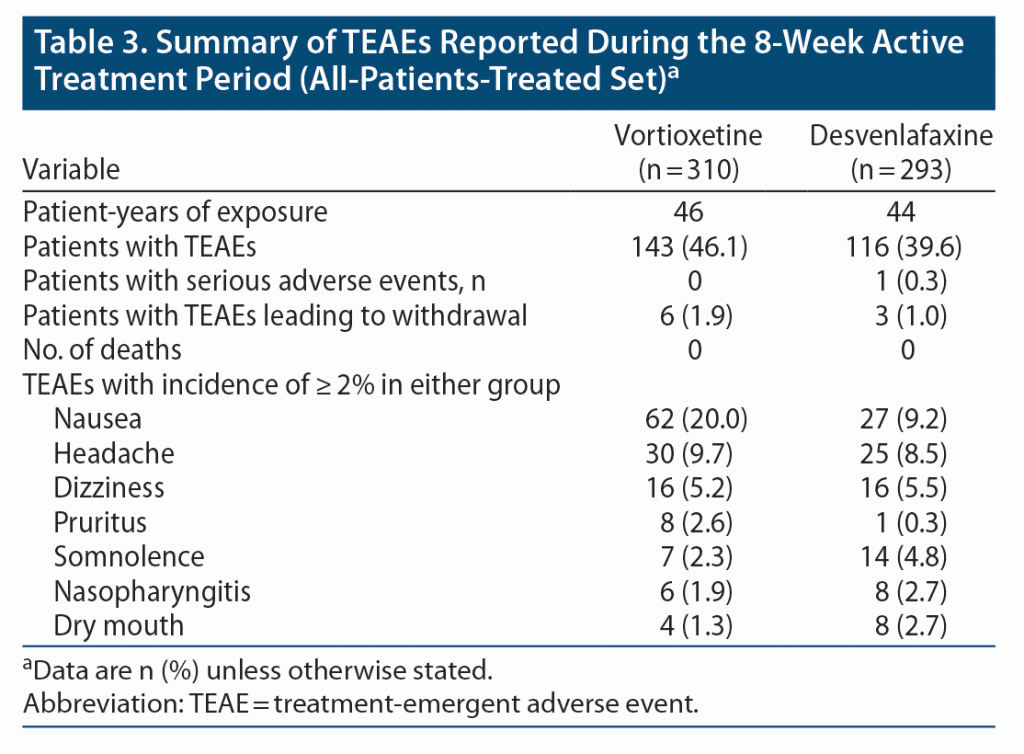
During the 8-week treatment period, TEAEs were reported in 46.1% of patients in the vortioxetine group and 39.6% of those in the desvenlafaxine group (Table 3). TEAEs were mostly mild or moderate (> 98% of all TEAEs in each group). In both groups, the most common TEAEs were nausea, headache, and dizziness. The incidence of nausea was higher in vortioxetine-treated patients (20.0% vs 9.2% for desvenlafaxine). The incidence of other TEAEs was generally similar between groups.
One serious adverse event was reported (severe vomiting lasting for 3 days in 1 patient in the desvenlafaxine group, considered unrelated to treatment). The incidence of TEAEs leading to withdrawal from the study was low (1.9% and 1.0% in the vortioxetine and desvenlafaxine groups, respectively). The majority (≥ 97%) of patients in both groups did not experience suicidal ideation or behavior during the treatment period.
DISCUSSION
VIVRE is the first head-to-head study of vortioxetine versus an SNRI in patients with MDD experiencing partial response to SSRI monotherapy; published data are notably lacking concerning the comparative efficacy of antidepressants in this patient population. VIVRE assessed treatment effects across the spectrum of symptoms experienced by patients with MDD and, as such, provides clinically relevant information about the role of vortioxetine versus SNRIs in this setting.
The results support earlier use of vortioxetine in the treatment algorithm in patients with MDD failing to respond to initial SSRI therapy, prior to an SNRI. Comparable efficacy was observed for vortioxetine and desvenlafaxine in terms of improvement in depressive symptoms as assessed by change in MADRS total score over the 8 weeks of treatment. However, significantly more patients treated with vortioxetine achieved symptomatic and functional remission assessed using the version of the CGI-S that measures depression severity and its impact on global patient functioning.41 Vortioxetine-treated patients also experienced significantly greater improvements in daily and social functioning assessed using the FAST than those who received desvenlafaxine. These findings are clinically important, as improvements in global, daily, and social functioning are positively linked to maintaining antidepressant response and preventing relapse in patients with MDD.13,46,47
Vortioxetine-treated patients also reported significantly greater satisfaction with their medication as assessed using the Q-LES-Q than those who received desvenlafaxine. Satisfaction with antidepressant medication combines patient perceptions of treatment efficacy and tolerability and has been shown to directly correlate with treatment adherence in patients with MDD.11,12 Non-adherence to antidepressant therapy remains a major challenge in clinical practice, being associated with suboptimal clinical outcomes.13 In a network meta-analysis of head-to-head studies in patients with MDD,48 vortioxetine was found to offer the best balance of efficacy and acceptability among the 21 antidepressants included.
The VIVRE study findings add to the growing body of evidence supporting significantly greater efficacy of vortioxetine versus SNRIs on clinically relevant outcomes for patients with MDD. In the SOLUTION study,32 significantly greater reduction in depressive symptoms as assessed by MADRS total score was seen in patients who received vortioxetine 10 mg/d versus those who received venlafaxine XR 150 mg/d. Significantly more vortioxetine- than venlafaxine-treated patients also achieved treatment success, defined by the dual outcome of ≥ 50% reduction in MADRS total score from baseline and no TEAEs during the 8-week treatment period.33
The significantly greater effect of vortioxetine versus desvenlafaxine on daily functioning replicates earlier findings versus another SNRI, duloxetine. In the CONNECT study,34,35 which objectively measured improvement in patient functioning using the University of California San Diego Performance-based Skills Assessment (UPSA), significantly greater improvement in UPSA composite score was seen with vortioxetine compared with duloxetine. Vortioxetine, but not duloxetine, also demonstrated a robust effect versus placebo on a novel dual-outcome composite measure of depressive symptoms and functional capacity (defined as a change from baseline of ≥ 50% for MADRS total score and ≥ 7 points on the UPSA) in that study.35
MDD is known to be one of the major causes of absenteeism and presenteeism in the workplace.49 Patients with MDD experience significant impairments in their ability to function at work, particularly in terms of planning and executing tasks.50 Over half (60%) of all patients were in paid employment at baseline in the VIVRE study. Working patients treated with vortioxetine experienced significantly greater improvements in overall functioning and domains of autonomy (ie, activities of daily life) and interpersonal relationships (ie, social life) than those who received desvenlafaxine and were more likely to achieve symptomatic and functional remission assessed using the CGI-S. Remission has been shown to be significantly associated with improved work performance and productivity in patients with MDD.51 Our findings are consistent with those of previous studies showing significant improvements in functional and occupational outcomes in working patients with MDD treated with vortioxetine.28–30
The superior benefits of vortioxetine versus desvenlafaxine are most likely due to its multimodal mechanism of action. Vortioxetine is a 5-HT3, 5-HT7, and 5-HT1D receptor antagonist, a 5-HT1B receptor partial agonist, and a 5-HT1A receptor agonist as well as an inhibitor of the 5-HT transporter.16,52,53 Vortioxetine therefore not only inhibits 5-HT reuptake, but also directly and indirectly modulates the downstream release of other neurotransmitters relevant to the neurobiology of depression through its effects on specific 5-HT receptors, including norepinephrine, dopamine, acetylcholine, histamine, γ-aminobutyric acid, and glutamate.18,19 For example, norepinephrine has been implicated in the regulation of motivation and energy,54 while dopamine appears important for motivation and reward processing.55,56 Tvortioxetine
The main study limitation is the relatively short duration of follow-up (8 weeks), as patients with MDD generally require long-term treatment. However, long-term functional benefits have previously been demonstrated in working patients with MDD treated with vortioxetine for up to 1 year, including those with partial response to prior antidepressant therapy.29,30 The ability to request dose adjustment in the desvenlafaxine group without an increase actually being implemented may have introduced expectation bias, potentially impacting treatment outcomes.57
In conclusion, the VIVRE study demonstrates that vortioxetine confers superior clinical benefits versus SNRI treatment in patients with MDD with only partial response to SSRI monotherapy, including a greater likelihood of achieving symptomatic and functional remission, better daily and social functioning, and greater treatment satisfaction. Our findings support earlier use of vortioxetine in the treatment algorithm in patients with MDD.
Submitted: January 3, 2023; accepted April 12, 2023.
Published online: May 24, 2023.
