Objective: Evidence indicates that behavioral or drug therapy may not target underlying pathophysiologic mechanisms for chronic insomnia, possibly due to previously unrecognized high rates (30%-90%) of sleep apnea in chronic insomnia patients. Although treatment studies with positive airway pressure (PAP) demonstrate decreased severity of chronic sleep maintenance insomnia in patients with co-occurring sleep apnea, sleep-onset insomnia has not shown similar results. We hypothesized advanced PAP technology would be associated with decreased sleep-onset insomnia severity in a sample of predominantly psychiatric patients with comorbid sleep apnea.
Methods: We reviewed charts of 74 severe sleep-onset insomnia patients seen from March 2011 to August 2015, all meeting American Academy of Sleep Medicine Work Group criteria for a chronic insomnia disorder and all affirming behavioral and psychological origins for insomnia (averaging 10 of 18 indicators/patient), as well as averaging 2 or more psychiatric symptoms or conditions: depression (65.2%), anxiety (41.9%), traumatic exposure (35.1%), claustrophobia (29.7%), panic attacks (28.4%), and posttraumatic stress disorder (20.3%). All patients failed continuous or bilevel PAP and were manually titrated with auto-adjusting PAP modes (auto-bilevel and adaptive-servo ventilation). At 1-year follow-up, patients were compared through nonrandom assignment on the basis of a PAP compliance metric of > 20 h/wk (56 PAP users) versus < 20 h/wk (18 partial PAP users).
Results: PAP users showed significantly greater decreases in global insomnia severity (Hedges’ g = 1.72) and sleep-onset insomnia (g = 2.07) compared to partial users (g = 1.04 and 0.91, respectively). Both global and sleep-onset insomnia severity decreased below moderate levels in PAP users compared to partial users whose outcomes persisted at moderately severe levels.
Conclusions: In a nonrandomized controlled retrospective study, advanced PAP technology (both auto-bilevel and adaptive servo-ventilation) were associated with large decreases in insomnia severity for sleep-onset insomnia patients who strongly believed psychological factors caused their sleeplessness. PAP treatment of sleep-onset insomnia merits further investigation.
Objective: Evidence indicates that behavioral or drug therapy may not target underlying pathophysiologic mechanisms for chronic insomnia, possibly due to previously unrecognized high rates (30%-90%) of sleep apnea in chronic insomnia patients. Although treatment studies with positive airway pressure (PAP) demonstrate decreased severity of chronic sleep maintenance insomnia in patients with co-occurring sleep apnea, sleep-onset insomnia has not shown similar results. We hypothesized advanced PAP technology would be associated with decreased sleep-onset insomnia severity in a sample of predominantly psychiatric patients with comorbid sleep apnea.
Methods: We reviewed charts of 74 severe sleep-onset insomnia patients seen from March 2011 to August 2015, all meeting American Academy of Sleep Medicine Work Group criteria for a chronic insomnia disorder and all affirming behavioral and psychological origins for insomnia (averaging 10 of 18 indicators/patient), as well as averaging 2 or more psychiatric symptoms or conditions: depression (65.2%), anxiety (41.9%), traumatic exposure (35.1%), claustrophobia (29.7%), panic attacks (28.4%), and posttraumatic stress disorder (20.3%). All patients failed continuous or bilevel PAP and were manually titrated with auto-adjusting PAP modes (auto-bilevel and adaptive-servo ventilation). At 1-year follow-up, patients were compared through nonrandom assignment on the basis of a PAP compliance metric of > 20 h/wk (56 PAP users) versus < 20 h/wk (18 partial PAP users).
Results: PAP users showed significantly greater decreases in global insomnia severity (Hedges’ g = 1.72) and sleep-onset insomnia (g = 2.07) compared to partial users (g = 1.04 and 0.91, respectively). Both global and sleep-onset insomnia severity decreased below moderate levels in PAP users compared to partial users whose outcomes persisted at moderately severe levels.
Conclusions: In a nonrandomized controlled retrospective study, advanced PAP technology (both auto-bilevel and adaptive servo-ventilation) were associated with large decreases in insomnia severity for sleep-onset insomnia patients who strongly believed psychological factors caused their sleeplessness. PAP treatment of sleep-onset insomnia merits further investigation.
Prim Care Companion CNS Disord 2016;18(5):doi:10.4088/PCC.16m01980
© Copyright 2016 Physicians Postgraduate Press, Inc.
aSleep & Human Health Institute, Albuquerque, New Mexico
bMaimonides Sleep Arts & Sciences, Ltd, Albuquerque, New Mexico
cLos Alamos Medical Center, Sleep Laboratory, Los Alamos, New Mexico
dDepartment of Psychology, Mississippi State University, Starkville
eDepartment of Psychology, Baylor College of Medicine, Houston, Texas
*Corresponding author: Barry Krakow, MD, Sleep & Human Health Institute, 6739 Academy NE, Ste 380, Albuquerque, NM 87109 ([email protected]).
Chronic insomnia is extremely prevalent in primary care and psychiatric patients.1-4 Pharmacotherapy is commonly prescribed to address this vexing condition,5,6 and many individuals rely on over-the-counter sleep aids7,8 or substances.9,10 Despite widespread use of anti-insomnia drugs, therapeutic effects vary6,10; in contrast, many sleep medicine specialists and psychologists advocate cognitive-behavioral therapy for insomnia (CBT-I), a gold-standard psychological treatment.11 In primary care practice, 2 opposing arguments emerge regarding practical treatment considerations: (1) CBT-I is potent, yet largely inaccessible12 and (2) drug therapy is readily available, yet yields unsatisfactory results in many patients.13,14
Much as drug and behavioral therapies are effective for some patients,11,15,16 commentaries17-19 and reviews20-25 raise questions as to whether these paradigms attend to all underlying mechanisms of chronic insomnia. Research on the pathophysiology of chronic insomnia mostly investigates psychophysiologic factors influencing learned sleeplessness11 or sleep-regulating central nervous system receptors.26
Since 2001, we theorized another pathophysiologic mechanism for insomnia related to sleep-disordered breathing,27,28 which requires treatment distinct from CBT-I or sedatives. This mechanism is supported by mounting evidence on the high prevalence of comorbid obstructive sleep apnea (OSA) and upper airway resistance syndrome (UARS) in chronic insomnia patients23 at rates from 30% to 90% in diverse cohorts, including chronic hypnotic users,29-31 trauma survivors with posttraumatic stress disorder,27 elderly individuals,32 postmenopausal women,33 and community samples of treatment-seeking insomniacs.34,35 Consequently, sleep breathing treatment for comorbid insomnia and OSA/UARS appears to be a novel intervention in which positive airway pressure (PAP) therapy and additional modalities have all demonstrated medium to large effects on insomnia outcomes,36-40 measured on the Insomnia Severity Index (ISI).41
These therapeutic observations align with current theory on how sleep breathing events cause sleep maintenance insomnia, the most common sleep complaint in psychiatric patients.42 Difficulty staying asleep (the phrase used by patients with sleep maintenance insomnia), previously attributed solely to central nervous system hyperarousal or psychiatric distress,15,16 has also been related to the disruptive sleep fragmentation effects of OSA/UARS in 1 small study.43 In 20 classic insomnia patients with no clinical breathing symptoms and who reported psychological factors as the main cause of sleeplessness,43 19 cases of covert OSA (n = 11) and UARS (n = 8) were diagnosed, and 90% of the sample’s awakenings in the sleep laboratory (mean = 25 per patient) were preceded and provoked by sleep breathing events (ie, apneas, hypopneas, flow limitation). Reversal of sleep breathing events lessens sleep fragmentation and improves middle of the night insomnia,36,44 thus sleep maintenance insomnia may prove to be a clinically relevant target for sleep breathing treatments.
Sleep breathing treatments for sleep-onset insomnia (SOI), in contrast, have not yielded similar results to those observed in sleep maintenance insomnia patients with sleep-disordered breathing. Only 1 study37 on insomnia patients with OSA/UARS treated with PAP showed significant improvement in subjective and objective sleep-onset latency; whereas, 3 studies44-46 showed no change in sleep-onset latency. As SOI operates via well-described mechanisms11,47,48 related to psychophysiologic conditioning, poor sleep hygiene, or psychiatric distress,11,15,16 sleep breathing treatments would not be expected to resolve sleep-onset complaints.19
Moreover, scant research explicates how sleep breathing problems might cause SOI other than the obvious finding of breathing events at sleep onset, leading to arousals or awakenings.49 One theory was elaborated indirectly from a small study of untreated OSA patients, wherein Broström et al50 indicated “fear of dying” was commonly reported; Luyster et al23 cited this work in speculating how waking fear leads to psychophysiologic conditioning. Another theory (Respiratory Threat Matrix Model of Chronic Insomnia) hypothesizes racing thoughts—the omnipresent complaint linked to sleep-onset difficulties—emerge into consciousness at bedtime and thwart sleep initiation, thereby preventing both sleep and ensuing breathing events.51 Speculatively, abnormal breathing (eg, apneas, hypopneas, or flow limitation), given the life-sustaining nature of breathing itself, may transform sleep into an aversive stimulus in susceptible individuals.51

- Positive airway pressure (PAP) may provide combined therapeutic effects for patients with comorbid insomnia and sleep-disordered breathing.
- Patients treated for this comorbidity (proposed “complex insomnia” diagnosis) may fail or reject standard continuous PAP due to the anxiety triggered by expiratory pressure intolerance.
- The results of this study suggest future investigations should test whether advanced PAP devices using dual pressures are more efficacious in the treatment of global insomnia and sleep-onset insomnia in complex insomnia patients.
Although standard PAP has proven efficacious in sleep maintenance insomnia treatment,36-40,44-46,52 we often observe greater severity of continuous PAP (CPAP)-induced “claustrophobic tendencies”53-55 in SOI patients, which culminates in CPAP failure or rejection. Anecdotally, we observe a reduction in claustrophobic tendencies in patients undergoing trials with advanced PAP technology: auto-adjusting dual-pressure devices, auto-bilevel (ABPAP) or adaptive servo-ventilation (ASV-PAP), the latter mode for the diagnosis of complex sleep apnea.39 We speculate these technologies provide better results at sleep initiation, because they effectively eradicate the common problem of expiratory pressure intolerance (EPI)—the unpleasant, uncomfortable sensation provoked by attempting to exhale against the inward force of positive airway pressure.56 This distressing sensation may trigger claustrophobic tendencies53-55 in susceptible individuals, such as psychiatric patients.39 Pressure intolerance is common with CPAP, the standard fixed-pressure mode, which in past studies44-46 theoretically caused discomfort at sleep onset and negated potential therapeutic effects of PAP.39 Speculatively, CPAP-induced pressure intolerance may remind individuals of the potential underlying threat to respiration from OSA/UARS.51
In our experience, ABPAP or ASV function as a rescue strategy to promote expedient adaptation instead of requiring patients to endure repeated unpleasant exposure to standard CPAP for weeks or months. Even with extended CPAP use, adherence is not guaranteed.57 To the contrary, the experiences of repeated failed CPAP attempts can produce adverse psychophysiologic responses to any subsequent efforts to try PAP,58 and, in some cases, acute phobic responses are elicited.59
Some insomnia patients never tolerate CPAP, as recently described in a case series of 273 former CPAP failure patients (all abandoned treatment) of whom 72% subsequently reinitiated a device after completing an overnight titration with ABPAP or ASV; this advanced PAP technology was associated with elimination or prevention of claustrophobic tendencies (B.K., unpublished data, 2016). To examine manually titrated auto-adjusting PAP technology in the treatment of SOI, the current report gathered retrospective data on consecutive OSA/UARS patients who presented with a predominant pattern of severe SOI. All patients failed CPAP or BPAP at various stages and subsequently qualified for and were currently using ABPAP or ASV. We hypothesized regular PAP users would show significantly greater decreases in global or total insomnia severity and SOI compared to partial users.
METHODS
Study Subjects and Design
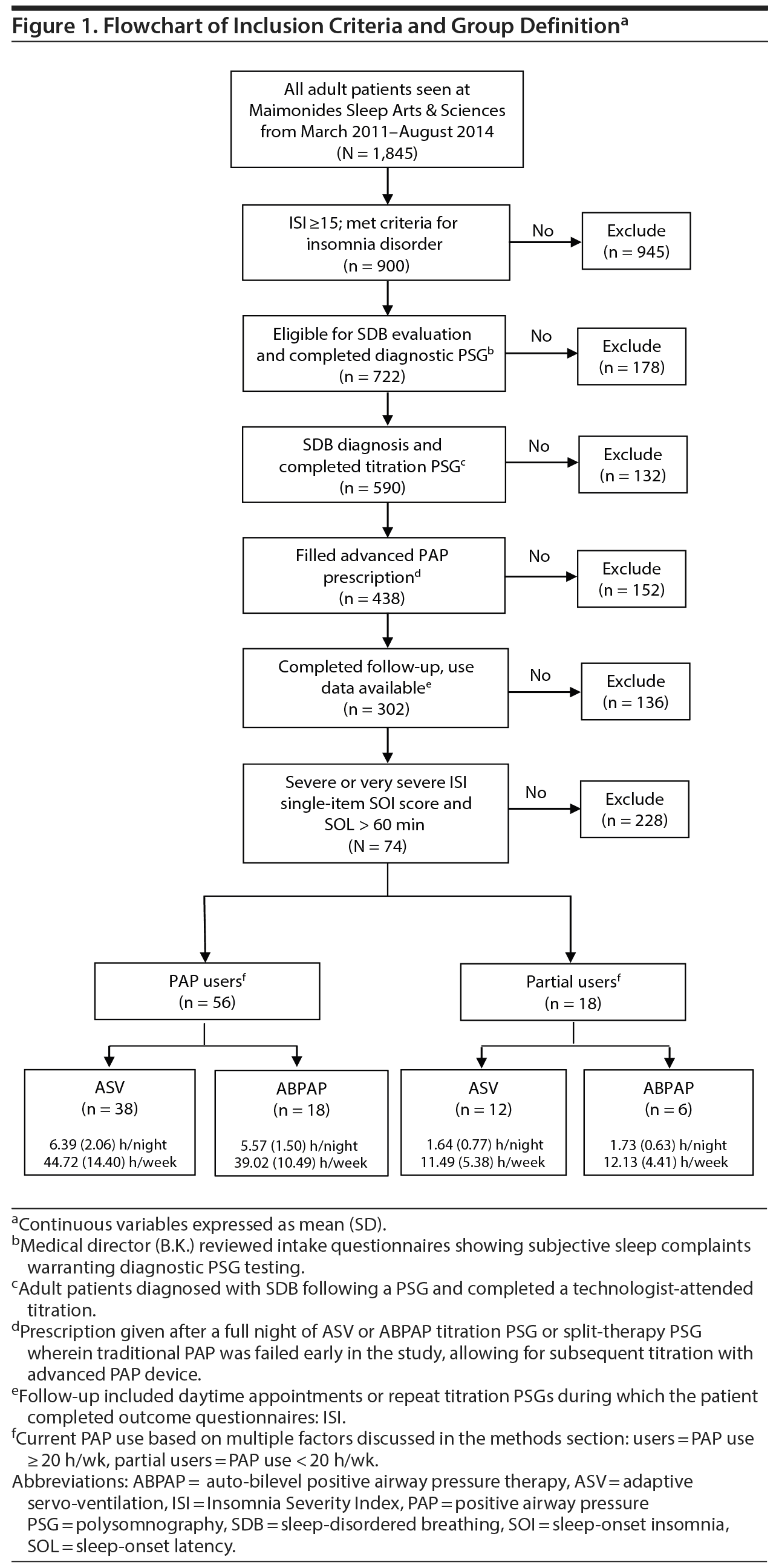
We reviewed charts of a consecutive series of treatment-seeking chronic insomnia patients at Maimonides Sleep Arts & Sciences, Ltd (March 2011 to August 2015), a private, community-based sleep medical center that specializes in the treatment of mental health patients with sleep disorders.29,60,61 All patients completed online intake surveys to measure degree of insomnia and related findings of psychophysiologic conditioning (see Supplementary Appendix 1), poor sleep hygiene, and psychiatric distress. The online intake also elicits subjective estimates of sleep-onset latency, total sleep time, total time in bed, and time awake after first sleep onset. All patients met the following inclusion criteria (Figure 1): (1) insomnia lasting longer than 6 months (chronic) with a subjective emphasis (chief insomnia complaint) of prolonged sleep-onset latency (> 60 minutes) and severe SOI symptoms; (2) severe to very severe rating on the ISI single-item SOI score; (3) self-reported daytime impairment qualifying patients for a research diagnosis of chronic insomnia disorder62; (4) self-reported multiple indicators of psychophysiologic insomnia, poor sleep hygiene, or psychiatric symptoms as presumptive causes of SOI; (5) objective diagnosis of OSA or UARS; (6) failed CPAP therapy (see Procedures for explanation of CPAP failure); (7) manual in-laboratory titration of auto-adjusting PAP technology and initiated use of ABPAP or ASV; and (8) returned for follow-up. The Los Alamos Medical Center Institutional Review Board exempted this study from medical ethical approval, as testing and therapy were routine and data were deidentified.
Additional information collected from the online intake included subjective report of past psychological disorder diagnoses and treatment; however, no formal psychiatric interviewing or instruments were used to evaluate historical complaints of psychiatric conditions. The information used in this retrospective study relies entirely on subjective reports from the patients’ intake questionnaires. As part of the intake procedures, individuals were asked to report past psychiatric conditions, use of psychotropic medications, and psychotherapy experience. Individuals were also asked to provide information concerning history of trauma and claustrophobia.
Procedures
All patients underwent diagnostic polysomnography and were scored per standards from the American Academy of Sleep Medicine (AASM).63 An OSA diagnosis required an Apnea Hypopnea Index ≥ 5 events/h, and a diagnosis of UARS required an Apnea Hypopnea Index < 5 and Respiratory Disturbance Index ≥ 15.63 Titration protocols followed AASM guidelines56 up to a point in which ABPAP or ASV was instituted, and then the algorithm for manual titration of auto-adjusting technology was applied as described previously in chronic insomnia patients.39 Patients completed ISI at baseline and follow-up, approximately 1.2 years after PAP initiation, at which time objective data downloads determined hours of use.
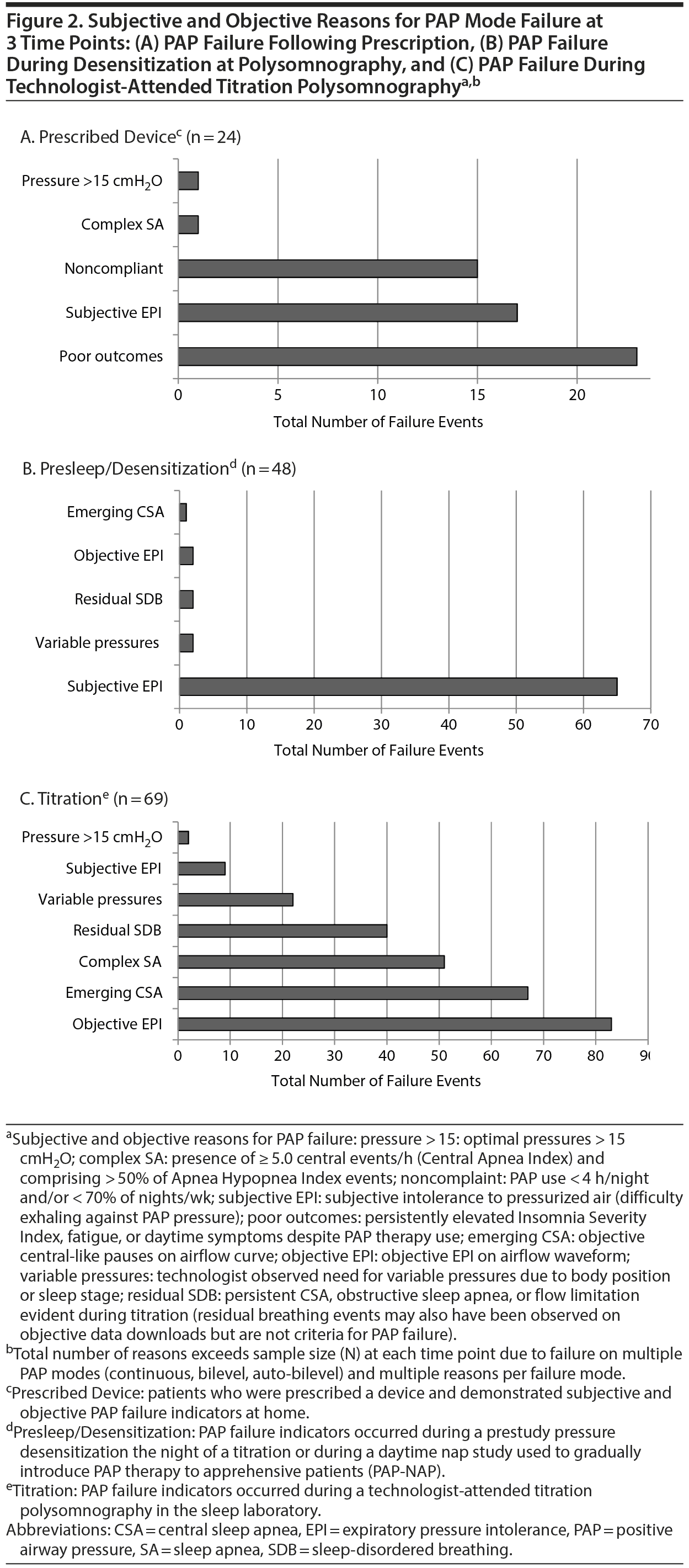
Failure with PAP therapy occurred in all patients for multiple reasons, most conspicuously due to experiences associated with claustrophobic tendencies or due to poor results with a device (Figure 2). In brief, 24 patients prescribed PAP failed due to poor outcomes, noncompliance, and subjective EPI (Figure 2A). Another 48 failed PAP during presleep desensitization in the sleep laboratory before a titration. Desensitization failure was caused by subjective EPI, at which point patients verbalized discomfort, choking, or suffocating feelings; requested a temporary halt to the procedure; and then received the option to try a different pressure delivery mode. Some patients described the EPI experience as traumatic or verging on a panic attack (Figure 2B). Nearly every patient (n = 69) also failed at least 1 traditional PAP mode (CPAP, APAP, BPAP) during a technologist-attended titration in which objective expiratory pressure intolerance eventually worsened their breathing, resulting in central apneas or other residual breathing events (persisting despite increased pressures) (Figure 2C). Among all 74 patients, 403 specific indicators (> 5 per patient) of failure or rejection manifested with various PAP modes, which ultimately led to prescriptions for ResMed ABPAP (n = 24) or ResMed ASV (n = 50) (ResMed, San Diego, California), and both types of devices received insurance coverage.
Data Analysis
Descriptive statistics characterized sociodemographic data, standard self-report sleep metrics, relevant medication use, psychiatric history, patterns of psychophysiologic conditioning, poor sleep hygiene, and proportions of CPAP failure indicators. Objective data downloads provided hours of PAP use to divide the sample into 2 groups on the basis of the general clinical practice guideline that deems compliance as ≥ 20 hours/wk and noncompliance as < 20 hours/wk. The compliant patient group was termed PAP users and the noncompliant group partial users. Changes in global insomnia severity and SOI severity were tested with repeated-measures analysis of variance for within- and between-subjects analyses. Continuous variables were expressed as mean (SD) and dichotomous variables as percentages. Hedges’ g was calculated to assess effect sizes for unequal or small samples. Statistical significance was .05. Data were analyzed using SPSS version 23.0.
RESULTS
Baseline Characteristics
All 74 patients diagnosed with OSA/UARS included PAP users (n = 56) and partial users (n = 18). Sociodemographic data indicate a sample of predominantly male, white, mildly obese, married or living with a partner, middle-aged adults (Table 1); initial outcome analyses demonstrated no significant findings for these sociodemographics. Despite prior attempts at CPAP or BPAP therapies, intake total mean (SD) ISI scores were 21.74 (3.68), at the cutoff for severe insomnia (ISI = 22), even as the majority of patients presented using prescription (68%) or over-the-counter (51%) sleep aids. Baseline difficulty falling asleep (SOI) as reported on the ISI averaged between severe and very severe categories (3.43 [0.50]) on the 0 to 4 scale. Subjective baseline sleep indices showed patients reported more than 2 hours to fall asleep and averaged less than 5 hours total sleep. There were no significant differences between PAP users and partial users except for the latter group’s lower use of over-the-counter sleep aids and shorter time in bed.
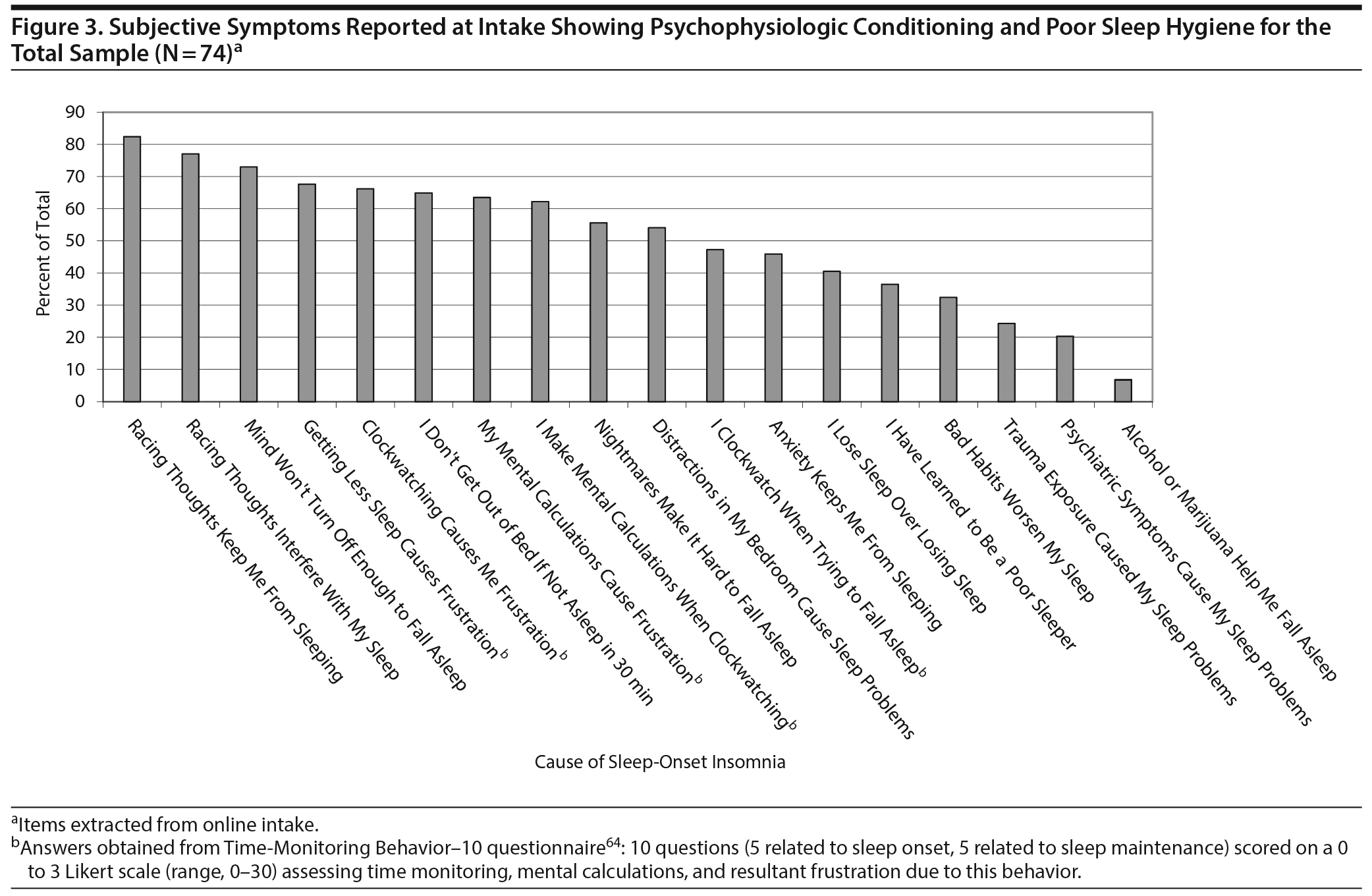
Figure 3 illustrates pervasive indicators of psychophysiologic conditioning and poor sleep hygiene, which patients directly attributed to insomnia in general or SOI in particular, such as learning to be a poor sleeper, losing sleep over losing sleep, and time monitoring behavior. Patients endorsed a mean (SD) of 9.49 (3.68) of a possible 18 indicators for maladaptive behaviors. Patients completed 3 distinct queries for bedtime ruminations, each of which was consistently endorsed by 71% to 82% of the sample as the most prevalent etiologic factor in SOI. As another presumptive explanation for their SOI, patients averaged by self-report more than 2 psychiatric disorders, symptoms, or conditions: depression (65.2%), anxiety (41.9%), traumatic exposure (35.1%), claustrophobia (29.7%), panic attacks (28.4%), posttraumatic stress disorder (20.3%), obsessive-compulsive disorder (9.5%), and bipolar disorder (5.4%), albeit no formal psychiatric evaluations were conducted in these patients.
Adherence to PAP Therapy
PAP users (n = 56) averaged a mean (SD) of 6.13 (1.92) h/night and 42.89 (13.45) h/wk, and partial users (n = 18) 1.67 (0.71) h/night and 11.70 (4.95) h/wk. There were no significant differences on the basis of ABPAP or ASV modes.
Changes in Insomnia Severity
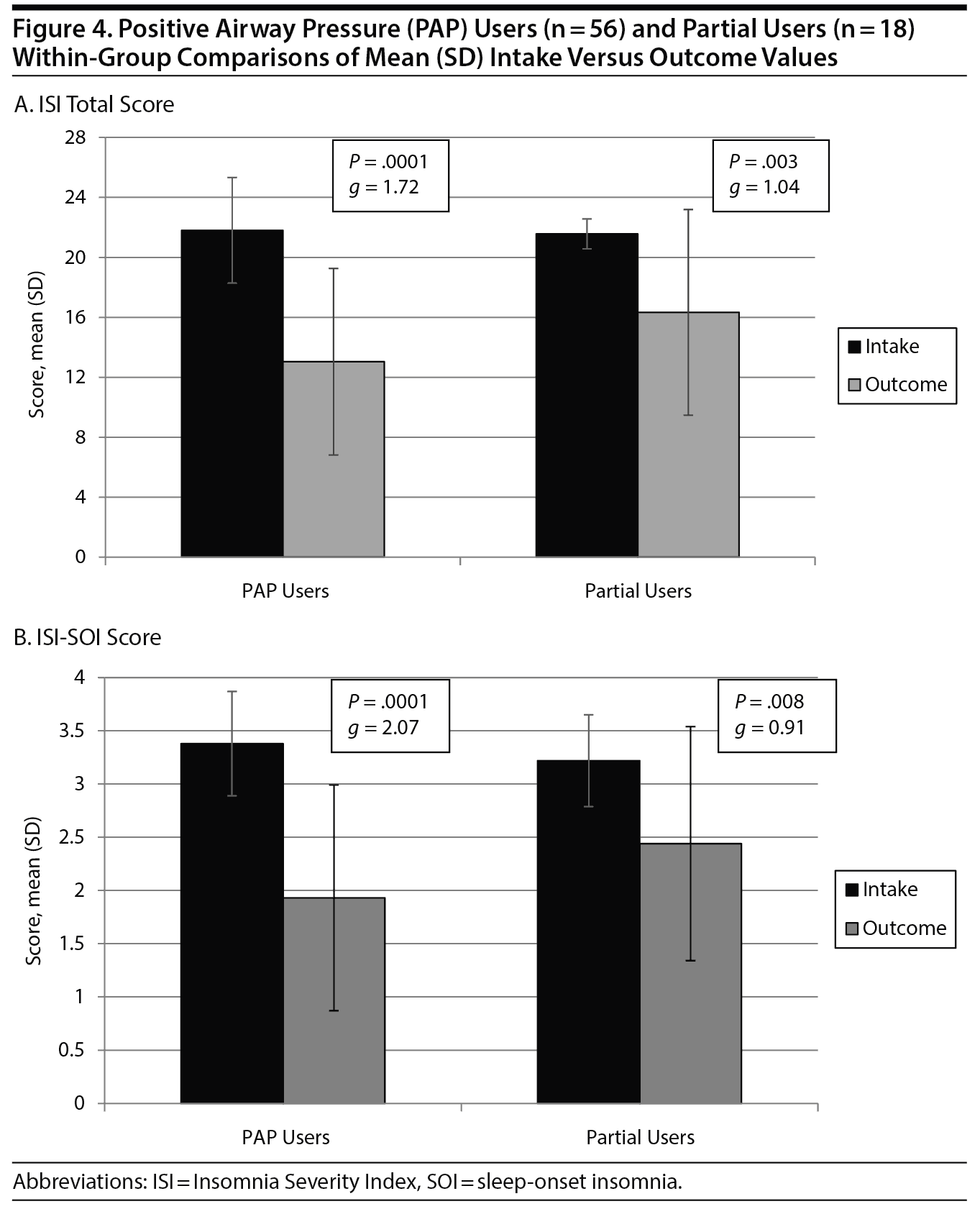
For changes in total ISI scores (Figure 4A), a significant group ×— time interaction was observed (F1,72 = 4.472; P = .04), with PAP users showing greater improvement from intake to follow-up (21.8 to 13.0, g = 1.72) compared to partial users (21.6 to 16.3, g = 1.04). Clinical insomnia severity decreased in the PAP user group to a mean outcome ISI of 13.0, which is below the currently applied ISI cutoff score of 15, equivalent to less than a moderate level of insomnia, whereas partial users remained above 15, with a mean outcome ISI of 16.3, indicative of moderate insomnia levels.
Measured by more stringent ISI clinical cutoffs, we calculated subclinical insomnia (< 11) and nonclinical insomnia (< 8). Among PAP users, 22 (39.3%) were subclinical and 10 (17.9%) were nonclinical at follow-up, whereas in the partial users, only 4 (22.2%) were subclinical and 2 (11.1%) were nonclinical.
For changes in the single-item SOI score (Figure 4B), a significant (group ×— time) interaction was observed (F1,72 = 9.597; P = .03), with PAP users showing superior results compared to partial users (g = 2.07 vs g = 0.91, respectively). For changes in clinical severity, PAP users decreased from an intake range of severe to very severe SOI to a score < 2, equivalent to less than a moderate level of difficulty falling asleep, whereas partial users showed improvement but remained at the moderately severe level.
Using an experimental ISI single-item SOI score of < 2 as the cutoff for clinical SOI, we noted 20 PAP users (35.7%) and 4 partial users (22.2%) attained an outcome SOI score < 2.
Supplemental Analyses
The use of ABPAP or ASV manifested equally large effects, with no statistical differences for mode type in either regular or irregular users. Controlling for over-the-counter sleep aids did not change results. However, at the clinical level, 51 of 74 patients were taking prescription or over-the-counter medication (Supplementary eFigure 1) for insomnia at intake, including 40 users (ASV = 27, ABPAP = 13) and 11 partial users (ASV = 6, ABPAP = 5). At follow-up, 32 of the 51 sleep aid users reported no change in insomnia medication at follow-up, whereas 16 reported a decrease in medication, including 11 users (ASV = 7, ABPAP = 4) and 5 partial users (ASV = 2, ABPAP = 3). An additional 3 patients reported an increase in medication (users = 3, ASV = 3). Three patients not using medication at intake started medication for insomnia (users = 3, ASV = 3). Proportions of change were no different between groups or types of modes, but the sample sizes were too small to detect differences.
Last, the time-in-bed analysis was confounded by the fact that more time spent in bed most likely equated to more time using PAP therapy. Thus, a patient spending more or less time in bed would appear to have a greater chance for more or less PAP use, respectively. When controlling for the time in bed variable, the analyses still manifest differences between the 2 groups.
DISCUSSION
Marked clinical changes in global insomnia severity as well as sharp decreases in SOI occurred in association with use of advanced auto-adjusting PAP technology in a predominant sample of psychiatric patients with chronic SOI of which 68% were currently using prescription sleeping pills and 51% were using over-the-counter sleep aids. Clinical improvements in the regular PAP users were consistently greater than in the nonrandomized control group of partial users. Whether these findings are a result of the use of advanced PAP technology that ameliorated patients’ difficulties with EPI and potential claustrophobic tendencies can only be determined with prospective randomized controlled studies.
It is noteworthy these patients strongly attributed their sleep-onset difficulties to numerous psychological factors, which may explain their reliance on medications; yet, despite the use of sleep aids, they presented with severe residual insomnia, which appeared to respond well to the physiologic intervention of PAP therapy. Notwithstanding, it has already been proven that other nontechnological-based treatments could yield similar benefits.11,15,16
Therefore, a question is raised as to whether combined physiologic, psychological, and pharmacologic interventions could produce additive benefits if each therapy were proven to further decrease symptoms not relieved by any individual treatment.51 Regardless, in our sample of patients, insomnia was not cured, only reduced in severity, and, therefore, further insomnia interventions such as CBT-I or sedatives would most likely yield further gains in the treatment of their residual insomnia.11,46,48,65,66
Some patients indicated racing thoughts dissipated with PAP, but these anecdotal statements were not intended for measurement due to the retrospective design. Nonetheless, these assertions are congruent with the aforementioned Respiratory Threat Matrix Model of Chronic Insomnia.51 Researching this theory is difficult given the subtlety of the mental functions described in the model. Nevertheless, prospective randomized controlled studies with larger sample sizes could assess whether cognitive activation at bedtime increases or decreases with PAP, regardless of underlying pathophysiologic mechanisms. The potential relationship between racing thoughts and claustrophobic tendencies in these types of patients also merits investigation, including how various PAP delivery modes affect these complaints. Finally, complementary studies could examine recently reported observations that sleep apnea patients with SOI are less likely to attain adherence with PAP therapy.44
Limitations
To our knowledge, although this study is the first to demonstrate a marked association between PAP use and improvement in SOI symptoms, the research suffers from several weaknesses in its retrospective, nonrandomized controlled design in a medium-sized sample of patients. Selection bias occurred for several reasons, as we focused only on patients using PAP therapy; therefore, sleep-onset insomniacs who presented to our center and chose other pathways such as psychological treatments or other medications were excluded, and some patients refused sleep testing or dropped out of care. Some patients completed all steps that could have included them in the study, but with no follow-up measures, we could not determine PAP therapy use. Another possible confound is the use of only the most recent follow-up. While we make the assumption that treatment-seeking patients attend clinic appointments much of the time to solve a problem, it is also true that such patients are sticking with PAP therapy and therefore are probably receiving some benefit; the former issue of problem-solving might underestimate the benefits of PAP, whereas, the latter issue of maintaining treatment might overestimate the benefits of PAP.
Our sample of patients also did not complete formal psychiatric evaluations to confirm and clarify their psychiatric distress and its potential impact on sleep, and, during the time period covered by the retrospective design, we did not track changes in their psychiatric diagnoses or treatments, both of which could have affected their insomnia outcomes. Moreover, use of sleep diaries to track progress would have yielded more precise information about patient responses. Most importantly, without a prospective, randomized controlled protocol and without intercurrent information on the longitudinal progression for each patient—for example, any other medications or treatments that might have favorably influenced insomnia—we can only note the association between advanced PAP technology and observed decreases in SOI. Patients may have initiated or adjusted medications for insomnia during this time period, but our data only include their baseline and final follow-up drugs, whereas no longitudinal tracking of medications occurred. Conceivably, patients also may have accessed salient information to gain knowledge about psychological therapies for insomnia, including educational material at our sleep center. Last, speculatively, PAP may provide relaxation benefits as a placebo, irrespective of its evidence-based, therapeutic effects.
In sum, emerging research on comorbid OSA/UARS and insomnia (proposed “complex insomnia” designation)27 is opening new therapeutic pathways via physiologic interventions for a vexing health condition, commonly explicated through psychological theories11,47 and conventionally treated with medications.5-8,67 The current study, while at a lower level of evidence, suggests the need for research with larger samples to determine the full impact or lack thereof regarding sleep breathing treatments on 3 cardinal complaints of chronic insomnia disorder patients: difficulty falling asleep, difficulty staying asleep, and early morning awakenings. This research must include formal psychiatric evaluations to clarify the impact of mental health symptoms and disorders on insomnia outcomes. Prospective studies that examine multimodal therapies such as the combination of PAP therapy and CBT-I or sedatives will most likely prove the most illuminating and clinically relevant.65,66
Submitted: May 3, 2016; accepted August 1, 2016.
Published online: September 29, 2016.
Potential conflicts of interest: Dr Krakow owns and operates www.nightmaretreatment.com, www.ptsdsleepclinic.com, www.sleeptreatment.com, www.sleepdynamictherapy.com, www.soundsleepsoundmind.com, and www.nocturiacures.com; is the medical director of a national durable medical equipment company Classic Sleep Care for which his sole functions are consultation and quality assurance (he has no patient encounters and does not benefit from the sale of any durable medical equipment); and markets and sells 3 books for sleep disorder patients: Insomnia Cures, Turning Nightmares into Dreams, and Sound Sleep, Sound Mind: 7 Keys to Sleeping Through the Night. He owns and operates one commercial sleep center, Maimonides Sleep Arts & Sciences, Ltd, and conducts continuing medical education/continuing education unit educational programs for medical and mental health providers to learn about sleep disorders. Sometimes, these programs involve the attendee paying a fee directly to his center. Other times, he conducts the workshops at other locations, which may be paid for by vendors such as Respironics and ResMed, or other institutions such as the US Army Medical Department Center and School, VA Medical Center, and regional sleep center conferences. He is president of a nonprofit sleep research center, the Sleep & Human Health Institute (www.shhi.org), that occasionally provides consultation services or receives grants for pilot studies, the most recent of which were ResMed ~$400,000 January 2015 (funding for randomized controlled trial of treatment in insomnia patients) and Respironics $50,000 January 2009 (study on prevalence of sleep disordered breathing in insomnia patients). Dr Nadorff, Mr Ulibarri, and Ms McIver report no conflicts of interest related to the subject of this article.
Funding/support: None.
Acknowledgments: The authors are grateful to the Los Alamos Medical Center and the Los Alamos Medical Center Sleep Laboratory for administrative and clinical assistance in the completion of this research project.
Supplementary material: See accompanying pages.
REFERENCES
1. Doroudgar S, Chou TI, Yu J, et al. Evaluation of trazodone and quetiapine for insomnia: an observational study in psychiatric inpatients. Prim Care Companion CNS Disord. 2013;15(6):doi:10.4088/PCC.13m01558. PubMed
2. Krystal AD, McCall WV, Fava M, et al. Eszopiclone treatment for insomnia: effect size comparisons in patients with primary insomnia and insomnia with medical and psychiatric comorbidity. Prim Care Companion CNS Disord. 2012;14(4):doi:10.4088/PCC11m01296. PubMed
3. Tian H, Abouzaid S, Gabriel S, et al. Resource utilization and costs associated with insomnia treatment in patients with major depressive disorder. Prim Care Companion CNS Disord. 2012;14(5):doi:10.4088/PCC.12m01374. PubMed
4. Falloon K, Arroll B, Elley CR, et al. The assessment and management of insomnia in primary care. BMJ. 2011;342:d2899. PubMed doi:10.1136/bmj.d2899
5. Hajak G, Geisler P. Experience with zolpidem ‘ as needed’ in primary care settings. CNS Drugs. 2004;18(suppl 1):35-40, discussion 41, 43-45. PubMed doi:10.2165/00023210-200418001-00007
6. Omvik S, Pallesen S, Bjorvatn B, et al. Patient characteristics and predictors of sleep medication use. Int Clin Psychopharmacol. 2010;25(2):91-100. PubMed doi:10.1097/YIC.0b013e328334e5e6
7. Wilson JF. In the clinic. Insomnia. Ann Intern Med. 2008;148(1):ITC13-1-ITC13-16. PubMed
8. Culpepper L, Wingertzahn MA. Over-the-counter agents for the treatment of occasional disturbed sleep or transient insomnia: a systematic review of efficacy and safety. Prim Care Companion CNS Disord. 2015;17(6):doi:10.4088/PCC.15r01798. PubMed
9. Brower KJ, Aldrich MS, Robinson EA, et al. Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry. 2001;158(3):399-404. PubMed doi:10.1176/appi.ajp.158.3.399
10. Johnson JE. Insomnia, alcohol, and over-the-counter drug use in old-old urban women. J Community Health Nurs. 1997;14(3):181-188. PubMed doi:10.1207/s15327655jchn1403_5
11. Bootzin RR, Perlis ML. Nonpharmacologic treatments of insomnia. J Clin Psychiatry. 1992;53(suppl 6):37-41. PubMed
12. Karlin BE, Trockel M, Taylor CB, et al. National dissemination of cognitive behavioral therapy for insomnia in veterans: therapist- and patient-level outcomes. J Consult Clin Psychol. 2013;81(5):912-917. PubMed doi:10.1037/a0032554
13. Kripke DF. Chronic hypnotic use: deadly risks, doubtful benefit. Sleep Med Rev. 2000;4(1):5-20. PubMed doi:10.1053/smrv.1999.0076
14. Mattila T, Stoyanova V, Elferink A, et al. Insomnia medication: do published studies reflect the complete picture of efficacy and safety? Eur Neuropsychopharmacol. 2011;21(7):500-507. PubMed doi:10.1016/j.euroneuro.2010.10.005
15. Roehrs T, Roth T. Insomnia pharmacotherapy. Neurotherapeutics. 2012;9(4):728-738. PubMed doi:10.1007/s13311-012-0148-3
16. Roth T, Franklin M, Bramley TJ. The state of insomnia and emerging trends. Am J Manag Care. 2007;13(suppl 5):S117-S120. PubMed
17. Pigeon WR, Sateia MJ. Is insomnia a breathing disorder? Sleep. 2012;35(12):1589-1590. PubMed
18. Collop N. I don’ t sleep because I can’ t breathe. Sleep Med. 2013;14(9):807. PubMed doi:10.1016/j.sleep.2013.04.012
19. Lanier WL, Ramar K. Sleep medication failure and newly diagnosed obstructive sleep apnea: the role of brain function modulation by muscle afferent activity. Mayo Clin Proc. 2014;89(12):1591-1595. PubMed doi:10.1016/j.mayocp.2014.10.009
20. Bayon V, Léger D. Insomnia and sleep apnea [in French]. Rev Mal Respir. 2014;31(2):181-188. PubMed doi:10.1016/j.rmr.2013.09.020
21. Al-Jawder SE, Bahammam AS. Comorbid insomnia in sleep-related breathing disorders: an under-recognized association. Sleep Breath. 2012;16(2):295-304. PubMed doi:10.1007/s11325-011-0513-1
22. Wickwire EM, Collop NA. Insomnia and sleep-related breathing disorders. Chest. 2010;137(6):1449-1463. PubMed doi:10.1378/chest.09-1485
23. Luyster FS, Buysse DJ, Strollo PJ Jr. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. J Clin Sleep Med. 2010;6(2):196-204. PubMed
24. Benetó A, Gomez-Siurana E, Rubio-Sanchez P. Comorbidity between sleep apnea and insomnia. Sleep Med Rev. 2009;13(4):287-293. PubMed doi:10.1016/j.smrv.2008.09.006
25. Lavie P. Insomnia and sleep-disordered breathing. Sleep Med. 2007;8(suppl 4):S21-S25. PubMed doi:10.1016/S1389-9457(08)70005-4
26. Zisapel N. Drugs for insomnia. Expert Opin Emerg Drugs. 2012;17(3):299-317. PubMed doi:10.1517/14728214.2012.690735
27. Krakow B, Melendrez D, Pedersen B, et al. Complex insomnia: insomnia and sleep-disordered breathing in a consecutive series of crime victims with nightmares and PTSD. Biol Psychiatry. 2001;49(11):948-953. PubMed doi:10.1016/S0006-3223(00)01087-8
28. Krakow B, Melendrez D, Warner TD, et al. To breathe, perchance to sleep: sleep-disordered breathing and chronic insomnia among trauma survivors. Sleep Breath. 2002;6(4):189-202. PubMed doi:10.1055/s-2002-36593
29. Krakow B, Ulibarri VA, McIver ND. Pharmacotherapeutic failure in a large cohort of patients with insomnia presenting to a sleep medicine center and laboratory: subjective pretest predictions and objective diagnoses. Mayo Clin Proc. 2014;89(12):1608-1620. PubMed doi:10.1016/j.mayocp.2014.04.032
30. Krakow B, Ulibarri VA, Romero EA. Patients with treatment-resistant insomnia taking nightly prescription medications for sleep: a retrospective assessment of diagnostic and treatment variables. Prim Care Companion J Clin Psychiatry. 2010;12(4):doi:10.4088/PCC.09m00873. PubMed
31. Krakow B, Ulibarri VA, Romero E. Persistent insomnia in chronic hypnotic users presenting to a sleep medical center: a retrospective chart review of 137 consecutive patients. J Nerv Ment Dis. 2010;198(10):734-741. PubMed doi:10.1097/NMD.0b013e3181f4aca1
32. Lichstein KL, Riedel BW, Lester KW, et al. Occult sleep apnea in a recruited sample of older adults with insomnia. J Consult Clin Psychol. 1999;67(3):405-410. PubMed doi:10.1037/0022-006X.67.3.405
33. Guilleminault C, Palombini L, Poyares D, et al. Chronic insomnia, postmenopausal women, and sleep disordered breathing, part 1: frequency of sleep disordered breathing in a cohort. J Psychosom Res. 2002;53(1):611-615. PubMed doi:10.1016/S0022-3999(02)00445-2
34. Ong JC, Gress JL, San Pedro-Salcedo MG, et al. Frequency and predictors of obstructive sleep apnea among individuals with major depressive disorder and insomnia. J Psychosom Res. 2009;67(2):135-141. PubMed doi:10.1016/j.jpsychores.2009.03.011
35. Wong SH, Ng BY. Review of sleep studies of patients with chronic insomnia at a sleep disorder unit. Singapore Med J. 2015;56(6):317-323. PubMed doi:10.11622/smedj.2015089
36. Glidewell RN, Renn BN, Roby E, et al. Predictors and patterns of insomnia symptoms in OSA before and after PAP therapy. Sleep Med. 2014;15(8):899-905. PubMed doi:10.1016/j.sleep.2014.05.001
37. Krakow B, Melendrez D, Lee SA, et al. Refractory insomnia and sleep-disordered breathing: a pilot study. Sleep Breath. 2004;8(1):15-29. PubMed doi:10.1007/s11325-004-0015-5
38. Krakow B, Melendrez D, Sisley B, et al. Nasal dilator strip therapy for chronic sleep-maintenance insomnia and symptoms of sleep-disordered breathing: a randomized controlled trial. Sleep Breath. 2006;10(1):16-28. PubMed doi:10.1007/s11325-005-0037-7
39. Krakow B, Ulibarri VA, Romero EA, et al. Adaptive servo-ventilation therapy in a case series of patients with co-morbid insomnia and sleep apnea. J Sleep Disord Treat Care. 2013;2:1-10.
40. Nguyên XL, Rakotonanahary D, Chaskalovic J, et al. Insomnia related to sleep apnoea: effect of long-term auto-adjusting positive airway pressure treatment. Eur Respir J. 2013;41(3):593-600. PubMed doi:10.1183/09031936.00080011
41. Bastien CH, Valliרres A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297-307. PubMed doi:10.1016/S1389-9457(00)00065-4
42. Winokur A. The relationship between sleep disturbances and psychiatric disorders: introduction and overview. Psychiatr Clin North Am. 2015;38(4):603-614. PubMed doi:10.1016/j.psc.2015.07.001
43. Krakow B, Romero E, Ulibarri VA, et al. Prospective assessment of nocturnal awakenings in a case series of treatment-seeking chronic insomnia patients: a pilot study of subjective and objective causes. Sleep. 2012;35(12):1685-1692. PubMed
44. Björnsdóttir E, Janson C, Sigurdsson JF, et al. Symptoms of insomnia among patients with obstructive sleep apnea before and after two years of positive airway pressure treatment. Sleep. 2013;36(12):1901-1909. PubMed
45. Guilleminault C, Palombini L, Poyares D, et al. Chronic insomnia, premenopausal women and sleep disordered breathing, part 2: comparison of nondrug treatment trials in normal breathing and UARS post menopausal women complaining of chronic insomnia. J Psychosom Res. 2002;53(1):617-623. PubMed doi:10.1016/S0022-3999(02)00463-4
46. Guilleminault C, Davis K, Huynh NT. Prospective randomized study of patients with insomnia and mild sleep disordered breathing. Sleep. 2008;31(11):1527-1533. PubMed
47. Sateia MJ, Doghramji K, Hauri PJ, et al. Evaluation of chronic insomnia: an American Academy of Sleep Medicine review. Sleep. 2000;23(2):243-308. PubMed
48. Yang CM, Liao YS, Lin CM, et al. Psychological and behavioral factors in patients with comorbid obstructive sleep apnea and insomnia. J Psychosom Res. 2011;70(4):355-361. PubMed doi:10.1016/j.jpsychores.2010.12.005
49. Guilleminault C, Eldridge FL, Dement WC. Insomnia with sleep apnea: a new syndrome. Science. 1973;181(4102):856-858. PubMed doi:10.1126/science.181.4102.856
50. Broström A, Johansson P, Strömberg A, et al. Obstructive sleep apnoea syndrome-patients’ perceptions of their sleep and its effects on their life situation. J Adv Nurs. 2007;57(3):318-327. PubMed doi:10.1111/j.1365-2648.2006.04110.x
51. Krakow B. Sound Sleep, Sound Mind: 7 Keys to Sleeping Through the Night. Hoboken, NJ: John Wiley & Sons, Inc; 2007.
52. Krakow B, Lowry C, Germain A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. 2000;49(5):291-298. PubMed doi:10.1016/S0022-3999(00)00147-1
53. Chasens ER, Pack AI, Maislin G, et al. Claustrophobia and adherence to CPAP treatment. West J Nurs Res. 2005;27(3):307-321. PubMed doi:10.1177/0193945904273283
54. Edmonds JC, Yang H, King TS, et al. Claustrophobic tendencies and continuous positive airway pressure therapy non-adherence in adults with obstructive sleep apnea. Heart Lung. 2015;44(2):100-106. PubMed doi:10.1016/j.hrtlng.2015.01.002
55. Means MK, Edinger JD. Graded exposure therapy for addressing claustrophobic reactions to continuous positive airway pressure: a case series report. Behav Sleep Med. 2007;5(2):105-116. PubMed doi:10.1080/15402000701190572
56. Kushida CA, Chediak A, Berry RB, et al; Positive Airway Pressure Titration Task Force; American Academy of Sleep Medicine. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157-171. PubMed
57. Krakow B, Ulibarri VA, Foley-Shea MR, et al. Adherence and subthreshold adherence in sleep apnea subjects receiving positive airway pressure therapy: a retrospective study evaluating differences in adherence versus use. Respir Care. 2016;61(8):1023-1032. PubMed doi:10.4187/respcare.04538
58. Edinger JD, Radtke RA. Use of in vivo desensitization to treat a patient’s claustrophobic response to nasal CPAP. Sleep. 1993;16(7):678-680. PubMed
59. Casas I, de la Calzada MD, Guitart M, et al. Diagnosis and treatment of the phobia due to treatment with air using nasal continuous pressure [in Spanish]. Rev Neurol. 2000;30(6):593-596. PubMed
60. Krakow B, Ulibarri V, Melendrez D, et al. A daytime, abbreviated cardio-respiratory sleep study (CPT 95807-52) to acclimate insomnia patients with sleep disordered breathing to positive airway pressure (PAP-NAP). J Clin Sleep Med. 2008;4(3):212-222. PubMed
61. Krakow B, Ulibarri VA. Prevalence of sleep breathing complaints reported by treatment-seeking chronic insomnia disorder patients on presentation to a sleep medical center: a preliminary report. Sleep Breath. 2013;17(1):317-322. PubMed doi:10.1007/s11325-012-0694-2
62. Edinger JD, Bonnet MH, Bootzin RR, et al; American Academy of Sleep Medicine Work Group. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567-1596. PubMed
63. Berry RB, Budhiraja R, Gottlieb DJ, et al; American Academy of Sleep Medicine; Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J Clin Sleep Med. 2012;8(5):597-619. PubMed
64. Krakow B, Krakow JJ, Ulibarri VA, et al. Nocturnal time monitoring behavior (“clock-watching”) in patients presenting to a sleep medical center with insomnia and posttraumatic stress symptoms. J Nerv Ment Dis. 2012 Sep;200(9):821-5. PubMed doi: 10.1097/NMD.0b013e318266bba3
65. Edinger JD, Simmons B, Goelz K, et al. A pilot test of an online cognitive-behavioral insomnia therapy for patients with comorbid insomnia and sleep apnea. Sleep. 2015;38[abstract supplement]:A236.
66. Ong JC, Crawford MR, Kong A, et al. Management of obstructive sleep apnea and comorbid insomnia: a mixed-methods evaluation [published online ahead of print December 15, 2015]. Behav Sleep Med. PubMed doi:10.1080/15402002.2015.1087000
67. Bertisch SM, Herzig SJ, Winkelman JW, et al. National use of prescription medications for insomnia: NHANES 1999-2010. Sleep. 2014;37(2):343-349. PubMed
Please sign in or purchase this PDF for $40.00.
Save
Cite