The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
LESSONS LEARNED AT THE INTERFACE OF MEDICINE AND PSYCHIATRY
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord 2020;22(6):20f02708
To cite: Paudel S, Vyas CM, Stern TA. A prescription for deprescribing antipsychotics: managing polypharmacy in schizophrenia. Prim Care Companion CNS Disord. 2020;22(6):20f02708.
To share: https://doi.org/10.4088/PCC.20f02708
© Copyright 2020 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts
‡Drs Paudel and Vyas contributed equally to this work.
*Corresponding author: Shreedhar Paudel, MD, MPH, Department of Psychiatry, Massachusetts General Hospital, 55 Fruit St, Boston, MA 02114 ([email protected]).
Do any of your patients with schizophrenia receive more than 1 antipsychotic medication? Have you ever wondered why they are taking multiple medications? Have you been concerned that tapering and discontinuing “unnecessary medications” will be harmful to your patients? Have you been frustrated by attempts to discontinue antipsychotics and wished that there was a better strategy to help you and your patients? If you have, then the following case vignette and discussion should serve to enhance your understanding of the risks and benefits of polypharmacy and the strategies for tapering and discontinuing antipsychotics in those with schizophrenia.
CASE VIGNETTE
Ms A, a 44-year-old single college graduate, was working full time; however, she had a history of schizophrenia and generalized anxiety disorder (with multiple psychiatric hospitalizations and 1 suicide attempt by a carbon monoxide overdose). Her care was being transferred to a new physician for continued treatment. At the time of transfer, she was taking lurasidone (120 mg/day) and clozapine (275 mg/day), with a clozapine level of 149 ng/mL and a norclozapine level of 189 ng/mL). Other psychotropics included lorazepam (0.5 mg twice/day as needed), armodafinil (250 mg/day), escitalopram (20 mg/day), propranolol (20 mg/day as needed), prazosin (2 mg nightly), and melatonin (5–10 mg nightly). She denied hallucinating or having delusions. She could concentrate on her work. She was active in self-care (she walked regularly, practiced yoga, and engaged in mild physical exercise). She had persistent social anxiety but no depression or euphoria. She reported no nightmares or flashbacks and denied binging or purging. She had no obsessive thinking or compulsive behaviors. She complained of difficulty losing weight, tiredness, sleeping more, and difficulty staying awake despite taking armodafinil.
At the conclusion of the evaluation, the physician asked Ms A, “Why are you taking both lorazepam and armodafinil?” She responded, as expected, “Lorazepam to sleep and control anxiety and armodafinil to make myself awake during the day.” She was also asked, “Why do you take both clozapine and lurasidone?” Again, her answer was direct, “My psychiatrist asked me to take them.” After the final question, “Do you feel that you are overmedicated?” she looked perplexed and said, “I don’t know, you are the doctor. But, I might be.”
The physical examination revealed that she was overweight; she was calm and cooperative; she had normal speech, language, eye contact, and mood and a full range of affect; she had no involuntary movements, cognitive deficits, or thoughts to hurt herself or others; and she had insight into her illness and its management. Her laboratory reports included complete metabolic panel, complete blood cell count and differential, thyroid function, hemoglobin A1C, and cholesterol, the results of which were all within normal limits. She was not suicidal.
During the follow-up visit 1 month later, the potential risks and benefits of tapering her unnecessary medications and a contingency plan, if her symptoms returned, were discussed in detail. With careful shared decision-making, lurasidone was slowly tapered and discontinued over the next 4 months. Armodafinil was tapered several months into the lurasidone taper and was discontinued 1 month after lurasidone was stopped. After lurasidone and armodafinil were discontinued, Ms A’s as-needed use of lorazepam and propranolol decreased significantly. At her 7-month follow-up appointment, she was still doing well, had stopped taking lorazepam and propranolol, had minimal anxiety, and had no symptoms of a mood or thought disorder. She became emotional and said, “I feel like a normal person again.”
Although her new physician was apprehensive about further medication tapers, with Ms A’s excitement after stopping 4 unnecessary medications, they agreed to see how she would do without clozapine. Since she was insightful, well educated, working full time, had a supportive family, was not using any illicit substances, and had not had symptoms of psychosis for more than 2 years, a plan was created to taper clozapine and monitor her condition. Before making the decision to lower the clozapine dose, her mother was invited to join the appointment, and her input was received. At that point, Ms A’s clozapine level was 140 ng/mL with a total level of 286 ng/mL. The daily dose was decreased from 275 mg to 250 mg and then to 200 mg/day 2 months later. However, Ms A reported the onset of paranoia and worsening anxiety within the next 6 weeks, leading to an increase in her clozapine dose (to 225 a day). Within a week of the dose increase, her symptoms resolved. Nine months later, she continues to work full time, plans to take college courses, thinks about finding a partner, engages in self-care activities, and volunteers in a program for those with schizophrenia and cancer at a local hospital. She has minimal anxiety in social situations and has no mood or psychotic symptoms. As she was unable to lose weight despite tapering off lurasidone, discussions on the risks and benefits of using aripiprazole with or without clozapine were started with Ms A.
DISCUSSION
Why Is Antipsychotic Polypharmacy
Common in Those With Schizophrenia?
Antipsychotic polypharmacy is the use of more than 1 antipsychotic medication in an individual. Antipsychotic polypharmacy is highly prevalent (~30%) among those diagnosed with schizophrenia.1 However, antipsychotic polypharmacy is still considered a controversial practice due to inconsistent findings on its risks and benefits. Combinations of antipsychotics may offer a clinical advantage over monotherapy; however, given the variety of clinical circumstances one can encounter, identification of optimal treatment is often challenging. Schizophrenia is a complex multidimensional disorder that often leads to hallucinations, delusions, and disordered mood, behavior, and thinking, which may ultimately impair physical well-being.2 The broad range of symptoms spanning multiple psychopathological domains is thought to involve distinct neuroreceptors. Thus, a combination of antipsychotics with different receptor-binding profiles often explains the beneficial results seen in those with schizophrenia. Also, some evidence suggests that individuals with schizophrenia are more likely to receive antipsychotic polypharmacy than those with other neuropsychiatric disorders.3 A recent study4 exploring the risk of antipsychotic polypharmacy reported that symptom severity and number of psychiatric hospitalizations and psychiatric emergency visits in those with schizophrenia were associated with the use of antipsychotic polypharmacy. Further, increasing age, geographic location, and patient care options (inpatient vs outpatient, urban vs rural) may play a significant role in the use of antipsychotic polypharmacy among those with schizophrenia.1
What Are the Risks and Benefits
of Polypharmacy in Schizophrenia?
Antipsychotic medications are the cornerstone of schizophrenia management and are often used in combination. Appropriate combination of antipsychotic medications may boost the therapeutic response, counter the side effects, and provide effective control of treatment-resistant psychosis in those with schizophrenia.5 For example, although clozapine is associated with significant weight gain and metabolic syndrome, randomized trials show that the combination of aripiprazole and clozapine may lead to weight loss and greater reductions in total and low-density lipoprotein cholesterol levels among those who were suboptimally treated with clozapine.6,7 This strategy could be implemented among overweight/obese individuals diagnosed with schizophrenia. Further, aripiprazole augmentation in clozapine-treated patients with refractory schizophrenia showed significantly greater improvements in the negative symptom domains.8
Compared to monotherapy, antipsychotic polypharmacy has also shown protective results on long-term outcomes associated with schizophrenia. A recent study9 found that use of polypharmacy (clozapine plus aripiprazole) was associated with a 14% to 23% lower risk of psychiatric or all-cause hospitalization compared with monotherapy (clozapine alone) for the maintenance therapy of schizophrenia. These results also indicate that combination of 2 antipsychotics with different receptor profiles may have a modest clinical benefit on long-term clinical outcomes associated with schizophrenia. Some clinical circumstances affect tolerability and drive the need for combination of antipsychotics over monotherapy.10 However, results of a systematic review11 of open-label and efficacy-focused studies showed that the risk of intolerability-related discontinuation was similar between groups with antipsychotic polypharmacy and monotherapy.
Antipsychotic polypharmacy is associated with adverse drug reactions, drug-drug interactions, nonadherence, and deficits in everyday functioning.12–14 Combined use of antipsychotics is a major contributor of high-dose prescriptions that may eventually lead to neurocognitive deficits in later life.15,16 A growing body of evidence suggests that antipsychotic polypharmacy may also increase the risk of metabolic syndrome,17 which may increase all-cause mortality.18 The use of antipsychotic polypharmacy is especially concerning if appropriate combinations are not used. Polypharmacy is an area of potential concern for older adults because of a higher risk of adverse drug reactions.19 Aging significantly affects the pharmacokinetics, pharmacodynamics, and drug-drug interactions of antipsychotic medications, and these issues contribute to problems (such as lower treatment efficacy, treatment nonadherence, or treatment resistance) in those with schizophrenia. Research20 also suggests that polypharmacy is a risk factor for underprescribing. Given this finding, some patients may not receive necessary medications, likely impacting their safety and well-being.
Is There an Art to Deprescribing
for Those With Schizophrenia?
Deprescribing is a systematic process involving identifying and discontinuing drugs after careful assessment of overall health, the well-being of a patient, therapy goals, physical well-being, and adherence to the ongoing treatment regimen.21,22 Physicians should also perform a psychosocial and behavioral risk assessment to identify self-harm and suicidal thoughts at the time of deprescribing medications used for management of schizophrenia.23 After considering all these factors, a physician can discuss the potential risks and benefits of tapering and discontinuing medications and provide contingency plans if their patient experiences an increase in symptoms. A systematic method to deprescribing antipsychotic medications highlighted the importance of a recovery-focused approach that included patient-centered care, shared decision-making, and promoting hope and recovery.24 Another study25 reported that deprescribing antipsychotic medications can be feasible without adversely affecting clinical outcomes, such as hospitalization rates and employment status over a 5-year follow-up period. Similarly, a recent review26 highlighted the importance of deprescribing antipsychotic medications despite a paucity of evidence and multiple challenges related to physician and patient factors.
How Can Strategies Surrounding
Polypharmacy in Schizophrenia Be Optimized?
Use of polypharmacy is often reasonable for the management of schizophrenia, as it addresses different aspects of treatment nonadherence/resistance, insufficient response of psychotic and cognitive symptoms, comorbid psychiatric disorders, and side effects of antipsychotic monotherapy. However, evidence-based knowledge (in the form of continued physician education, clinical guidelines, and algorithms) appropriate for the management of schizophrenia is still lacking, as is the avoidance of irrational polypharmacy. Physicians should focus on simple drug regimens with once- or twice-daily dosing, use drugs with clear clinical indications, and know about a variety of adverse drug effects.27 Electronic data entry and feedback have reduced polypharmacy as well as drug-drug interactions seen in primary care populations.28 In many hospitals, feedback procedures, such as a system prompts/alerts when prescribers use 2 or more drugs from the same class, reduce antipsychotic polypharmacy. A recent guideline29 by a team of experts highlighted the importance of appropriate timing and inclusion of the patient’s family, friends, and mental health team in the deprescribing process and the development of a plan for relapse identification, prevention, and management while implementing antipsychotic deprescribing.
What Risks Are Associated
With the Chronic Use of Antipsychotics?
The chronic use of antipsychotics entails a difficult trade-off between the benefit of reducing the psychotic symptoms and the risk of developing long-term adverse side effects. As a class, the first-generation antipsychotics work through dopamine D2 neuroreceptor blockade. Within this class, medications with high a binding potency for dopaminergic neuroreceptors (such as haloperidol) are more likely to be associated with extrapyramidal side effects (eg, dystonic reactions, tardive dyskinesia), while medications with low binding potency for dopaminergic neuroreceptors (such as chlorpromazine) tend to be associated with anticholinergic effects (eg, dry mouth, blurred vision, dry eyes, constipation). The newer second-generation antipsychotics, especially clozapine and olanzapine, tend to cause problems related to metabolic syndrome, such as obesity and type 2 diabetes mellitus. Overall, all antipsychotic medications are associated with an increased likelihood of sedation, seizures, sexual dysfunction, cardiac arrhythmia, and sudden cardiac death.
What Are the Symptoms of Withdrawal
From Antipsychotics When They
Are Rapidly Tapered or Discontinued?
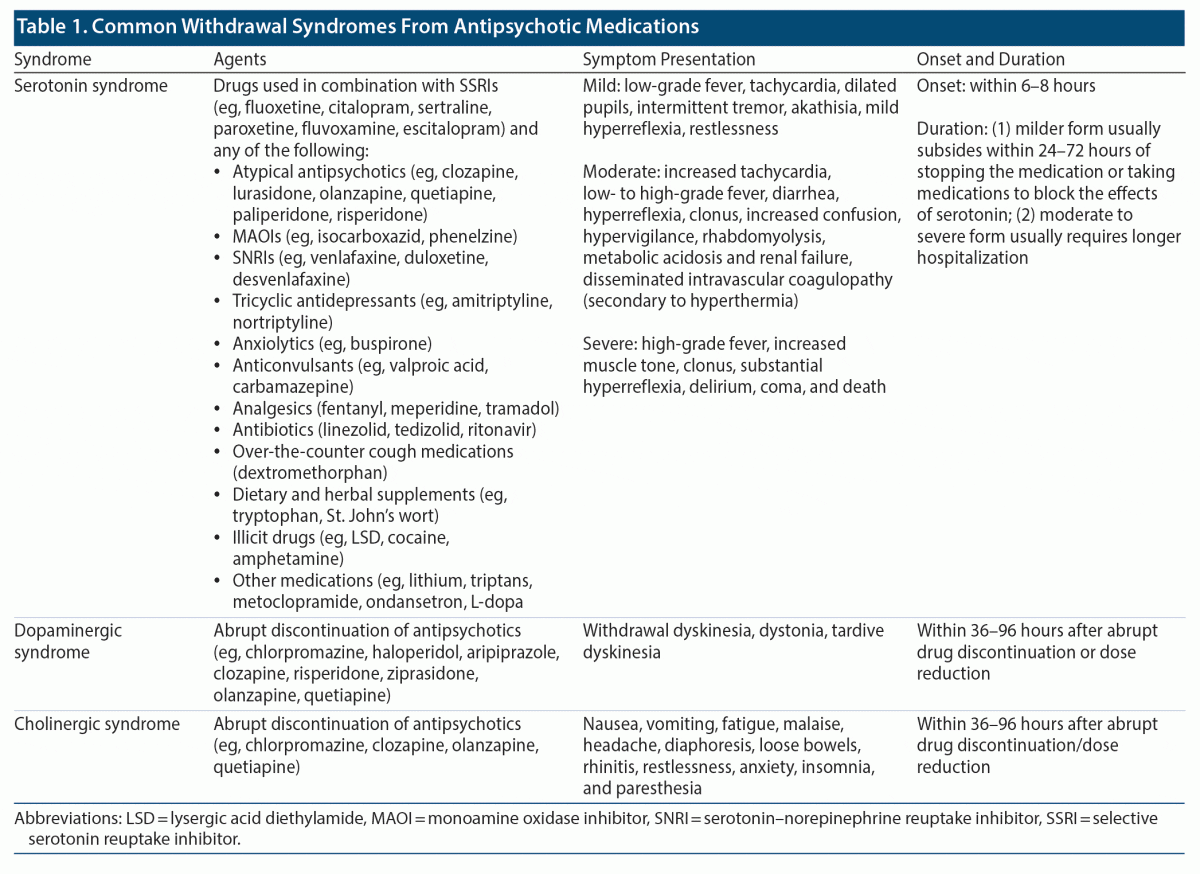
Antipsychotics are often prescribed for long-term care of people with schizophrenia. However, there are a variety of clinical circumstances in which stopping an antipsychotic medication should be considered. Withdrawal symptoms typically occur when antipsychotics are abruptly discontinued. Abrupt discontinuation of antipsychotics (such as clozapine) often results in rebound psychosis, which could be more severe than before the treatment was started. Prior evidence has indicated that abrupt withdrawal of clozapine and concomitant use of citalopram can result in serotonin syndrome (eg, with coma, hypersalivation, hyperreflexia, and stimulus-induced clonus).30 Citalopram is often used as an adjuvant in those with first-episode schizophrenia for negative symptoms.31 Depending on the pharmacologic action of the antipsychotic medication, several withdrawal syndromes can occur if stopped abruptly (Table 1). Dopaminergic syndrome, a common withdrawal syndrome resulting from abrupt discontinuation of antipsychotics, is characterized by akathisia, dystonia, and tardive dyskinesia.32 Another example is a cholinergic syndrome that has resulted from abrupt discontinuation of chlorpromazine, olanzapine, or quetiapine.32 Cholinergic syndrome often presents with nausea, vomiting, headache, restlessness, anxiety, insomnia, fatigue, and paresthesia. Overall, antipsychotic medications should be reduced slowly, ideally over weeks to months, by physicians.
How Quickly Can Antipsychotics Be Tapered
to Avoid Withdrawal Symptoms or Neuroleptic Malignant Syndrome?
Clear guidelines on the rate of tapering antipsychotic medication in those with stable schizophrenia are lacking. American Psychiatric Association (APA) practice guidelines33 recommend reducing antipsychotic doses around the extrapyramidal symptom threshold but within the therapeutic range of the medication during the stable phase of schizophrenia. Similarly, the APA guideline also recommends the rate of an antipsychotic medication taper be 10% of the baseline dose every 6 weeks to minimize the withdrawal symptoms. Several studies25,34 with small sample sizes report successful reduction of antipsychotic medication dose by 50%–67% over 4 weeks to a few years. Reduction of the dose by 25%–30% every 3 months, as tolerated, was the approach by Steingard,25 while reduction of the targeted dose within the first 4 weeks was employed by Ozawa et al.34 The evidence on the rate of tapering the injectable antipsychotics is still lacking, but the approach should be about the same as that for oral medication. Using the oral form of the same injectable antipsychotic medication, on an as-needed basis, usually helps with the tapering of injectables. As the APA suggested, maintaining the minimal effective dose promoting symptom control, appropriate functioning, and quality of life should be the main goals.
What Are Obstacles to Deprescribing
Antipsychotics in Those With Schizophrenia?
Deprescribing unnecessary antipsychotics is a way to prevent long-term adverse drug events and avoid unnecessary health care utilization. However, several obstacles make the deprescribing process difficult for primary care physicians. The existing evidence supports that barriers to deprescribing are formidable—ranging from patient expectations and fear of adverse outcomes, cultural diversity in prescribing practice, and influence of the patient’s socioeconomic and comorbidity factors to maintenance of the patient-physician relationship, access to a nonpharmaceutical regimen, and a myriad of organizational factors (such as targeted funding, fragmentation of care, and competing demands of medical practice).35–37
How Can Physicians Negotiate With Patients to Taper and Discontinue Antipsychotic Medications When the Medications No Longer Appear to Be Working?
Despite the need for tapering and discontinuing antipsychotic medications when they no longer appear to be working, the physician should develop a tapering plan for effective clinical communication to optimize their patient’s understanding and experience of the deprescribing process and avoid unintended negative consequences. Clinicians should have a reasonable plan of action that may help them negotiate with patients, especially with those who have used the antipsychotic medication for a long time. Besides explaining the reasons for tapering antipsychotic medications, patient input in the tapering process has also emerged as an important theme. For example, physicians may offer a series of options, eg, stay on the regimen but decrease the pill count per day or taper the dose gradually and allow the patient to select the choice based on personal preference. By doing this, the patient may feel more respected, which will eventually make him/her more adherent to the tapering plan. Negotiating about the tapering process is not always successful but could result in contentious and frustrating conversations. Primary care physicians should reassure their patients that the tapered and discontinued medications will always be reintroduced if the symptoms become intolerable. The patient should be informed that they will be under close clinical supervision during a taper; weekly or biweekly in-person clinic visits may also optimize adherence to the tapering process. Patients should also be provided with resources to talk to someone on the clinical team as needed, as specific questions or concerns can emerge.
CASE DISCUSSION
Ms A was in her stable phase of schizophrenia treatment, had no history of substance use, was working full time, was receiving regular psychotherapy (including as-needed cognitive-behavioral therapy for psychosis), and had been practicing yoga and exercise regularly. Her physician felt that a combination of clozapine and lurasidone was inappropriate and was causing more side effect burden in his patient. He discussed the risks and benefits of Ms A’s polypharmacy with her and the risks and benefits of deprescribing some of her unnecessary medications. Shared decision-making was used throughout the treatment process, and Ms A was committed to regular monitoring and supervision by her physician.
Use of brief clinical tools and patient-reported outcome measures to monitor the clinical outcomes while changing the antipsychotic medication dose will provide added benefits. Simple and brief tools like the Clinical Global Impressions–Improvement and Severity of Illness scales38 can be very helpful. In the case of Ms A, besides standard clinical interview, no other standard tools were used on a regular basis. At the time of her first evaluation, she was on a 120-mg daily dose of lurasidone, which was decreased by 20% to 30% each month and was stopped after 4 months. She tolerated deprescription of antipsychotic polypharmacy with improved quality of life without adversely impacting her symptoms. After tapering off lurasidone, Ms A was able to stop armodafinil within a month, as she was no longer drowsy during the day.
Hope is a very important factor in psychiatric recovery, which Ms A developed during the process of deprescribing. Similarly, after stopping 1 of the 2 antipsychotic medications and a wakefulness-inducing agent, she was also able to stop lorazepam and propranolol without increasing her anxiety. This case highlights the importance of deprescribing antipsychotic polypharmacy and its significant benefits including reducing the number of medications used to address the adverse effects of the medications. On the other hand, our case reveals the apprehension of a physician for a complete deprescription of antipsychotic medications. As discussed previously, lack of specific guidelines and evidence in clinical practice of antipsychotic deprescription most likely played a significant role in the physician’s anxiety about tapering clozapine. However, collaboration between Ms A and her physician in the deprescribing process led to reduction of clozapine from 275 mg/day to 200 mg/day in a 2-month period. With a good follow-up plan, Ms A was able to report worsening anxiety and paranoia after decreasing the dose of clozapine from 250 to 200 mg/day, and her physician was able to help resolve the symptoms by adjusting the clozapine dose to 225 mg/day in 6 weeks. This difficulty in tapering the further dose of clozapine demonstrates that patients might need to continue the minimal effective dose of antipsychotic medications to prevent decompensation. As Ms A could not lose weight despite stopping lurasidone and could not tolerate a lower dose of clozapine, the options of switching to aripiprazole or the combination of aripiprazole and clozapine were discussed with her.
CONCLUSION
Antipsychotic polypharmacy is common among patients with schizophrenia. To minimize the side effect burden, unnecessary medications can be tapered, and the dose of antipsychotic medications can be reduced to its lowest effective dose during the stabilization phase of schizophrenia treatment. Although standard guidelines for appropriate deprescribing of antipsychotic medications are lacking, patient-tailored slow tapers to the lowest effective dose seem feasible. Our case is a great example for primary care physicians who employ shared decision-making, recovery-oriented practice, and patient-centered approaches can be invaluable tools to make a proper clinical judgment with regard to deprescribing unnecessary antipsychotic medications while managing patients with schizophrenia spectrum disorders.
Submitted: June 3, 2020; accepted July 31, 2020.
Published online: December 17, 2020.
Potential conflicts of interest: Dr Stern is an employee of the Academy of Consultation-Liaison Psychiatry (as editor of Psychosomatics). Drs Paudel and Vyas report no conflicts of interest related to the subject of this article.
Funding/support: None.
Additional information: All information has been de-identified to protect anonymity.
REFERENCES
1. Gallego JA, Bonetti J, Zhang J, et al. Prevalence and correlates of antipsychotic polypharmacy: a systematic review and meta-regression of global and regional trends from the 1970s to 2009. Schizophr Res. 2012;138(1):18–28. PubMed CrossRef
2. Schizophrenia. National Institute of Mental Health website.https://www.nimh.nih.gov/healthtopics/schizophrenia. Accessed November 9, 2020.
3. Armstrong KS, Temmingh H. Prevalence of and factors associated with antipsychotic polypharmacy in patients with serious mental illness: findings from a cross-sectional study in an upper-middle-income country. Br J Psychiatry. 2017;39(4):293–301. PubMed CrossRef
4. Paudel S, Dahal R, Mathias J, et al. Evaluation of risk factors for antipsychotic polypharmacy in inpatient psychiatry units of a community hospital: a retrospective analysis. Community Ment Health J. 2019;55(5):750–754. PubMed CrossRef
5. Englisch S, Zink M. Combined antipsychotic treatment involving clozapine and aripiprazole. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(6):1386–1392. PubMed CrossRef
6. Fleischhacker WW, Heikkinen ME, Olié JP, et al. Effects of adjunctive treatment with aripiprazole on body weight and clinical efficacy in schizophrenia patients treated with clozapine: a randomized, double-blind, placebo-controlled trial. Int J Neuropsychopharmacol. 2010;13(8):1115–1125. PubMed CrossRef
7. Henderson DC, Fan X, Copeland PM, et al. Aripiprazole added to overweight and obese olanzapine-treated schizophrenia patients. J Clin Psychopharmacol. 2009;29(2):165–169. PubMed CrossRef
8. Chang JS, Ahn YM, Park HJ, et al. Aripiprazole augmentation in clozapine-treated patients with refractory schizophrenia: an 8-week, randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2008;69(5):720–731. PubMed CrossRef
9. Tiihonen J, Taipale H, Mehtälä J, et al. Association of antipsychotic polypharmacy vs monotherapy with psychiatric rehospitalization among adults with schizophrenia. JAMA Psychiatry. 2019;76(5):499–507. PubMed CrossRef
10. Freudenreich O, Goff DC. Antipsychotic combination therapy in schizophrenia: a review of efficacy and risks of current combinations. Acta Psychiatr Scand. 2002;106(5):323–330. PubMed CrossRef
11. Galling B, Roldán A, Rietschel L, et al. Safety and tolerability of antipsychotic co-treatment in patients with schizophrenia: results from a systematic review and meta-analysis of randomized controlled trials. Expert Opin Drug Saf. 2016;15(5):591–612. PubMed CrossRef
12. Barnes TR, Paton C. Antipsychotic polypharmacy in schizophrenia: benefits and risks. CNS Drugs. 2011;25(5):383–399. PubMed CrossRef
13. Fenton WS, Blyler CR, Heinssen RK. Determinants of medication compliance in schizophrenia: empirical and clinical findings. Schizophr Bull. 1997;23(4):637–651. PubMed CrossRef
14. Harrington M, Lelliott P, Paton C, et al. The results of a multi-centre audit of the prescribing of antipsychotic drugs for in-patients in the UK. Psychiatric Bulletin. 2002;26(11):414–418. CrossRef
15. Hori H, Noguchi H, Hashimoto R, et al. Antipsychotic medication and cognitive function in schizophrenia. Schizophr Res. 2006;86(1–3):138–146. PubMed CrossRef
16. Milton J, Lawton J, Smith M, et al. Hidden high-dose antipsychotic prescribing: effects of p.r.n. doses. Psychiatric Bulletin. 1998;22(11):675–677. CrossRef
17. Correll CU, Frederickson AM, Kane JM, et al. Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res. 2007;89(1–3):91–100. PubMed CrossRef
18. Weinmann S, Read J, Aderhold V. Influence of antipsychotics on mortality in schizophrenia: systematic review. Schizophr Res. 2009;113(1):1–11. PubMed CrossRef
19. Dagli RJ, Sharma A. Polypharmacy: a global risk factor for elderly people. J Int Oral Health. 2014;6(6):i–ii. PubMed
20. Cantlay A, Glyn T, Barton N. Polypharmacy in the elderly. InnovAiT: Education and inspiration for general practice. 2016;9(2):69–77. CrossRef
21. Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA Intern Med. 2015;175(5):827–834. PubMed CrossRef
22. Lin SK. Antipsychotic polypharmacy: a dirty little secret or a fashion? Int J Neuropsychopharmacol. 2020;23(2):125–131. PubMed CrossRef
23. Garfinkel D, Ilhan B, Bahat G. Routine deprescribing of chronic medications to combat polypharmacy. Ther Adv Drug Saf. 2015;6(6):212–233. PubMed CrossRef
24. Gupta S, Cahill JD. A prescription for “deprescribing” in psychiatry. Psychiatr Serv. 2016;67(8):904–907. PubMed CrossRef
25. Steingard S. Five year outcomes of tapering antipsychotic drug doses in a community mental health center. Community Ment Health J. 2018;54(8):1097–1100. PubMed CrossRef
26. Gupta S, Steingard S, Garcia EF, et al. Deprescribing antipsychotic medications in psychotic disorders: how and why? Curr Psychiatry Rev. 2018;14(1):26–32. CrossRef
27. Zink M, Englisch S, Meyer-Lindenberg A. Polypharmacy in schizophrenia. Curr Opin Psychiatry. 2010;23(2):103–111. PubMed CrossRef
28. Lapane KL, Waring ME, Schneider KL, et al. A mixed method study of the merits of e-prescribing drug alerts in primary care. J Gen Intern Med. 2008;23(4):442–446. PubMed CrossRef
29. Gupta S, Cahill JD, Miller R. Deprescribing antipsychotics: a guide for clinicians. BJPsych Adv. 2018;24(5):295–302. CrossRef
30. Stevenson E, Schembri F, Green DM, et al. Serotonin syndrome associated with clozapine withdrawal. JAMA Neurol. 2013;70(8):1054–1055. PubMed CrossRef
31. Goff DC, Freudenreich O, Cather C, et al. Citalopram in first episode schizophrenia: the DECIFER trial. Schizophr Res. 2019;208:331–337. PubMed CrossRef
32. Keks N, Schwartz D, Hope J. Stopping and switching antipsychotic drugs. Aust Prescr. 2019;42(5):152–157. PubMed CrossRef
33. Keepers GA, Fochtmann LJ, Anzia JM, et al. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. Am J Psychiatry. 2020;177(9):868–872. PubMed CrossRef
34. Ozawa C, Bies RR, Pillai N, et al. Model-guided antipsychotic dose reduction in schizophrenia: a pilot, single-blind randomized controlled trial. J Clin Psychopharmacol. 2019;39(4):329–335. PubMed CrossRef
35. Anderson K, Stowasser D, Freeman C, et al. Prescriber barriers and enablers to minimising potentially inappropriate medications in adults: a systematic review and thematic synthesis. BMJ Open. 2014;4(12):e006544. PubMed CrossRef
36. Sinnott C, Mc Hugh S, Browne J, et al. GPs’ perspectives on the management of patients with multimorbidity: systematic review and synthesis of qualitative research. BMJ Open. 2013;3(9):e003610. PubMed CrossRef
37. Wallis KA, Andrews A, Henderson M. Swimming against the tide: primary care physicians’ views on deprescribing in everyday practice. Ann Fam Med. 2017;15(4):341–346. PubMed CrossRef
38. Guy W, ed. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: US Department of Health, Education, and Welfare Public Health Service Alcohol, Drug Abuse, and Mental Health Administration; 1976.
Please sign in or purchase this PDF for $40.00.
Save
Cite