Relevant financial relationships: Dr McIntyre has received research grant support from the Canadian Institutes of Health Research, the Global Alliance for Chronic Diseases, and the National Natural Science Foundation of China and has received speaker/consultation fees from AbbVie, Alkermes, atai Life Sciences, Axsome Therapeutics, Bausch Health, Eisai, Intra-Cellular Therapies, Janssen, Kris Pharma, Lundbeck, Mitsubishi Tanabe, Neumora Therapeutics, Neurocrine Biosciences, NewBridge Pharmaceuticals, Novo Nordisk, Otsuka, Pfizer, Purdue, Sanofi, Sunovion, and Takeda. He is also a CEO of Braxia Scientific Corp. Dr Florea, Mr Pedersen, and Dr Christensen are employees of H. Lundbeck A/S.
Funding/support: The VIVRE study was funded by H. Lundbeck A/S.
Role of the sponsor: H. Lundbeck A/S personnel were involved in the design and conduct of the study and in the management, analysis, and interpretation of the results and reviewed this manuscript prior to submission.
Previous presentation: Poster presented at the annual meeting of the American Society of Clinical Psychopharmacology (ASCP); May 31–June 3, 2022; Scottsdale, Arizona.
Acknowledgments: The authors thank the patients and investigators who participated in this study. The principal study investigators and their respective research centers are listed in Supplementary Appendix 1. Editorial assistance in the preparation of this manuscript was provided by Jennifer Coward, BSc, of Piper Medical Communications Ltd, funded by H. Lundbeck A/S.
ORCID: Roger S. McIntyre: https://orcid.org/0000-0003-4733-2523; Ioana Florea: https://orcid.org/0000-0002-4888-9406; Michael Cronquist Christensen: https://orcid.org/0000-0002-3605-7223
Supplementary material: Available at Psychiatrist.com.
CLINICAL POINTS
- Head-to-head studies of antidepressants in patients with major depressive disorder (MDD) are rare, particularly in those with inadequate response to prior therapy. This study assessed the efficacy of the multimodal antidepressant vortioxetine versus that of the serotonin-norepinephrine reuptake inhibitor desvenlafaxine in patients with MDD experiencing partial response to treatment with a selective serotonin reuptake inhibitor.
- Vortioxetine was non-inferior to desvenlafaxine in terms of reduction in depression symptom severity assessed using the Montgomery-Asberg Depression Rating Scale.
- Vortioxetine-treated patients were significantly more likely to achieve symptomatic and functional remission and reported significantly greater improvements in daily and social functioning and significantly greater satisfaction with their medication than those who received desvenlafaxine.
References (57)

- Cleare A, Pariante CM, Young AH, et al; Members of the Consensus Meeting. Evidence-based guidelines for treating depressive disorders with antidepressants: a revision of the 2008 British Association for Psychopharmacology guidelines. J Psychopharmacol. 2015;29(5):459–525. PubMed CrossRef
- Kennedy SH, Lam RW, McIntyre RS, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder, Section 3: pharmacological treatments. Can J Psychiatry. 2016;61(9):540–560. PubMed CrossRef
- Bauer M, Severus E, Möller HJ, et al; WFSBP Task Force on Unipolar Depressive Disorders. Pharmacological treatment of unipolar depressive disorders: summary of WFSBP guidelines. Int J Psychiatry Clin Pract. 2017;21(3):166–176. PubMed CrossRef
- American Psychological Association. Clinical practice guideline for the treatment of depression across three age cohorts. APA website. https://www.apa.org/depression-guideline. February 2019. Accessed 8 March, 2023.
- Malhi GS, Bell E, Bassett D, et al. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry. 2021;55(1):7–117. PubMed CrossRef
- Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905–1917. PubMed CrossRef
- Price J, Cole V, Goodwin GM. Emotional side-effects of selective serotonin reuptake inhibitors: qualitative study. Br J Psychiatry. 2009;195(3):211–217. PubMed CrossRef
- Read J, Cartwright C, Gibson K. Adverse emotional and interpersonal effects reported by 1829 New Zealanders while taking antidepressants. Psychiatry Res. 2014;216(1):67–73. PubMed CrossRef
- Goodwin GM, Price J, De Bodinat C, et al. Emotional blunting with antidepressant treatments: a survey among depressed patients. J Affect Disord. 2017;221:31–35. PubMed CrossRef
- Demyttenaere K, Reines EH, Lönn SL, et al. Satisfaction with medication is correlated with outcome but not persistence in patients treated with placebo, escitalopram, or serotonin-norepinephrine reuptake inhibitors: a post hoc analysis. Prim Care Companion CNS Disord. 2011;13(4):PCC.10m01080. PubMed CrossRef
- Sansone RA, Sansone LA. Antidepressant adherence: are patients taking their medications? Innov Clin Neurosci. 2012;9(5-6):41–46. PubMed
- Aljumah K, Ahmad Hassali A, AlQhatani S. Examining the relationship between adherence and satisfaction with antidepressant treatment. Neuropsychiatr Dis Treat. 2014;10:1433–1438. PubMed CrossRef
- Lam RW, McIntosh D, Wang J, et al; CANMAT Depression Work Group. Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 1. disease burden and principles of care. Can J Psychiatry. 2016;61(9):510–523. PubMed CrossRef
- Carvalho AF, Sharma MS, Brunoni AR, et al. The safety, tolerability and risks associated with the use of newer generation antidepressant drugs: a critical review of the literature. Psychother Psychosom. 2016;85(5):270–288. PubMed CrossRef
- Dale E, Bang-Andersen B, Sánchez C. Emerging mechanisms and treatments for depression beyond SSRIs and SNRIs. Biochem Pharmacol. 2015;95(2):81–97. PubMed CrossRef
- Sanchez C, Asin KE, Artigas F. Vortioxetine, a novel antidepressant with multimodal activity: review of preclinical and clinical data. Pharmacol Ther. 2015;145:43–57. PubMed CrossRef
- Gonda X, Sharma SR, Tarazi FI. Vortioxetine: a novel antidepressant for the treatment of major depressive disorder. Expert Opin Drug Discov. 2019;14(1):81–89. PubMed CrossRef
- Stahl SM. Modes and nodes explain the mechanism of action of vortioxetine, a multimodal agent (MMA): modifying serotonin’s downstream effects on glutamate and GABA (gamma amino butyric acid) release. CNS Spectr. 2015;20(4):331–336. PubMed CrossRef
- Riga MS, Sánchez C, Celada P, et al. Involvement of 5-HT3 receptors in the action of vortioxetine in rat brain: focus on glutamatergic and GABAergic neurotransmission. Neuropharmacology. 2016;108:73–81. PubMed CrossRef
- Thase ME, Mahableshwarkar AR, Dragheim M, et al. A meta-analysis of randomized, placebo-controlled trials of vortioxetine for the treatment of major depressive disorder in adults. Eur Neuropsychopharmacol. 2016;26(6):979–993. PubMed CrossRef
- Baldwin DS, Florea I, Jacobsen PL, et al. A meta-analysis of the efficacy of vortioxetine in patients with major depressive disorder (MDD) and high levels of anxiety symptoms. J Affect Disord. 2016;206:140–150. PubMed CrossRef
- McIntyre RS, Harrison J, Loft H, et al. The effects of vortioxetine on cognitive function in patients with major depressive disorder: a meta-analysis of three randomized controlled trials. Int J Neuropsychopharmacol. 2016;19(10):pyw055. PubMed CrossRef
- Florea I, Loft H, Danchenko N, et al. The effect of vortioxetine on overall patient functioning in patients with major depressive disorder. Brain Behav. 2017;7(3):e00622. PubMed CrossRef
- Christensen MC, Florea I, Lindsten A, et al. Efficacy of vortioxetine on the physical symptoms of major depressive disorder. J Psychopharmacol. 2018;32(10):1086–1097. PubMed CrossRef
- Christensen MC, McIntyre RS, Florea I, et al. Vortioxetine 20 mg/day in patients with major depressive disorder: updated analysis of efficacy, safety, and optimal timing of dose adjustment. CNS Spectr. 2023;28(1):90–97. PubMed CrossRef
- Iovieno N, Papakostas GI, Feeney A, et al. Vortioxetine versus placebo for major depressive disorder: a comprehensive analysis of the clinical trial dataset. J Clin Psychiatry. 2021;82(4):20r13682. PubMed CrossRef
- McIntyre RS, Loft H, Christensen MC. Efficacy of vortioxetine on anhedonia: results from a pooled analysis of short-term studies in patients with major depressive disorder. Neuropsychiatr Dis Treat. 2021;17:575–585. PubMed CrossRef
- McIntyre RS, Florea I, Tonnoir B, et al. Efficacy of vortioxetine on cognitive functioning in working patients with major depressive disorder. J Clin Psychiatry. 2017;78(1):115–121. PubMed CrossRef
- Chokka P, Bougie J, Proulx J, et al. Long-term functioning outcomes are predicted by cognitive symptoms in working patients with major depressive disorder treated with vortioxetine: results from the AtWoRC study. CNS Spectr. 2019;24(6):616–627. PubMed CrossRef
- Chokka P, Tvistholm AH, Bougie J, et al. Improvements in workplace productivity in working patients with major depressive disorder: results from the AtWoRC Study. J Occup Environ Med. 2020;62(3):e94–e101. PubMed CrossRef
- Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2010 and 2018). Pharmacoeconomics. 2021;39(6):653–665. PubMed CrossRef
- Wang G, Gislum M, Filippov G, et al. Comparison of vortioxetine versus venlafaxine XR in adults in Asia with major depressive disorder: a randomized, double-blind study. Curr Med Res Opin. 2015;31(4):785–794. PubMed CrossRef
- Wang G, Zhao K, Reynaud-Mougin C, et al. Successfully treated patients with vortioxetine versus venlafaxine: a simplified cost-effectiveness analysis based on a head-to-head study in Asian patients with major depressive disorder. Curr Med Res Opin. 2020;36(5):875–882. PubMed CrossRef
- Mahableshwarkar AR, Zajecka J, Jacobson W, et al. A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology. 2015;40(8):2025–2037. PubMed CrossRef
- Christensen MC, Loft H, McIntyre RS. Vortioxetine improves symptomatic and functional outcomes in major depressive disorder: a novel dual outcome measure in depressive disorders. J Affect Disord. 2018;227:787–794. PubMed CrossRef
- Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. PubMed
- Wechsler D. Wechsler Adult Intelligence Scale—Revised. San Antonio, TX: Psychological Corporation; 1981.
- Desvenlafaxine [package insert]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/021992s042lbl.pdf. Accessed 8 March, 2023.
- Thase ME, Kornstein SG, Germain JM, et al. An integrated analysis of the efficacy of desvenlafaxine compared with placebo in patients with major depressive disorder. CNS Spectr. 2009;14(3):144–154. PubMed CrossRef
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134(4):382–389. PubMed CrossRef
- Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4(7):28–37. PubMed
- Rosa AR, Sánchez-Moreno J, Martínez-Aran A, et al. Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clin Pract Epidemiol Ment Health. 2007;3(1):5. PubMed CrossRef
- Bonnín CM, Martínez-Arán A, Reinares M, et al. Thresholds for severity, remission and recovery using the functioning assessment short test (FAST) in bipolar disorder. J Affect Disord. 2018;240:57–62. PubMed CrossRef
- Endicott J, Nee J, Harrison W, et al. Quality of Life Enjoyment and Satisfaction Questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321–326. PubMed
- Posner K, Brown GK, Stanley B, et al. The Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–1277. PubMed CrossRef
- Habert J, Katzman MA, Oluboka OJ, et al. Functional recovery in major depressive disorder: focus on early optimized treatment. Prim Care Companion CNS Disord. 2016;18(5). PubMed CrossRef
- Oluboka OJ, Katzman MA, Habert J, et al. Functional recovery in major depressive disorder: providing early optimal treatment for the individual patient. Int J Neuropsychopharmacol. 2018;21(2):128–144. PubMed CrossRef
- Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–1366. PubMed CrossRef
- Evans-Lacko S, Knapp M. Global patterns of workplace productivity for people with depression: absenteeism and presenteeism costs across eight diverse countries. Soc Psychiatry Psychiatr Epidemiol. 2016;51(11):1525–1537. PubMed CrossRef
- McIntyre RS, Cha DS, Soczynska JK, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. 2013;30(6):515–527. PubMed CrossRef
- Sarfati D, Stewart K, Woo C, et al. The effect of remission status on work functioning in employed patients treated for major depressive disorder. Ann Clin Psychiatry. 2017;29(1):11–16. PubMed
- Bang-Andersen B, Ruhland T, Jørgensen M, et al. Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem. 2011;54(9):3206–3221. PubMed CrossRef
- Mørk A, Pehrson A, Brennum LT, et al. Pharmacological effects of Lu AA21004: a novel multimodal compound for the treatment of major depressive disorder. J Pharmacol Exp Ther. 2012;340(3):666–675. PubMed CrossRef
- Moret C, Briley M. The importance of norepinephrine in depression. Neuropsychiatr Dis Treat. 2011;7(suppl 1):9–13. PubMed
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64(3):327–337. PubMed CrossRef
- Belujon P, Grace AA. Dopamine system dysregulation in major depressive disorders. Int J Neuropsychopharmacol. 2017;20(12):1036–1046. PubMed CrossRef
- Cohen EA, Hassman HH, Ereshefsky L, et al. Placebo response mitigation with a participant-focused psychoeducational procedure: a randomized, single-blind, all placebo study in major depressive and psychotic disorders. Neuropsychopharmacology. 2021;46(4):844–850. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite