ABSTRACT
Objective: To evaluate the status of management of insomnia disorder, describe gaps in current recognition and treatment, identify current guidance for optimal management, and develop up-to-date educational recommendations for primary care providers.
Participants: Four insomnia experts representing primary care, psychiatry, and clinical research were selected based on clinical expertise, educational qualifications, and research experience. A patient with insomnia was also included.
Consensus Process: The Insomnia Working Group met in March 2022 to review data on available therapies (including medications approved since publication of current guidelines) and share current best practices for evidence-based multimodal treatment of insomnia disorder.
Conclusions: Insomnia is highly prevalent but underdiagnosed and undertreated. It is increasingly recognized as a distinct disorder, not merely a symptom arising secondary to another medical or psychiatric illness. The subtypes of sleep disturbance—reports of difficulty falling or staying asleep, insufficient sleep duration, early waking—and the presence of next-day impairment and common comorbid conditions require a targeted, individualized approach to therapy. Challenges exist in treating insomnia with commonly used on- and off-label drugs, including low-dose antidepressants, benzodiazepines, and benzodiazepine receptor agonists because of the risk of adverse effects, including impaired next-day functioning. The dual orexin receptor antagonists have a novel mechanistic target and offer an alternative pharmacologic choice. Optimal outcomes for insomnia require a comprehensive approach that includes lifestyle and behavioral strategies to mitigate maladaptive thoughts and behaviors related to sleep and selection of pharmacotherapy based on individual patient complaints and characteristics.
Prim Care Companion CNS Disord 2023;25(1):22nr03385
To cite: Rosenberg RP, Benca R, Doghramji P, et al. A 2023 update on managing insomnia in primary care: insights from an expert consensus group. Prim Care Companion CNS Disord. 2023;25(1):22nr03385.
To share: https://doi.org/10.4088/PCC.22nr03385
© 2023 Physicians Postgraduate Press, Inc.
aNeuroTrials Research, Inc, Atlanta, Georgia
bWake Forest School of Medicine, Winston-Salem, North Carolina
cCollegeville Family Practice, Collegeville, Pennsylvania
dUrsinus College, Collegeville, Pennsylvania
eDepartment of Psychiatry, University of Michigan College of Medicine, Ann Arbor, Michigan
*Corresponding author: Russell P. Rosenberg, PhD, FAASM, NeuroTrials Research, Inc, 5887 Glenridge Dr NE, Ste 400, Atlanta, GA 30328 ([email protected]).
A consensus panel including experts in primary care, psychiatry, and clinical research convened in March 2022 to identify gaps in the recognition, diagnosis, and management of insomnia and to review new data on available and emerging sleep medications. What follows are their recommendations, based on current evidence, for improving treatment for an insomnia disorder in the primary care setting.
Insomnia, Despite Its Myriad of Personal and Societal Consequences, Is Underrecognized in Primary Care
Insomnia disorder is a distinct chronic condition characterized by reports of difficulty initiating or maintaining sleep, which is present even when the patient has adequate opportunity and an appropriate environment in which to sleep, and has a significant impact on next-day functioning.1 Insomnia is underrecognized and undertreated, resulting in a significant health care burden (increased morbidity and mortality) and poorer quality of life for those who experience it.2 Insomnia disorder is highly prevalent in the general population, with estimates of 10% among adults,3 and is particularly prevalent in primary care patients, with estimates ranging from 10%–20%.4
The medical, psychiatric, psychosocial, and economic burdens of insomnia disorder—and the resulting diminished quality of life—are significant. Poor sleep is not only a problem at nighttime; its impact also involves diminished daytime functioning and work performance, fatigue, and social isolation.4,5
In the United States, the costs of insomnia are estimated to be as high as $100 billion, most of which reflect indirect costs due to lost productivity (presenteeism/absenteeism), accidents (often involving drowsy driving), and disproportionate use of health care resources.6–8 One study9 found that the costs of managing patients with insomnia compared to those without insomnia were 46% higher after 1 year; the costs were 80% higher if a comorbidity was present.
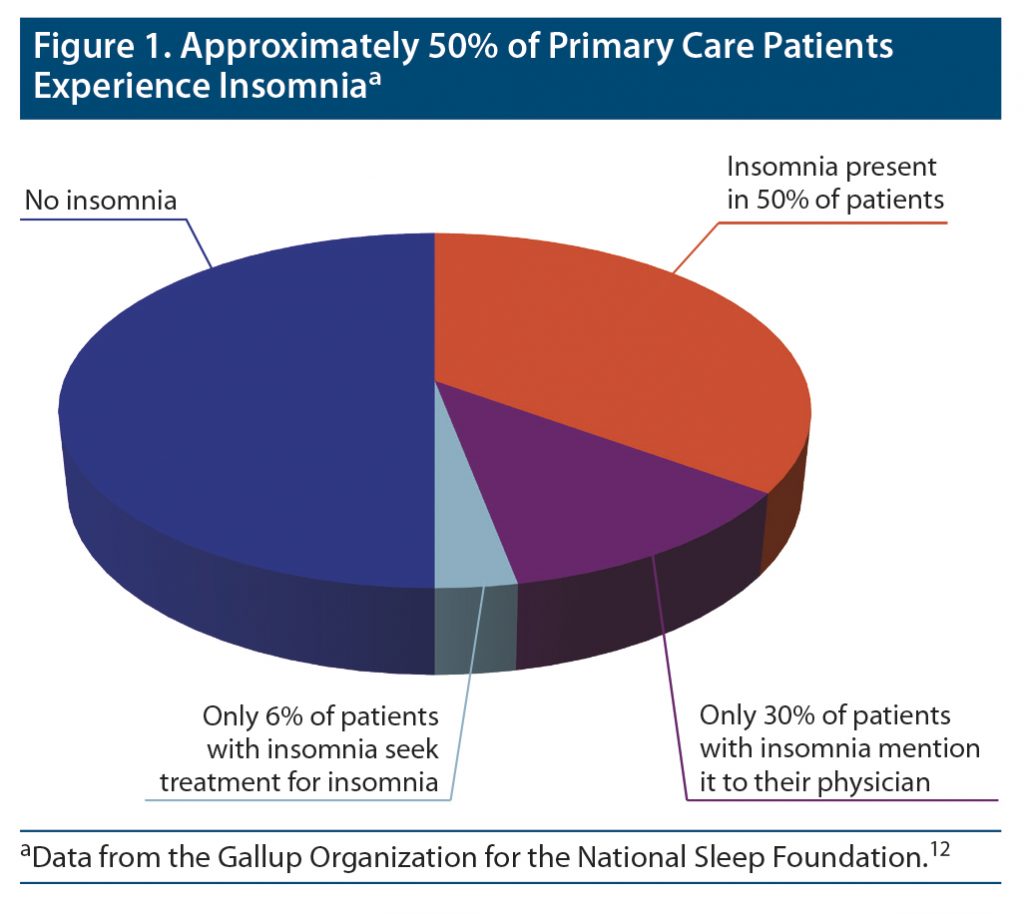
People who experience disturbed sleep often fail to consult their primary care clinicians about the issue.2 Although approximately half of patients in primary care experience insomnia, only a fraction of this group, about 25%–50%, discuss sleep issues with their primary care providers, and an even smaller percentage seek treatment for insomnia.10,11 A random population survey conducted by the National Sleep Foundation showed that although almost 50% of primary care patients experienced insomnia, only 30% of them mentioned the problem to a physician, and only 6% reported that they had sought the help of a physician for their sleep difficulty (Figure 1).12 Compounding the problem, primary care clinicians appear to be remiss in asking about sleep disturbances. In another National Sleep Foundation survey (N = 1,506 US adults), 70% of respondents reported that their clinicians never asked them about their sleep.13
The Importance of Comorbid Insomnia in Psychiatric and Medical Conditions
In the past decade, the definition of insomnia, as reflected in disease nosology systems and diagnostic criteria, has evolved. The result is that insomnia is regarded as a distinct disorder that has profound implications for treatment.
The insomnia landscape fundamentally shifted with the publication in 2013 of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM-5).14 In the previous version, chronic insomnia was defined as a primary condition or as secondary to another condition. Such a classification is inaccurate because regarding insomnia as secondary implies that it will resolve when the comorbidity is treated.15 Within that paradigm, management of insomnia is considered a lower priority in favor of treating its comorbidities first.2,16 This misperception has contributed to significant undertreatment of sleep disturbances.10 In the DSM-5, however, the distinction between primary and secondary insomnia was eliminated, and the term insomnia disorder was adopted. Insomnia disorder is therefore now recognized as a condition that often coexists with, and is not merely secondary to, another condition such as depression.17 Similarly, in a marked departure from earlier versions, the third edition of the International Classification of Sleep Disorders (ICSD-3) also consolidated primary and comorbid insomnia into a single entity.18
The presence of insomnia in a patient with other medical or psychiatric diseases is an important consideration for the primary care clinician. Insomnia is typically seen comorbidly with a variety of medical and psychiatric conditions encountered in the primary care setting. One survey found that people with insomnia have a mean of 3.2 comorbidities19; these include cardiovascular disease, hypertension, diabetes, gastrointestinal disorders, respiratory disorders (including asthma and sleep apnea), neurologic disorders, and cancer.2,5,10,16,20–23 Depression, however, is the most common comorbidity likely to occur with insomnia24,25 and has been shown to be the most common comorbidity seen in the primary care setting.26
There are currently limited data regarding sleep dysfunction in patients who have been acutely diagnosed with COVID-19, whether asymptomatic or symptomatic or whether requiring hospitalization or not, and among those hospitalized, whether requiring intensive care admission due to respiratory failure or not. There is evidence that suggests the acute as well as long-term impact of COVID-19 on sleep can be profound.27 Various validated questionnaires and surveys have been employed to assess the frequency and severity of sleep problems associated with COVID-19. While there are inherent weaknesses in survey methodology, these data are useful. Donzella et al28 reported that individuals with a positive COVID test result were more likely to report trouble sleeping 3 or more times per week since the start of the pandemic. It is unclear whether the sleep difficulties were due to the viral infection or secondary factors (anxiety, changes in social factors, or economic concerns). An international study29 regarding COVID and sleep problems found the rates of insomnia symptoms (36.7%) and insomnia disorder (17.4%) were very high during the first wave of the pandemic.
Insomnia is not only frequently comorbid with psychiatric conditions, but is also a risk factor for future development or exacerbation of a psychiatric disorder. Compared to individuals without insomnia, the risks that a person with insomnia will develop depression, anxiety, alcohol abuse disorder, or psychosis are 2.83, 3.23, 1.35, and 1.28 times higher, respectively.30 Sleep disturbance is also associated with a > 50% increased risk for Alzheimer’s disease.31 More than 40 studies have shown a clear relationship between insomnia and suicidal ideation, suicidal behaviors, and death.32 Aside from comorbidities, otherwise healthy older adults with insomnia symptoms (delayed sleep onset, poor sleep efficiency [ratio of total sleep time to time in bed]) have a mortality risk twice as high as those without insomnia.33
A growing body of evidence demonstrates that the relationship between insomnia and some comorbidities is bidirectional, that is, the insomnia worsens the comorbidity, and the comorbidity worsens insomnia.2,21 The status of one condition affects the status of the other in many dimensions: severity, choice of treatment, and outcomes.16
Additionally, clinical trials of both medications and cognitive-behavioral therapy for insomnia (CBT-I) have demonstrated that treating insomnia in depressed patients was associated with improved outcomes in depression and insomnia,34,35 anxiety and insomnia,36 pain and insomnia,37 and suicidal ideation.38 While data demonstrating that improvement in insomnia can benefit comorbidities are generally lacking, there is mounting evidence that treating insomnia in the presence of the comorbidity may improve outcomes for not only the insomnia, but also the comorbidity. Clinicians should avoid the risk of trivializing insomnia and prioritizing other medical conditions, thus delaying an accurate diagnosis and downplaying the need for insomnia treatment.2,4 At the very least, the presence of insomnia indicates a patient at higher risk for poor health outcomes.
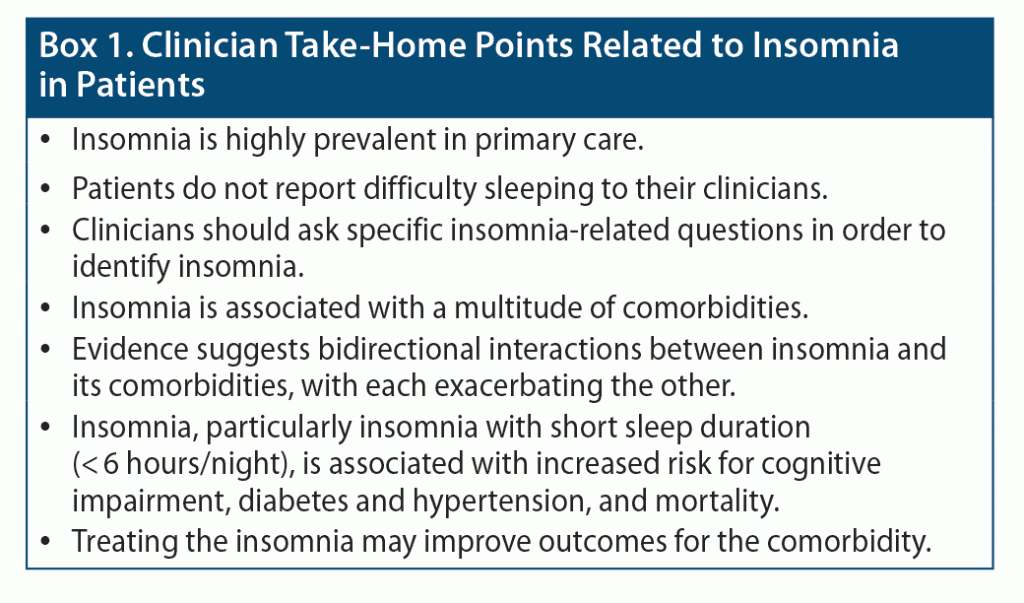
Finally, there is evidence that patients with insomnia and short sleep duration (< 6 hours/night) are at higher risk for impaired neurocognitive functioning, increased cortisol levels, hypertension, diabetes, and death.39 Identifying these patients and treating their insomnia may help to mitigate risk. See Box 1 for clinician take-home points.
Insomnia Screening and Diagnosis
Given the high prevalence of insomnia demonstrated in their patients, primary care clinicians should consider sleep to be a vital sign and routinely screen for insomnia.40 To improve screening, it may be helpful for clinicians to ensure that their patient intake forms and electronic records are designed to routinely prompt a discussion of sleep patterns and next-day function. See Box 2 for simple questions that can highlight the possibility of insomnia and provoke further questions, if needed.
If insomnia is suspected, further evaluation is indicated. A detailed history obtained during the interview is key to understanding the patient’s subjective experience of insomnia.4 In line with the 11th edition of the International Classification of Diseases (ICD-11) criteria, questions should explore the nature of the sleep disturbance (delayed sleep onset, trouble staying asleep, early morning awakening, nonrestorative sleep), sleep routines and potentially maladaptive habits, impaired daytime functioning, and the potential presence of contributing comorbidities.21,41 Other potentially useful sources of information include a sleep diary and an interview with the patient’s bed partner. Data from smartphone or fitness apps may be suggestive,21 but the objective accuracy of data from such devices has not been validated.
The ICD-11, approved in February 2022, defines insomnia disorder as characterized by “persistent difficulty with sleep initiation, duration, consolidation, or quality that occurs despite adequate opportunity and circumstances for sleep, and results in some form of daytime impairment” (Table 1).1 Next-day impairment can involve fatigue, poor functioning, reduced alertness, and, in many cases, exacerbation of existing comorbidities such as depression, any of which can significantly reduce the affected individual’s quality of life.2,42
Polysomnography is not usually necessary, nor is it recommended for the initial objective assessment of insomnia. However, additional clinical screening tools and laboratory and sleep studies may be needed to rule out other conditions that can disrupt sleep, such as mood disorders, pain, restless leg syndrome, or obstructive sleep apnea.21
Validated tools are available to help clinicians assess the severity of insomnia disorder and monitor the response to treatment. Perhaps most useful is the brief Insomnia Severity Index (ISI),43 a 7-item patient self-reported questionnaire that reliably assesses both nighttime and daytime aspects of sleep disturbance occurring within the past month. Questions use a 5-point scale (from “no problem” to “very severe problem”) to evaluate patterns of sleep onset, sleep maintenance, and early awakening; difficulties with daytime functioning; whether the sleep problems are noticed by others; and the degree of distress resulting from insomnia. Results classify insomnia as not present, mild, moderate, or severe.43
The Emerging Science of Sleep and Consequences for Treatment Selection
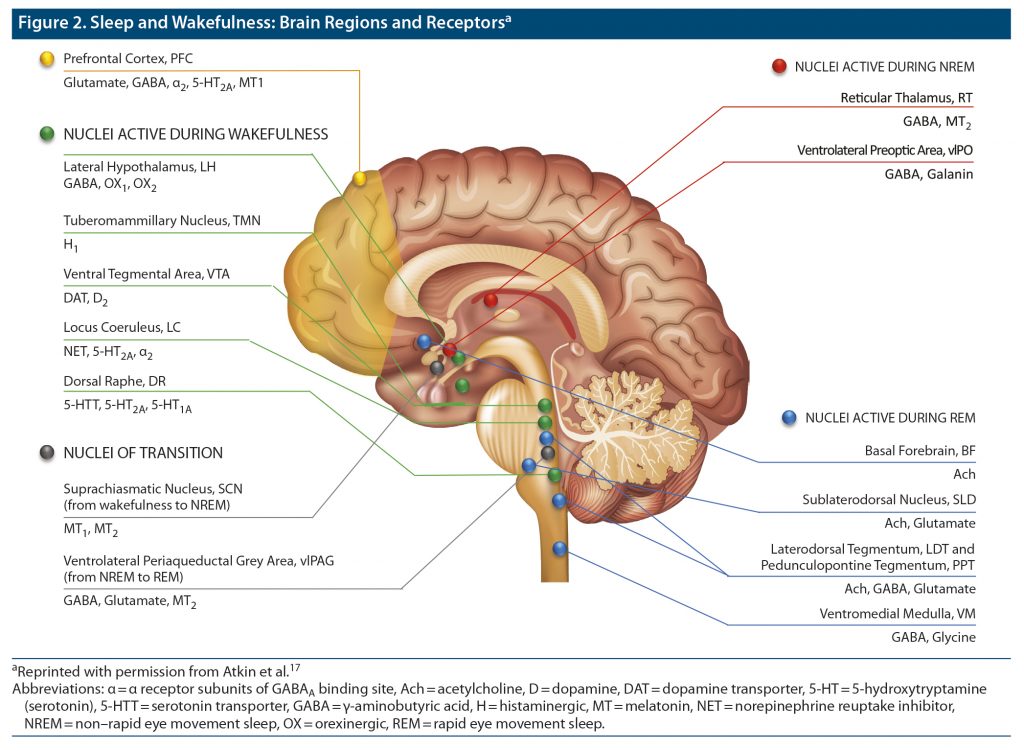
Research continues to reveal the complex neurophysiologic processes involved in the regulation of sleep and wakefulness. A basic awareness of these mechanisms can help clinicians better understand how different drugs target various neurotransmitter pathways to improve the symptoms of insomnia.17
Different brain structures and their associated neurotransmitters play various complementary roles at different stages in the sleep process (Figure 2).17 Neurons in the thalamus, hypothalamus, hippocampus, basal ganglia, and brain stem produce γ-aminobutyric acid (GABA), a widespread inhibitory neurotransmitter that reduces arousal. Within the hypothalamus, the suprachiasmatic nucleus receives information about light exposure that regulates the circadian rhythms that govern the sleep/wake cycle and triggers production or suppression of melatonin. During rapid eye movement sleep, the brain stem reduces muscle activity, while the thalamus relays sensory information for processing by the cerebral cortex.
The act of falling asleep involves 2 separate but complementary (and equally necessary) neurochemical processes: decreasing wakefulness-promoting activity and increasing sleep-promoting activity. Inhibiting wakefulness results from activity by such factors as adenosine, nitric oxide, and GABAergic neurons in the hypothalamus.44
At the end of the night, the process reverses: sleep-promoting activity diminishes, and wakefulness is triggered and maintained by a group of neurotransmitters (glutamate, norepinephrine, dopamine, serotonin, acetylcholine, histamine, and orexin A and B).45–47 Many of these neurotransmitters and neurotransmitter pathways may serve as potential therapeutic targets for managing various forms of sleep disturbance in individual patients.
Treating Insomnia: Nonpharmacologic Approaches
Optimal management of insomnia disorder calls for a multimodal strategy that reduces time to sleep onset (sleep latency), reduces awakening during the night, increases sleep duration, and allows wakefulness after adequate sleep duration. The goal of treating insomnia disorder is thus 2-fold: to restore the duration and quality of sleep, while alleviating the daytime impairment caused by the disorder.48 To achieve optimal outcomes, clinicians should adopt an approach incorporating lifestyle changes, improved sleep hygiene, cognitive-behavioral techniques, and the safe, judicious use of appropriately selected medications. Guidelines urge a shared decision-making approach when designing a treatment strategy, which involves discussing the risks, benefits, and costs of treatment and considering the patients’ needs, values, and preferences.10
Common sense lifestyle strategies such as following a nutritious diet, getting adequate exercise, maintaining normal body weight, consuming alcohol and caffeine in moderation, and avoiding use of tobacco or illicit drugs can improve patients’ sleep as well as their overall physical and mental health.49 Sleep hygiene consists of creating a calm, quiet bedroom environment and adopting daily routines conducive to initiating and maintaining sleep by reducing or avoiding daytime naps, evening consumption of caffeine or alcohol, and exposure to electronic screens or late-night TV. As useful as lifestyle strategies and sleep hygiene techniques may be, they are often inadequate in themselves, but should be included as part of a broader therapeutic strategy.21,50
The current major sleep guidelines from the American Academy of Sleep Medicine (AASM), American College of Physicians, and European Sleep Research Society all strongly recommend the use of CBT-I as the first-line approach for treating insomnia.41,48,51 CBT-I offers a combination of nonpharmacologic interventions, including cognitive strategies and behavioral methods (sleep hygiene, stimulus control, sleep restriction, relaxation) targeted to address sleep disturbances.52 A typical course of CBT-I lasts 6 to 8 weeks.
Past evidence shows that CBT-I alone offers greater long-term efficacy than do insomnia medications with few adverse effects,41,48 although to date there are no trials comparing CBT-I with any of the dual orexin receptor antagonists (DORAs), which are discussed below. CBT-I has been shown to improve not just nighttime sleep but also daytime functioning in terms of mood, fatigue, concentration, and overall quality of life.53 If results are inadequate, CBT-I can be combined with medications. One drawback is that providers offering CBT-I are not widely available. However, CBT-I can be delivered through a variety of formats: individual or group sessions, face-to-face or online, and via patient self-education.54 Recently, based on data from clinical trials demonstrating efficacy and safety, the US Food and Drug Administration (FDA) granted clearance for use of Somryst, the first software-based program for CBT-I available as a prescription digital therapeutic.55
Although nonpharmacologic strategies have been found to improve sleep, in many cases patients may need medication to achieve more immediate or optimal outcomes. However, patients often have concerns about taking prescription drugs for insomnia. They may fear becoming addicted to the drug, or they may be discouraged by media reports about complex sleep-related behaviors associated with the benzodiazepine receptor agonists (BzRAs). Such concerns may prevent them from discussing sleep disturbances with their clinicians and may drive them to use over-the-counter (OTC) remedies. Patients should be counseled about the risks and benefits of OTC options, particularly antihistamines (such as diphenhydramine and doxylamine). Diphenhydramine and doxylamine have anticholinergic properties that can cause cognitive impairment, hangover effects, dizziness, or falls.56 Prolonged-release melatonin use was found to be safe in adults aged 18 to 80 years in a large randomized double-blind 6-month trial.57 Valerian is an herbal medicine used as a sleep aid; however, a recent meta-analysis58 showed that effectiveness in improving sleep quality was inconsistent, although no severe adverse events occurred. The inconsistent findings may be due to the variable quality of herbal extracts.58 The increasing availability of medical marijuana and nonprescription cannabinoid (CBD) products means that many patients in primary care will be using them to facilitate sleep. Mounting evidence supports the potential benefits of medical marijuana for certain conditions such as pain or anxiety, which are commonly comorbid with insomnia and may be listed as indications for cannabis in certain states.59 It is important for clinicians to keep current regarding data on the medical use of cannabis and to routinely question patients about their use of such products. It is important to consider, however, that insomnia can occur in association with cannabis withdrawal.
Treating Insomnia: Medical Management
The main agents used for insomnia disorder include the following60:
- Approved prescription drugs with indications for insomnia
- Prescription drugs used off label (low-dose sedating antidepressants, benzodiazepines)
- OTC sleep aids
- Supplements and alternative therapies.
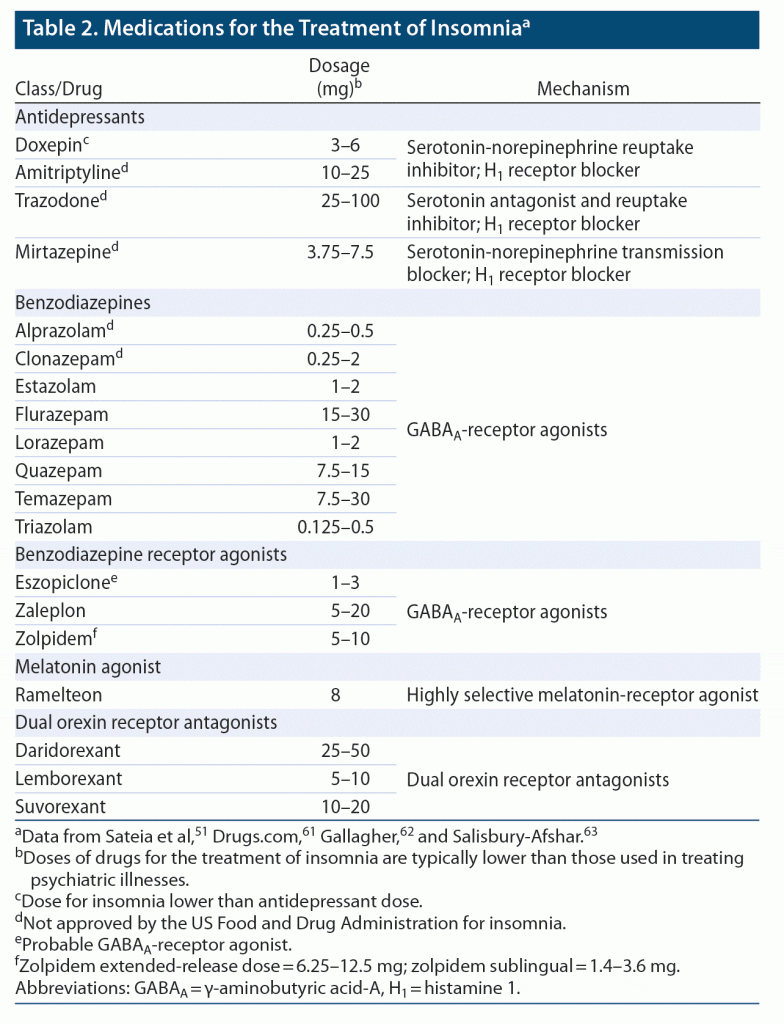
A summary of drugs commonly prescribed for insomnia is included in Table 2.51,61–63
AASM guidelines state that their recommendations for the use of approved as well as off-label insomnia drugs are weak because the available evidence is “sorely limited” and of low quality.51 The authors of the guidelines note that there is a discrepancy between FDA approval, which is based on trials that demonstrate statistically significant changes in both subjective and objective outcomes compared to placebo, and the AASM’s use of the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) approach, which mandates issuing a “weak” recommendation when the evidence for benefit versus harm is less clear and the quality of the evidence is lower. The guidelines thus emphasize that “clinicians must continue to exercise sound clinical judgment based not only on these recommendations, but also on clinical experience, prior patient response, patient preferences, and potential adverse effects.”51(p345) It should be noted that the most recently approved insomnia medications (the DORAs lemborexant and daridorexant) appeared on the market after the release of the most recent guidelines, which thus do not include recommendations concerning use of these agents.
In the United States, trazodone is the most frequently prescribed medication for insomnia in the primary care setting, with 34% more prescriptions than the next most commonly prescribed FDA-approved hypnotic.17 However, it is not FDA approved for insomnia, and clinical trial data are lacking to support the safety and efficacy of trazodone for insomnia. Importantly, although GABAergic agents such as benzodiazepines and BzRAs are widely prescribed, they can be associated with significant adverse effects that include diminished next-day functioning such as cognitive impairment, risk for dependence, accidents (car accidents, falls), tolerance, and rebound insomnia once the drug is discontinued.17
Because they sometimes have shorter half-lives and possibly somewhat different mechanisms than conventional benzodiazepines, the BzRAs (eszopiclone, zaleplon, zolpidem), the first of which was approved in 1992, initially offered hope for greater safety. These drugs carry a boxed warning for potential risk for complex sleep-related behaviors such as walking, eating, making phone calls, or driving in the middle of the night while not fully awake and without the patient’s recollection when fully awake.60
Concerns about the generally poor safety profile of GABAergic agents has, in part, led to increased interest in drugs that target other mechanisms involved in sleep and wakefulness.17 These include agents commonly used in psychiatric conditions, particularly antidepressants that affect the serotonin, norepinephrine, and histamine receptor pathways. Importantly, dosages of these drugs for treatment of insomnia are typically lower than those used to treat psychiatric illnesses (Table 2). For example, the dosage of doxepin for insomnia (3–6 mg/d) is far lower than that used to treat depression (up to 300 mg/d).
Other classes of drug are sometimes used off-label for insomnia. These include the calcium-channel modulator anticonvulsants gabapentin and pregabalin (indicated for seizures and neuropathic pain). The atypical antipsychotics (olanzapine, quetiapine, risperidone, and ziprasidone), indicated for schizophrenia and bipolar disorder, can have hypnotic effects due to their serotonergic and histaminergic activity.17 However, it is recommended that these agents not be used for insomnia disorder unless the patient has the comorbid disorder for which they are indicated.64
The newest drugs for insomnia are classified as DORAs. Orexin-A and orexin-B are neuropeptides involved in, among other actions, arousal behavior and inducing wakefulness.17 DORAs act by blocking 2 orexin receptors: OX1R and OX2R. DORAs reduce arousal but preserve the patient’s ability to awaken in response to auditory stimuli (such as alarms or a crying baby), although the clinical implications of this finding have not been delineated to date.65
The first DORA, suvorexant, was approved by the FDA in 2014. Studies show suvorexant facilitates sleep induction and sleep maintenance.66 Adverse effects include sleep paralysis, hypnagogic hallucinations, cataplexy, and suicidal ideation.10
Lemborexant was approved in 2019; data on its safety and efficacy were not available at the time current AASM insomnia guidelines were developed. However, a recent meta-analysis comparing the various classes of insomnia agents that included suvorexant, zolpidem (immediate release and extended release), zopiclone, eszopiclone, trazodone, flunitrazepam, estazolam, triazolam, brotizolam, temazepam, and ramelteon showed that lemborexant had the highest probability of being the best treatment on 3 of 4 outcome measures: sleep efficiency, sleep-onset latency, and total sleep time.67 This meta-analysis did not include daridorexant, as it was conducted prior to the drug’s approval. Lemborexant also effectively reduces wakefulness after sleep onset. Importantly, lemborexant was associated with significantly less risk of dizziness and postural instability compared to the BzRAs, and it was not associated with clinically meaningful residual morning sleepiness or reduced next-day functioning.10,67 No significant drug interactions were reported, suggesting that lemborexant may be safer to use in older patients than the BzRAs.67 However, given the potential risk of next-morning driving impairment, individuals who are more sensitive to the effects of lemborexant should be prescribed a lower dose.10
A third DORA, daridorexant, was approved in January 2022. Daridorexant is FDA approved in doses of 25 mg and 50 mg.68 In the pivotal trials, doses of 25 mg and 50 mg showed a statistically significant improvement in sleep parameters compared to placebo at both months 1 and 3.69 Daridorexant improves both sleep and daytime functioning with a favorable safety profile in adult patients, including the elderly.10,69 In a randomized trial, the 50 mg dose of daridorexant significantly reduced daytime sleepiness at 1 and 3 months (a secondary endpoint) as measured by the sleepiness domain score on the Insomnia Daytime Symptoms and Impacts Questionnaire.70 Daridorexant has the shortest half-life of the available DORAs (8 hours compared to 12 hours for suvorexant and 17–19 hours for lemborexant). Whether that short half-life contributes to reduced risk for impaired next-day functioning is not yet known.
Another agent in the pipeline is seltorexant, which is selective for the OXR2 receptor and is being evaluated for potential use in major depressive disorder and insomnia.10 Seltorexant has a very short half-life (2 to 3 hours). Whether selectivity for one type of orexin receptor offers better efficacy and safety than dual inhibition is yet to be established.
Choosing the Right Drug for the Right Patient: What the Evidence Shows
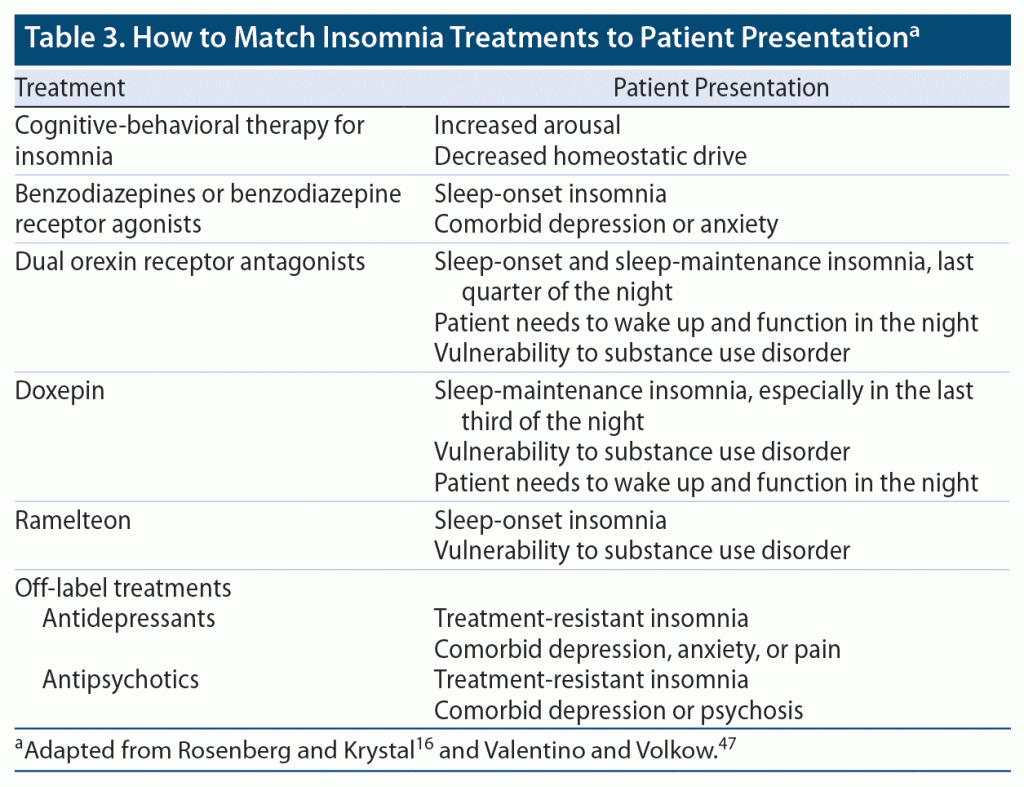
Drugs used in insomnia produce different effects on distinct types of sleep disturbance. The choice of agent should be individualized depending on various factors such as the patient’s age and gender, as well as the presence of comorbidities, the risk for adverse effects, and patient preference (Table 3).16,42,47,60
As discussed previously, clinicians who identify insomnia in a patient should be alert for the presence of a psychiatric disorder and, conversely, should always assess for insomnia in a patient with a psychiatric disorder. The choice of treatment should also take the presence of comorbidities into account. For example, a BzRA may be an appropriate choice for a patient with comorbid insomnia and depression or anxiety, while an atypical antipsychotic might be indicated for a patient with comorbid insomnia and schizophrenia. DORAs may be a better choice for patients with a substance abuse disorder since orexin mediates the reward and arousal effects of drugs of abuse, including opioids. Use of such substances can often result in disrupted sleep, while use of orexin antagonists can block arousal and reward, thus offering a potential 2-fold benefit for patients with comorbid substance abuse disorder and insomnia.47
Insomnia is also associated with an increased risk of cardiovascular disease, possibly because it is a risk factor for hypertension.21 Therapy that effectively controls blood pressure may contribute to improved sleep, and, conversely, insomnia agents that down-regulate the arousal and stress systems (such as trazodone, doxepin, or the DORAs) may contribute to lower blood pressure.71
The patient’s gender is also a consideration when designing therapy for insomnia. For example, prescribing information for zolpidem notes that women should receive the lower starting dose (5 mg).72 For pregnant or breastfeeding women, a nonpharmacologic therapy such as CBT-I may be a better option than any insomnia medication.10
Like doxepin73,74 and ramelteon,75,76 DORAs appear to be a viable choice for older adults. A prespecified subgroup analysis of pooled data from 2 efficacy and 3 safety randomized, double-blind, placebo-controlled, parallel-group trials showed that suvorexant improved sleep maintenance and onset over 3 months of nightly treatment with few safety concerns.77 A 1-month randomized, double-blind, placebo-controlled study78 of lemborexant in adults aged ≥ 55 years with insomnia (N = 1,006) showed that lemborexant 5 mg and 10 mg significantly improved sleep latency, sleep efficiency, and wake time to sleep onset compared to placebo. Lemborexant 5 mg did not differ from placebo on cognitive performance testing, and both lemborexant doses caused less postural instability than zolpidem.
DORAs also appear to be a viable option for patients with Alzheimer’s disease who typically demonstrate reduced total sleep time and early awakening. A recent controlled trial found that, compared to placebo, treatment with suvorexant in patients with Alzheimer’s disease resulted in significantly longer sleep times and reduced awakening after sleep onset.22
The appropriate management strategy for sleep disturbance comorbid with COVID-19 remains unclear. Well-controlled randomized double-blind studies with this population have not been conducted with either older or more recently approved drugs to treat insomnia disorder. Use of CBT-I or newer orexin antagonists could be considered and expected to be as effective as with other insomnia comorbid medical disorders.
Regardless of the choice of therapy, patients should be monitored frequently to assess treatment efficacy and identify potential adverse effects.59 If results are unsatisfactory, the drug dosage may need to be adjusted, or a switch to another drug within the class with a different half-life or to a drug with a different mechanism may be needed. Patients should also be counseled not to stop taking prescription sleep aids abruptly, especially benzodiazepines and BzRAs, due to the risk for rebound insomnia or withdrawal symptoms such as anxiety.2,42,59
Ideally, a strategy that combines healthy lifestyle habits with judicious, well-monitored use of medication targeted for the individual patient’s specific insomnia complaint (and any existing comorbidities) will result in optimal outcomes. While many patients presenting with insomnia symptoms may require only short-term or intermittent medication, others with insomnia may need long-term treatment with a sleep agent. Data support the use of DORAs as a safe and effective longer-term option.
CONCLUSIONS
Insomnia disorder is not just a problem at night. It has a profound negative effect on daytime functioning and overall health and is often underreported in the primary care setting. Primary care clinicians should proactively screen for evidence of insomnia, especially in patients presenting with medical or psychiatric illnesses. There is evidence that insomnia may contribute to worse outcomes in comorbid conditions and that treating the insomnia can result in improvements in the comorbid illness and quality of life. Effective treatment combines pharmacologic and nonpharmacologic strategies aimed at reducing time to sleep onset, maintaining sleep for an adequate duration, and preventing early awakening, while improving daytime function. A new class of drugs, the DORAs, improves sleep parameters through a novel mechanism that targets the wakefulness system, in contrast to commonly used hypnotics that cause sedation by increasing GABA transmission. A personalized approach to insomnia management should consider whether the patient has difficulty falling or staying asleep, comorbidities, and patient preferences.
Submitted: August 2, 2022; accepted October 6, 2022.
Published online: January 24, 2023.
Relevant financial relationships: Dr Rosenberg has received research grants from Apnimed, Eisai, Idorsia, and Jazz. Dr Benca receives grant support from Eisai and is a consultant for Eisai, Genomind, Indorsia, Jazz, Merck, and Sage Therapeutics. Dr Doghramji is on the advisory boards for Eisai, Idorsia, and Bayer Healthcare; is on the speaker’s bureaus of Eisai, Idorsia, AbbVie, and Jazz; and is a stock shareholder in Pfizer and CVS. Dr Roth is a consultant for Eisai, Indorsia, Janssen, Jazz, Merck, and Takeda.
Funding/support: This article was developed with support from an educational grant from Eisai.
Role of the sponsors: Eisai had no role in the design, analysis, interpretation, or publication of this article.
Acknowledgments: The authors thank Allison B. for providing the patient’s perspective during the panel discussion and Ron Schaumburg, MA, and Jacqueline Brooks, MBBCh, MRCPsych, for editorial assistance with the manuscript. Allison B., Ron Schaumburg, and Jacqueline Brooks have no conflicts of interest to declare.
Clinical Points
- Insomnia is highly prevalent in the primary care setting and is often comorbid with a multitude of medical and psychiatric illnesses; however, it remains underdiagnosed and undertreated.
- There is evidence to suggest that treating the insomnia can improve outcomes for the comorbidity, so clinicians should identify and treat insomnia whenever possible.
- Dual orexin receptor antagonists improve insomnia via a novel mechanism that targets the wakefulness system rather than targeting the GABA system to increase sedation, and data suggest that may be a good choice in select comorbidities.
References (78)

- World Health Organization. International Classification of Diseases, 11th Revision. 2022 release. Accessed April 20, 2022. https://www.who.int/standards/classifications/classification-of-diseases
- Roach M, Juday T, Tuly R, et al. Challenges and opportunities in insomnia disorder. Int J Neurosci. 2021;131(11):1058–1065. PubMed CrossRef
- Morin CM, Jarrin DC, Ivers H, et al. Incidence, persistence, and remission rates of insomnia over 5 years. JAMA Netw Open. 2020;3(11):e2018782. PubMed CrossRef
- Araújo T, Jarrin DC, Leanza Y, et al. Qualitative studies of insomnia: current state of knowledge in the field. Sleep Med Rev. 2017;31:58–69. PubMed CrossRef
- Byrne EM. The relationship between insomnia and complex diseases: insights from genetic data. Genome Med. 2019;11(1):57. PubMed CrossRef
- Etindele-Sosso FA. Insomnia, excessive daytime sleepiness, anxiety, depression and socioeconomic status among customer service employees in Canada. Sleep Sci. 2020;13(1):54–64. PubMed
- Gallup, Inc. The state of sleep in America 2022 report. Accessed April 20, 2022. www.gallup.com/analytics/390536/sleep-in-america-2022.aspx
- Kale HP, Qureshi ZP, Shah R, et al. Changes in healthcare resource use and costs in commercially insured insomnia patients initiating suvorexant. Adv Ther. 2021;38(10):5221–5237. PubMed CrossRef
- Anderson LH, Whitebird RR, Schultz J, et al. Healthcare utilization and costs in persons with insomnia in a managed care population. Am J Manag Care. 2014;20(5):e157–e165. PubMed
- Rosenberg R, Citrome L, Drake CL. Advances in the treatment of chronic insomnia: a narrative review of new nonpharmacologic and pharmacologic therapies. Neuropsychiatr Dis Treat. 2021;17:2549–2566. PubMed CrossRef
- Torrens Darder I, Argüelles-Vázquez R, Lorente-Montalvo P, et al. Primary care is the frontline for help-seeking insomnia patients. Eur J Gen Pract. 2021;27(1):286–293. PubMed CrossRef
- Gallup Organization for the National Sleep Foundation. Sleep in America: 1995. A National Survey of US Adults. A report prepared by the Gallup Organization for the National Sleep Foundation. Los Angeles, California: National Sleep Foundation; 1995.
- National Sleep Foundation. Accessed April 20, 2022. https://www.thensf.org/wp-content/uploads/2021/03/2005_summary_of_findings.pdf
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. 2003.
- Dopheide JA. Insomnia overview: epidemiology, pathophysiology, diagnosis and monitoring, and nonpharmacologic therapy. Am J Manag Care. 2020;26(suppl):S76–S84. PubMed CrossRef
- Rosenberg RP, Krystal AD. Diagnosing and treating insomnia in adults and older adults. J Clin Psychiatry. 2021;82(6):EI20008AH5C. PubMed CrossRef
- Atkin T, Comai S, Gobbi G. Drugs for insomnia beyond benzodiazepines: pharmacology, clinical applications, and discovery. Pharmacol Rev. 2018;70(2):197–245. PubMed CrossRef
- Sateia MJ. International Classification of Sleep Disorders-Third Edition: highlights and modifications. Chest. 2014;146(5):1387–1394. PubMed CrossRef
- Gadermann AM, Alonso J, Vilagut G, et al. Comorbidity and disease burden in the National Comorbidity Survey Replication (NCS-R). Depress Anxiety. 2012;29(9):797–806. PubMed CrossRef
- Acker KA, Carter P. Sleep-wake disturbances in oncology. Nurs Clin North Am. 2021;56(2):175–187. PubMed CrossRef
- Bollu PC, Kaur H. Sleep medicine: insomnia and sleep. Mo Med. 2019;116(1):68–75. PubMed
- Herring WJ, Ceesay P, Snyder E, et al. Polysomnographic assessment of suvorexant in patients with probable Alzheimer’s disease dementia and insomnia: a randomized trial. Alzheimers Dement. 2020;16(3):541–551. PubMed CrossRef
- Qureshi ZP, Thiel E, Nelson J, et al. Incremental healthcare utilization and cost burden of comorbid insomnia in Alzheimer’s disease patients. J Alzheimers Dis. 2021;83(4):1679–1690. PubMed CrossRef
- Breslau N, Roth T, Rosenthal L, et al. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39(6):411–418. PubMed CrossRef
- Roberts RE, Shema SJ, Kaplan GA, et al. Sleep complaints and depression in an aging cohort: a prospective perspective. Am J Psychiatry. 2000;157(1):81–88. PubMed CrossRef
- Arroll B, Fernando A 3rd, Falloon K, et al. Prevalence of causes of insomnia in primary care: a cross-sectional study. Br J Gen Pract. 2012;62(595):e99–e103. PubMed CrossRef
- Bhat S, Chokroverty S. Sleep disorders and COVID-19. Sleep Med. 2022;91:253–261. PubMed CrossRef
- Donzella SM, Kohler LN, Crane TE, et al. COVID-19 infection, the COVID-19 pandemic, and changes in sleep. Front Public Health. 2022;9:795320. PubMed CrossRef
- Morin CM, Bjorvatn B, Chung F, et al. Insomnia, anxiety, and depression during the COVID-19 pandemic: an international collaborative study. Sleep Med. 2021;87:38–45. PubMed CrossRef
- Hertenstein E, Feige B, Gmeiner T, et al. Insomnia as a predictor of mental disorders: a systematic review and meta-analysis. Sleep Med Rev. 2019;43:96–105. PubMed CrossRef
- Shi L, Chen SJ, Ma MY, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev. 2018;40:4–16. PubMed CrossRef
- Bernert RA, Joiner TE. Sleep disturbances and suicide risk: a review of the literature. Neuropsychiatr Dis Treat. 2007;3(6):735–743. PubMed
- Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults’ sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65(1):63–73 [Correction. Psychosom Med. 2003 Mar-Apr;65(2)]. PubMed CrossRef
- McCall WV, Blocker JN, D’Agostino R Jr, et al. Treatment of insomnia in depressed insomniacs: effects on health-related quality of life, objective and self-reported sleep, and depression. J Clin Sleep Med. 2010;6(4):322–329. PubMed CrossRef
- Cunningham JEA, Shapiro CM. Cognitive Behavioral Therapy for Insomnia (CBT-I) to treat depression: a systematic review. J Psychosom Res. 2018;106:1–12. PubMed CrossRef
- Pollack M, Kinrys G, Krystal A, et al. Eszopiclone coadministered with escitalopram in patients with insomnia and comorbid generalized anxiety disorder. Arch Gen Psychiatry. 2008;65(5):551–562. PubMed CrossRef
- Selvanathan J, Pham C, Nagappa M, et al. Cognitive behavioral therapy for insomnia in patients with chronic pain: systematic review and meta-analysis of randomized controlled trials. Sleep Med Rev. 2021;60:101460. PubMed CrossRef
- McCall WV, Benca RM, Rosenquist PB, et al. Reducing Suicidal Ideation Through Insomnia Treatment (REST-IT): a randomized clinical trial. Am J Psychiatry. 2019;176(11):957–965. PubMed CrossRef
- Vgontzas AN, Fernandez-Mendoza J, Liao D, et al. Insomnia with objective short sleep duration: the most biologically severe phenotype of the disorder. Sleep Med Rev. 2013;17(4):241–254. PubMed CrossRef
- Grandner MA, Malhotra A. Sleep as a vital sign: why medical practitioners need to routinely ask their patients about sleep. Sleep Health. 2015;1(1):11–12. PubMed CrossRef
- Riemann D, Baglioni C, Bassetti C, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26(6):675–700. PubMed CrossRef
- Taddei-Allen P. Economic burden and managed care considerations for the treatment of insomnia. Am J Manag Care. 2020;26(suppl):S91–S96. PubMed CrossRef
- Morin CM, Belleville G, Bélanger L, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep (Basel). 2011;34(5):601–608. PubMed CrossRef
- Brown RE, Basheer R, McKenna JT, et al. Control of sleep and wakefulness. Physiol Rev. 2012;92(3):1087–1187. PubMed CrossRef
- Saper CB, Fuller PM. Wake-sleep circuitry: an overview. Curr Opin Neurobiol. 2017;44:186–192. PubMed CrossRef
- Scammell TE, Arrigoni E, Lipton JO. Neural circuitry of wakefulness and sleep. Neuron. 2017;93(4):747–765. PubMed CrossRef
- Valentino RJ, Volkow ND. Drugs, sleep, and the addicted brain. Neuropsychopharmacology. 2020;45(1):3–5. PubMed CrossRef
- Qaseem A, Kansagara D, Forciea MA, et al; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline From the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. PubMed CrossRef
- Fatima Y, Bucks RS, Mamun AA, et al. Sleep trajectories and mediators of poor sleep: findings from the longitudinal analysis of 41,094 participants of the UK Biobank cohort. Sleep Med. 2020;76:120–127. PubMed CrossRef
- Irish LA, Kline CE, Gunn HE, et al. The role of sleep hygiene in promoting public health: a review of empirical evidence. Sleep Med Rev. 2015;22:23–36. PubMed CrossRef
- Sateia MJ, Buysse DJ, Krystal AD, et al. Clinical practice guideline for the pharmacologic treatment of chronic insomnia in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2017;13(2):307–349. PubMed CrossRef
- Li SQ, Sun XW, Zhang L, et al. Impact of insomnia and obstructive sleep apnea on the risk of acute exacerbation of chronic obstructive pulmonary disease. Sleep Med Rev. 2021;58:101444. PubMed CrossRef
- Morin CM, Beaulieu-Bonneau S, Bélanger L, et al. Cognitive-behavior therapy singly and combined with medication for persistent insomnia: impact on psychological and daytime functioning. Behav Res Ther. 2016;87:109–116. PubMed CrossRef
- Maire M, Linder S, Dvořák C, et al. Prevalence and management of chronic insomnia in Swiss primary care: cross-sectional data from the “Sentinella” practice-based research network. J Sleep Res. 2020;29(5):e13121. PubMed CrossRef
- Morin CM. Profile of Somryst prescription digital therapeutic for chronic insomnia: overview of safety and efficacy. Expert Rev Med Devices. 2020;17(12):1239–1248. PubMed CrossRef
- Johnson EO, Roehrs T, Roth T, et al. Epidemiology of alcohol and medication as aids to sleep in early adulthood. Sleep. 1998;21(2):178–186. PubMed CrossRef
- Wade AG, Ford I, Crawford G, et al. Nightly treatment of primary insomnia with prolonged release melatonin for 6 months: a randomized placebo-controlled trial on age and endogenous melatonin as predictors of efficacy and safety. BMC Med. 2010;8(1):51. PubMed CrossRef
- Shinjyo N, Waddell G, Green J. Valerian root in treating sleep problems and associated disorders: a systematic review and meta-analysis. J Evid Based Integr Med. 2020;25:X20967323. PubMed CrossRef
- Kaul M, Zee PC, Sahni AS. Effects of cannabinoids on sleep and their therapeutic potential for sleep disorders. Neurotherapeutics. 2021;18(1):217–227. PubMed CrossRef
- Neubauer DN. Pharmacotherapy for insomnia in adults. Up to Date. Updated September 14, 2021. Accessed April 20, 2021. www.uptodate.com/contents/pharmacotherapy-for-insomnia-in-adults
- Drugs.com. Schedule 4 (IV) drugs. Accessed April 20, 2022. https://www.drugs.com/schedule-4-drugs.html
- Gallagher A. FDA approves daridorexant for treatment of insomnia. Pharmacy Times. January 10, 2022. Accessed April 20, 2022. https://www.pharmacytimes.com/view/fda-approves-daridorexant-for-treatment-of-insomnia
- Salisbury-Afshar E. Management of insomnia disorder in adults. Am Fam Physician. 2018;98(5):319–322. PubMed
- Buysse DJ. Clinical pharmacology of other drugs used as hypnotics. In: Kryger MH, Roth T, Dement WC, eds. Principles and Practice of Sleep Medicine. 5th ed. Elsevier; 2011:492–509.
- Drake CL, Kalmbach DA, Cheng P, et al. Can the orexin antagonist suvorexant preserve the ability to awaken to auditory stimuli while improving sleep? J Clin Sleep Med. 2019;15(9):1285–1291. PubMed CrossRef
- Kishi T, Nomura I, Matsuda Y, et al. Lemborexant vs suvorexant for insomnia: a systematic review and network meta-analysis. J Psychiatr Res. 2020;128:68–74. PubMed CrossRef
- McElroy H, O’Leary B, Adena M, et al. Comparative efficacy of lemborexant and other insomnia treatments: a network meta-analysis. J Manag Care Spec Pharm. 2021;27(9):1296–1308. PubMed CrossRef
- QUVIVIQ. Prescribing Information. Idorsia. Accessed June 28, 2022. https://www.idorsia.us/documents/us/label/Quviviq_PI.pdf
- Mignot E, Mayleben D, Fietze I, et al; investigators. Safety and efficacy of daridorexant in patients with insomnia disorder: results from two multicenter, randomized, double-blind, placebo-controlled, phase 3 trials. Lancet Neurol. 2022;21(2):125–139 [Correction. Lancet Neurol. 2022 Jan 20]. PubMed CrossRef
- Fietze I, Bassetti C, Mayleben D, et al. Daridorexant is safe and improves both sleep and daytime functioning in elderly patients with insomnia. Sleep (Basel). 2021;44(suppl 2):A138–A139. CrossRef
- Bathgate CJ, Fernandez-Mendoza J. Insomnia, short sleep duration, and high blood pressure: recent evidence and future directions for the prevention and management of hypertension. Curr Hypertens Rep. 2018;20(6):52. PubMed CrossRef
- Ambien. Prescribing Information [package insert]. Sanofi-Aventis; 2022.
- Krystal AD, Durrence HH, Scharf M, et al. Efficacy and safety of doxepin 1 mg and 3 mg in a 12-week sleep laboratory and outpatient trial of elderly subjects with chronic primary insomnia. Sleep. 2010;33(11):1553–1561. PubMed CrossRef
- Lankford A, Rogowski R, Essink B, et al. Efficacy and safety of doxepin 6 mg in a four-week outpatient trial of elderly adults with chronic primary insomnia. Sleep Med. 2012;13(2):133–138. PubMed CrossRef
- Richardson GS, Zammit G, Wang-Weigand S, et al. Safety and subjective sleep effects of ramelteon administration in adults and older adults with chronic primary insomnia: a 1-year, open-label study. J Clin Psychiatry. 2009;70(4):467–476. PubMed CrossRef
- Roth T, Seiden D, Wang-Weigand S, et al. A 2-night, 3-period, crossover study of ramelteon’s efficacy and safety in older adults with chronic insomnia. Curr Med Res Opin. 2007;23(5):1005–1014. PubMed CrossRef
- Herring WJ, Connor KM, Snyder E, et al. Suvorexant in elderly patients with insomnia: pooled analyses of data from phase III randomized controlled clinical trials. Am J Geriatr Psychiatry. 2017;25(7):791–802. PubMed CrossRef
- Rosenberg R, Murphy P, Zammit G, et al. Comparison of lemborexant with placebo and zolpidem tartrate extended release for the treatment of older adults with insomnia disorder: a phase 3 randomized clinical trial. JAMA Netw Open. 2019;2(12):e1918254 [Correction. JAMA Netw Open. 2020 Apr 1;3(4):e206497]. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite