Energy drinks are non-alcoholic caffeinated beverages often marketed to reduce fatigue and increase alertness, energy, and attention. We present the case of a patient who developed acute liver failure and cerebral edema following a binge episode of energy drink consumption. The patient was hospitalized, required intubation and hemodialysis, and ultimately received an orthotopic liver transplant.
Case Report
A 21-year-old man with a reported history of attention-deficit/hyperactivity disorder and unspecified depressive and anxiety disorders was transferred from an outside hospital to our institution for evaluation for liver transplant due to acute liver failure. One week prior, the patient had consumed multiple energy drinks to stay awake for a 30 plus–hour video game streaming event. The following morning, the patient awoke with nausea and vomiting, which continued for several days, along with decreased food and water intake. He was initially evaluated at an urgent care facility, and his laboratory workup revealed alanine transaminase: 7,616 units/L, aspartate transaminase: 3,259 units/L, and total bilirubin: 13.7 mg/dL. Subsequently, the patient was sent to the nearest hospital and then was transferred to our institution. On arrival to our hospital, the patient was diagnosed with hepatic encephalopathy with an ammonia level of 339 µmol/L. The patient was evaluated by the hepatology service who suspected acute liver failure secondary to drug-induced liver injury from excessive energy drink consumption. The formal liver transplant evaluation process was initiated the day after admission, and the addiction psychiatry service was consulted as part of the liver transplant evaluation protocol.
Upon evaluation, there were varying reports of the types of energy drinks and the quantity consumed. The brands “Bang,” “Rockstar,” and “Full Throttle” were all listed at least once in the chart. Further history obtained from the patient and collateral sources revealed regular cannabis use. The patient had smoked cannabis at least 4 times during his week of illness prior to presentation. The patient denied alcohol use in the last several months, as well as history of binge drinking alcohol. He did report a remote history of cocaine and hallucinogen use briefly in adolescence.
On hospital day 2, the patient’s encephalopathy persisted, and the decision was made to intubate due to concerns for airway protection. A computer tomography scan of the head showed evidence of cerebral edema, and continuous electroencephalogram monitoring showed activity consistent with severe encephalopathy without epileptiform discharges. Hemodialysis was initiated to treat hyperammonemia and hepatic encephalopathy.
On hospital day 3, the patient was listed for liver transplant and underwent successful orthotopic liver transplantation. Although the patient’s encephalopathy and cerebral edema initially persisted following transplant, his liver enzymes began to trend downward, and his ammonia level normalized in the following days. Dialysis was discontinued on hospital day 4, and the patient’s mental status began to improve. He ultimately stabilized enough to be transferred out of the intensive care unit to the general medical ward and was subsequently discharged on hospital day 13 in the care of multiple family caregivers.
Discussion
Since their introduction in the 1990s, energy drink consumption has increased significantly in all age groups, especially adolescents and young adults.1 With an ever-growing market of brands, energy drink popularity has particularly increased among young people desiring a flavorful caffeine source, despite an increase in adverse health events.2
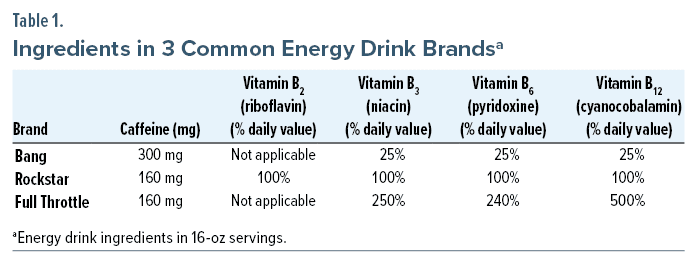
In this patient’s case, he reportedly drank 3 different brands of energy drinks, all of which offered an immense bolus of caffeine in a single 16-ounce can or bottle. Although caffeine toxicity is usually characterized by neurologic symptoms,3 such as confusion and tremors, severe toxicity can further cause organ dysfunction, specifically cardiac arrhythmias and cardiorespiratory failure.3,4 Caffeine-causing hepatic injury has been reported in several case reports4–7; however, these cases did not prove a causal link between caffeine and liver failure and failed to consider other etiologies significant in those cases (eg, drug use, alcohol use). Other reports of caffeine toxicity with available autopsies have shown no evidence of hepatic injury, further supporting the notion that caffeine by itself likely does not cause liver injury.4,7 The other significant components of energy drinks are B vitamins, particularly vitamins B2 (riboflavin), B3 (niacin), B6 (pyridoxine), and B12 (cyanocobalamin) (Table 1).8–10 Among these ingredients, niacin is the only vitamin that includes hepatotoxicity in its side effect profile and has been associated with drug- or toxin-induced hepatocellular injury patterns on pathologic examination.5 In the first published case of energy drink–induced hepatitis, it was found that consuming niacin in amounts as low as 300 mg/day could cause liver injury, despite previous reports suggesting 1 g/day of niacin was necessary to induce liver injury.6 Several case reports of acute hepatitis following energy drink consumption have attributed the niacin component as the underlying cause of injury.5,6,11,12
Although there are case reports associating energy drink consumption with acute hepatitis, energy drinks and acute liver failure have been linked to one another in the literature in only a handful of cases.5,6,11,12 While seemingly uncommon, the reported cases do lend credence to a relationship between liver dysfunction and chronic consumption of energy drinks ranging from weeks to years.5,6,11,12 However, our case is unique in that it describes a patient without a long-term history of energy drink consumption who reported drinking multiple energy drinks in short succession over the course of 24 hours. This is of particular interest because, to our knowledge, there are no other reported cases of binge energy drink consumption resulting in liver failure to such a degree as to warrant a liver transplant. This case emphasizes the importance of taking a thorough history of substance use, including caffeinated beverages such as energy drinks, when a patient, particularly an adolescent or young adult, presents with acute liver failure.
Moreover, our case further supports the notion that energy drinks are a public health concern that warrant further investigation.13 Despite energy drinks being readily available, their ingredients and other components are not well-studied. Recently, there has been a push for the US Food and Drug Administration to investigate caffeine content in certain energy drinks, but no such call has been made to look at other components.14
Conclusion
The clinical significance of this case is that an otherwise healthy individual may develop acute liver failure following a binge episode of energy drink consumption. As such, in patients with acute hepatic injury of unknown etiology, a thorough history of all substances used, both licit and illicit, should be part of the initial workup. Further investigation on energy drinks and their ingredients is needed to better understand the health impacts of these beverages.
Article Information
Published Online: January 16, 2024. https://doi.org/10.4088/PCC.23cr03613
© 2024 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2024;26(1):23cr03613
Submitted: July 27, 2023; accepted October 2, 2023.
To Cite: Jesse BD, Nguyen NV, Balasanova AA. Acute liver failure transplant after excessive energy drink consumption. Prim Care Companion CNS Disord. 2024;26(1):23cr03613.
Author Affiliations: College of Medicine, University of Nebraska Medical Center, Omaha, (Jesse, Nguyen); Department of Psychiatry, University of Nebraska Medical Center, Omaha (Balasanova).
Corresponding Author: Alëna A. Balasanova, MD, Department of Psychiatry, University of Nebraska Medical Center, 985578 Nebraska Medical Center, Poynter Hall, 5th Fl, Omaha, NE 68198 ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Ethical Approval: This case report was conducted in accordance with the Declaration of Helsinki. The collection and evaluation of all protected patient health information was performed in a Health Insurance Portability and Accountability Act (HIPAA)–compliant manner.
Patient Consent: The patient provided written informed consent for the information in the case report to be published. Information has been de-identified to protect anonymity.
Please sign in or purchase this PDF for $40.
Save
Cite