ABSTRACT
Importance: The prompt effective treatment of acute agitation among patients with schizophrenia or bipolar disorder can alleviate distressing symptoms for the patient and decrease the risk of escalation to aggression and the potential for serious harm to the patient, health care providers, and others.
Observations: A commonly used approach for the management of acute agitation has been the intramuscular administration of antipsychotic medications and/or benzodiazepines. However, US Food and Drug Administration–approved treatments with alternative routes of delivery now include inhaled loxapine powder and, more recently, dexmedetomidine sublingual film. Two formulations of intranasal olanzapine for acute agitation are in development.
Conclusions and Relevance: Intranasal formulations offer the potential for favorable pharmacokinetics and onset of action combined with ease of delivery obviating the need for injections and are thus consistent with patient-centered factors such as preference and self-administration. In this review, alternative methods of medication delivery are discussed, with an emphasis on the potential for intranasal administration to treat acute agitation in adult patients with schizophrenia or bipolar disorder.
Prim Care Companion CNS Disord 2024;26(1):23nr03596
Author affiliations are listed at the end of this article.
Acute agitation among patients with psychiatric conditions is seen in general emergency departments, psychiatric emergency departments, inpatient psychiatric units,1 long-term care,2 and community settings. In the glossary of technical terms contained in the original iteration of the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders, psychomotor agitation is defined as “excessive motor activity associated with a feeling of inner tension. The activity is usually non-productive and repetitious and consists of behaviors such as pacing, fidgeting, wringing of the hands, pulling of clothes, and inability to sit still.”3 Self-reported symptoms from patients with schizophrenia and bipolar disorder living in community settings include reports of feeling uneasy, restless, or nervous.4
Acute agitation is a unique state separate from but may progress to aggression or violence.1,5 Not all patients who are agitated become aggressive or violent, and not all events of aggression or violence are preceded by agitation, but escalation of severity of agitation can lead to aggression or violence.1 In a study6 of patients admitted to a psychiatric hospital, more than 90% experienced moderate to severe levels of aggressivity during an episode of agitation, and while most psychiatric patients are not violent, schizophrenia and bipolar disorder increase the risk of violence.7 Prevalence of aggressive or violent behavior is higher among patients with schizophrenia or bipolar disorder, approximately 10%–12% varying by population, than in the general population, approximately 2%. Patients with schizophrenia, bipolar disorder, and other psychotic disorders are responsible for the majority of aggressions/assaults in psychiatric inpatient wards, and a study reported that aggression was the presenting condition in 26% of psychiatric emergency department visits.7,8 In emergency care settings, agitation may also be associated with general medical conditions, as well as substance use or withdrawal (eg, alcohol, cocaine, methamphetamine, and other street drugs).9 In a prospective study10 of agitation among patients treated in an urban emergency department, causes of agitation were identified as alcohol related (83% of cases), drugs other than alcohol (12%), psychiatric (20%), and medical (11%). Finally, acute agitation can develop in other conditions, including Alzheimer’s disease or dementia, with studies2,11–13 reporting prevalence ranging from 22% to 68% in community and long-term care settings. The diverse etiologies and treatment options for the many causes of acute agitation are beyond the scope of this review, which will focus on acute agitation in the context of schizophrenia and bipolar disorder.
In addition to being an important clinical concern, agitation is uncomfortable for the individual.1,4 Hence, it is imperative to treat agitation in order to reduce distress and decrease the risk of escalation to aggressive or violent behavior. Delayed or inadequate treatment can lead to physical and psychological suffering for the patient, other patients or individuals in the care setting, and caregivers, including injury to health care providers.7 Moreover, inadequate treatment may result in other negative consequences for the patient, such as the intervention of police and the criminal justice system that could result in incarceration and potentially further delays in the effective treatment of any underlying psychiatric condition.
Several international guidelines support a broadly staged but flexible approach to the treatment of acute agitation.1,14–16 Initial steps include identification of the agitated patient, ongoing assessment of safety for the patient, staff, and others, along with initiation of an assessment of severity and cause of the agitation. Verbal de-escalation techniques in an appropriate environment are preferred over immediate pharmacologic intervention. However, when these strategies are not successful or feasible, medication may be an additional tool as part of the continued de-escalation efforts. The goal of medical therapy is to calm the patient without excessive sedation, using a fast-acting medication with minimal side effects.17 Patient consent and involvement in the selection of the type and route of administration as much as possible are critical principles for pharmacologic intervention.1,17 Whenever possible, noninvasive routes of administration are preferred over intramuscular (IM) injections. The use of physical restraint or seclusion may be indicated under certain conditions, though there is the potential for negative physical and psychological impacts for both agitated patients and health care providers.1,15
The early identification and treatment of agitation in mild or moderate states may help to lower the risk of escalation to aggressive or violent behavior requiring more invasive interventions.18 Unfortunately, evidence suggests that coercive measures continue to be common in clinical practice. A study of the management of acute agitation at psychiatric emergency departments in European countries reported that a majority (59.5%) of episodes involved the use of restraint or seclusion, suggesting that opportunities may have been missed to treat lower-acuity episodes that may have been responsive to less coercive interventions.18
Different strategies are used to manage acute agitation versus managing risk of future aggressive behavior. Hostility, as measured in the Positive and Negative Symptom Scale (PANSS), has been used as a marker for aggressive behavior and has been evaluated in analyses examining specific antihostility action of medications over a longer term.19 Long-term management of aggressive behavior is beyond the scope of this article, which will focus on acute agitation.
The current commonly used medical interventions for acute agitation have limitations.20 This review focuses on advances in formulations using alternative routes of administration that have the potential to offer effective and less invasive options with favorable safety profiles to treat acute agitation in patients with schizophrenia or bipolar disorder.
UNMET MEDICAL NEED
Current Usual Care
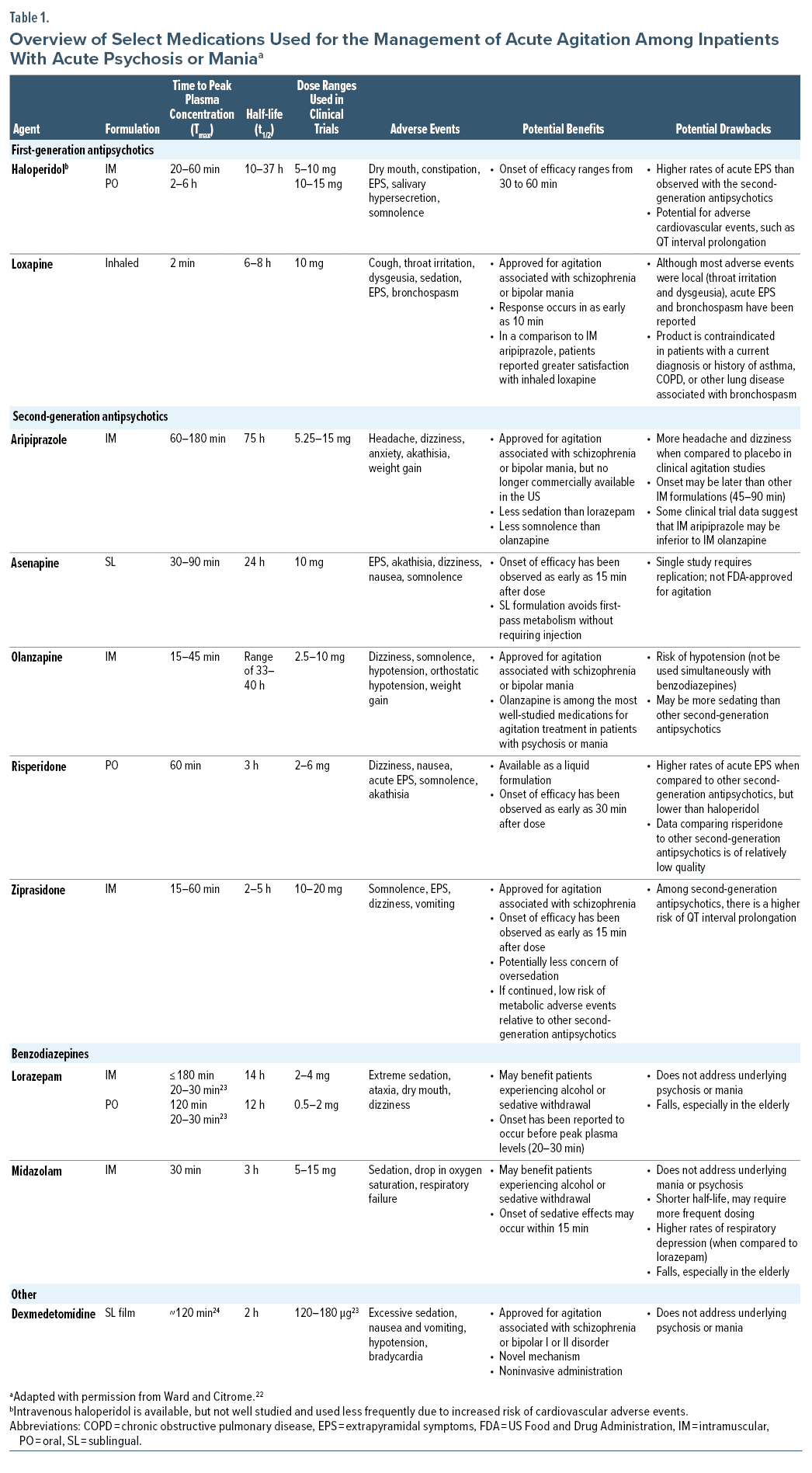
Current options for treatment of acute agitation include first-generation antipsychotics (eg, haloperidol, loxapine), second-generation antipsychotics (eg, aripiprazole, olanzapine, asenapine, ziprasidone, risperidone), and benzodiazepines (eg, lorazepam, midazolam), though not all treatments are approved by regulatory agencies for acute agitation (Table 1).21,22 Ketamine, a noncompetitive N-methyl-d-aspartate (NMDA) receptor antagonist, has been used for in-hospital and emergency department treatment of agitation, though its use is associated with elevated rates of sedation and need for intubation and potential for worsening of psychosis.25–29
For patients with schizophrenia or bipolar disorder, antipsychotics are preferred over benzodiazepines, as they address the underlying psychiatric disorder.21,23 If the patient cannot cooperate with oral medications, short-acting IM second-generation antipsychotics, such as IM ziprasidone or IM olanzapine, are preferred for the acute control of agitation; IM aripiprazole appears slightly less efficacious than other second-generation antipsychotics.21,23,30 IM second-generation antipsychotics are superior to IM haloperidol in terms of tolerability and perhaps efficacy, but administration of IM haloperidol remains common.29 For individuals with dementia, antipsychotics have been used “off-label” as a treatment of agitation, as-needed use of IM olanzapine and aripiprazole has been studied in registrational studies, and on-going use (not “as needed”) of oral brexpiprazole has received US Food and Drug Administration (FDA) approval for the treatment of agitation associated with dementia due to Alzheimer’s disease.31–34 Benzodiazepines are commonly used when an initial dose of an antipsychotic does not adequately control the agitation or if acute withdrawal from alcohol or sedatives is suspected.7,21 Although the FDA has approved non-IM formulations of loxapine (inhaled) and dexmedetomidine (sublingual) for the treatment of agitation associated with schizophrenia or bipolar disorder, both must be administered under the supervision of a health care professional.35,36
While IM formulations predominate in the treatment of acute agitation, unmet medical needs remain. In particular, there is a need for treatment options that can be used quickly in the community setting to help avoid progression to aggression. Considerable interest and research have been invested in formulations with alternative routes of delivery.
Three groupings of medication interventions based on formulation were identified by a panel of international experts on agitation20: IM/intravenous (IV), oral, and inhaled, and each formulation was evaluated and scored in terms of 9 attributes:
(1) Rapid onset of action
(2) Calmness without sedation
(3) Easy to administer
(4) Noninvasive route of administration
(5) Noncoercive and nontraumatic delivery
(6) Good safety profile
(7) Favorable tolerability
(8) Adequate patient preference
(9) Promotion of long-term adherence to treatment of the underlying condition
IM and IV formulations scored high in rapidness of action but relatively low otherwise, primarily because of their coercive and invasive nature. Oral formulations scored high for their ease of administration and noninvasive and nontraumatic characteristics but scored below average for rapidness of action. Inhaled formulations were found to have beneficial attributes of the other 2 groups, comparable time to onset to IM and IV formulations, and comparable safety/tolerability, noninvasive/nontraumatic delivery, and patient preference attributes to oral formulations. Of note, oral agents that are absorbed in the oral mucosa can also have a fast onset of action.20,37,38
As discussed here, intranasal formulations in development could share many advantages of inhaled or injected treatment options for agitation, while potentially avoiding some of the barriers for use at home or in community settings where a health care professional may not be present.
Patient Self-Management
A growing understanding of the negative impacts of coercive interventions on both patients and health care professionals places additional emphasis on the need for treatments that are noncoercive, noninvasive, and patient-centered.39,40 In a qualitative study among hospitalized patients with schizophrenia or bipolar disorder, patients reported that medication self-management increased their autonomy, confidence, self-reliance, appreciation, and satisfaction.41 Shared decision making with patients supports better engagement, decision making, and treatment adherence and thus improved outcomes.42
All currently approved treatment options with an indication for treatment of acute agitation require either administration or supervision by a health care professional, leaving an unmet need for approved agents that can be used at home. Indeed, patients are actively managing episodes of acute agitation with slower-acting medication on their own or with the help of family members and other care partners in the community setting.4 A European survey of 583 patients with schizophrenia and bipolar disorder of their experience of agitation in the community setting revealed that the majority (71%) were always or sometimes aware of becoming agitated and 61% were always or sometimes aware of agitation triggers. A majority, 55% of patients with schizophrenia and 66% of patients with bipolar disorder, also reported taking oral medications to cope with their episodes of agitation,4 illustrating common real-world self-treatment. This unmet medical need for a treatment approved for use in the community represents an opportunity to support patients to self-manage agitation, particularly in early stages, with the potential to relieve symptoms and reduce the risk of progression to higher severity states and a need for more intensive care.
Currently Available Rapid-Acting Formulations for Noninvasive Administration
Currently available rapid-acting formulations using noninvasive routes of administration have focused on pulmonary administration, such as with inhaled loxapine powder, and sublingual administration, such as with sublingual asenapine tablets (not specifically approved for agitation) and sublingual dexmedetomidine film.
Inhaled loxapine has been approved by the FDA for acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults since 2012.43 The formulation is delivered via a single-use handheld device that requires the patient to actively exhale and then inhale and hold their breath for up to 10 seconds to ensure dose delivery. Overall, inhaled loxapine is rapidly absorbed, with quick onset of action and comparable efficacy to IM ziprasidone, olanzapine, haloperidol, aripiprazole, and lorazepam, but can cause pulmonary adverse effects.35
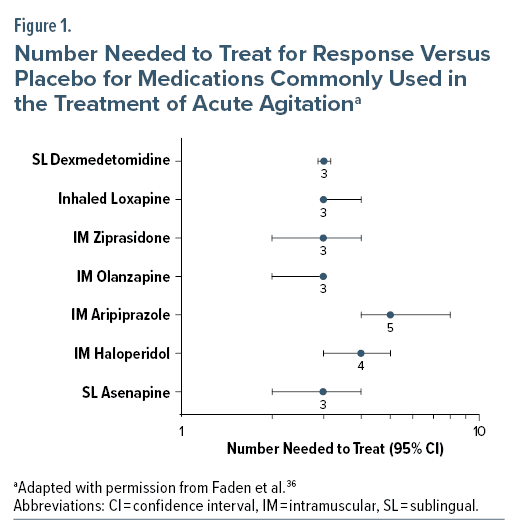
Clinical development of the formulation included a phase 2 trial and phase 3 trial in adult patients with schizophrenia and a phase 3 trial in adult patients with bipolar mania.35 The 2 phase 3 randomized, double-blind, multisite, placebo-controlled, parallel-group trials each enrolled > 300 participants who received inhaled loxapine 5 mg, 10 mg, or placebo.44,45 In the trial including patients with bipolar mania, both doses of loxapine were superior to placebo in reducing agitation as measured by change in the PANSS Excited Component (PANSS-EC) score at 2 hours (P < .0001 for both doses).44 Comparable results were observed in the trial of patients with schizophrenia (5-mg group vs placebo group, P = .0004; 10-mg group vs placebo group, P < .0001).45 Relative to placebo, reductions in PANSS-EC scores were observed for both dosages at the first time point after administration, 10 minutes in both trials (P ≤ .0003). Treatment effect size, as measured by the number needed to treat (NNT) versus placebo for response (Clinical Global Impressions—Improvement scale score of 1 or 2 at 2 hours post administration), was 3 (95% confidence interval [CI] 3–4) for inhaled loxapine pooled across all dosages and studies; this compares favorably to NNT values for IM treatments (Figure 1).46 Only the 10-mg dose strength is commercially available in the United States.43
With regards to safety and tolerability of inhaled loxapine, common adverse events (incidence ≥ 2% and greater than placebo) are dysgeusia (14% for the 10-mg group, 5% with placebo), sedation (12%, 10%), and throat irritation (3%, 0%).43 However, inhaled loxapine is not widely used. In the United States, its use is limited to health care settings that have immediate access on site to supplies and health care professionals competent in the management of acute bronchospasm, can be administered only after an assessment of pulmonary status, and can be administered only once in a 24-hour period, as mandated by a Risk Evaluation and Mitigation Strategy.43,47 Acquisition cost is also higher compared with generic IM medications.35
The most recent addition to the group of noninvasive formulations for treatment of acute aggression is dexmedetomidine sublingual film, approved by the FDA in April 2022 for the acute treatment of agitation associated with schizophrenia or bipolar I or II disorder in adults.24 It is the first FDA-approved treatment for acute agitation using a sublingual route of administration, though it has been available as an IV formulation since 1999 for use as an anesthetic agent.24,36,48 Dexmedetomidine is a α2-adrenergic receptor agonist and, unlike antipsychotics, does not have binding affinity to dopamine receptors.36,49
Results of 2 phase 3 clinical trials, 1 in patients with schizophrenia49 and 1 in patients with bipolar I/II disorder,50 showed significant improvements in PANSS-EC scores with 180-µg and 120-µg doses of dexmedetomidine compared to placebo at 2 hours, with significant differences in the least squares mean differences from placebo (P < .001 for both doses). Sublingual dexmedetomidine also demonstrated rapid onset of action, with statistically significant separation from placebo observed at 20 minutes in patients with bipolar disorder (P ≤ .009) and 20–30 minutes in patients with schizophrenia.49,50 A post hoc analysis of combined study results reported the NNT for response versus placebo was 3 (95% CI, 3–3).51 Across both studies, the most common adverse reactions (incidence ≥ 5% and at least twice the rate of placebo) are somnolence (23% for the 180-µg group, 22% for the 120-µg group, 6% with placebo), oral paresthesia or oral hypoesthesia (7%, 6%, 1%), dizziness (6%, 4%, 1%), dry mouth (4%, 7%, 1%), hypotension (5%, 5%, 0%), and orthostatic hypotension (5%, 3%, <1%).24 The cost of a dose of sublingual dexmedetomidine is higher than alternative IM formulations, which may impact uptake.36
A sublingual asenapine tablet is approved for the daily treatment of schizophrenia in adults and bipolar I disorder in adults and pediatric patients aged 10 to 17 years old, but it is not specifically approved for treatment of acute agitation.52 Sublingual asenapine has been assessed in a single-site randomized controlled trial versus placebo (n = 120, 1:1 asenapine 10 mg:placebo) for agitation.53 Change in PANSS-EC total score at 2 hours was statistically significantly greater in patients treated with asenapine versus placebo (P < .001). A significant difference in mean score between the asenapine and placebo arms was noted at 15 minutes. NNT for response versus placebo was 3 (95% CI, 2–4). The study results stated that no spontaneously reported adverse events were recorded in routine clinical records during the 2 hours of the study.53 Common adverse events associated with asenapine include akathisia, oral hypoesthesia, and somnolence in adults with schizophrenia and somnolence, oral hypoesthesia, dizziness, extrapyramidal symptoms, and akathisia in adults with bipolar I disorder.52 Although a generic version of sublingual asenapine is available and hence relatively inexpensive, its use as a treatment for acute agitation may be limited, as it is not approved for this purpose and the evidence base is limited.
Rapid-Acting Noninvasive Routes in Development
Intranasal delivery is a promising route of delivery that is being explored for acute agitation treatments, with 2 intranasal formulations of olanzapine in the initial stages of development. Olanzapine, in its rapidly acting IM formulation, is a well-studied antipsychotic used for the management of acute agitation in patients with psychosis or bipolar mania.7,22 A meta-analysis identified olanzapine as one of the most efficacious and safe treatments for agitation.54 The most commonly encountered adverse events (≥ 5% and at least twice that for placebo) associated with oral olanzapine monotherapy in adults with schizophrenia are postural hypotension, constipation, weight gain, dizziness, personality disorder, and akathisia and in adults with bipolar I disorder (manic or mixed episodes) are asthenia, dry mouth, constipation, increased appetite, somnolence, dizziness, and tremor.55 Long-term daily use of olanzapine has been associated with more adverse weight gain and metabolic events compared with other second-generation antipsychotics.22
INP105 (Impel NeuroPharma, Seattle, WA) is being studied for the acute treatment of agitation in adolescents and young adults with autism spectrum disorder (NCT05163717)56 and has been evaluated in healthy volunteers with a plan to further develop the intervention for the treatment of acute agitation in schizophrenia and bipolar disorder.22,57 The formulation delivers a powdered form of olanzapine using a proprietary propellant-powered intranasal delivery device. The phase 1 pharmacokinetic study in healthy adults compared INP105 to IM and oral disintegrating tablet (ODT) olanzapine formulations, with similar pharmacokinetic characteristics for the intranasal delivery as with IM administration. At equivalent doses, INP105 resulted in similar area under the curve (AUC) and Cmax as IM olanzapine and similar AUC but higher Cmax than olanzapine ODT. Median tmax was less for INP105 (15, 10, and 9.5 minutes for 5 mg, 10 mg, and 15 mg, respectively) than for olanzapine IM (20 and 15 minutes for 5 mg and 10 mg, respectively) or olanzapine ODT (120 minutes). Treatment-emergent adverse events (TEAEs) occurred in 74% of participants receiving INP105, with postural dizziness/dizziness, orthostatic hypotension/hypotension, nasal congestion, fatigue, headache, and restlessness being most the common TEAEs (> 5%) reported across all INP105-treated groups combined.57
NRL-4 (Neurelis, Inc., San Diego, CA) is being developed for the treatment of acute agitation associated with schizophrenia and bipolar disorder.58 The proprietary formulation contains olanzapine and an alkylsaccharide absorption enhancer, dodecyl maltoside (DDM, Intravail).59 Alkylsaccharides are safe, nontoxic, odorless, tasteless, nonmutagenic, and nonsensitizing at concentrations up to 25%.60 DDM is generally recognized as safe for oral administration.60 It is thought to enhance drug uptake by promoting the temporary loosening of tight junctions between cells of the nasal epithelial mucosa and potentially via transcellular transport mechanisms in nasal epithelial cells.59 DDM is already in use in 2 FDA-approved intranasal formulations of treatments for acute neurologic conditions: sumatriptan nasal spray (Tosymra),61 indicated for the acute treatment of migraine, and diazepam nasal spray (Valtoco),62 indicated for the treatment of seizure clusters associated with epilepsy. The administration device used with these products is a simple single-use device that does not require coordination with breath or other complex patient maneuvers, and presence of a health care provider is not necessary for administration.63 The clinical development program of NRL-4, including phase 1 clinical trials, is underway.
Intranasal Administration of CNS Drugs
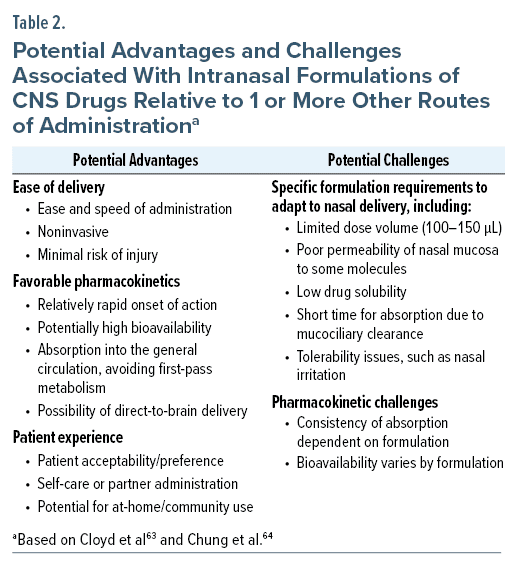
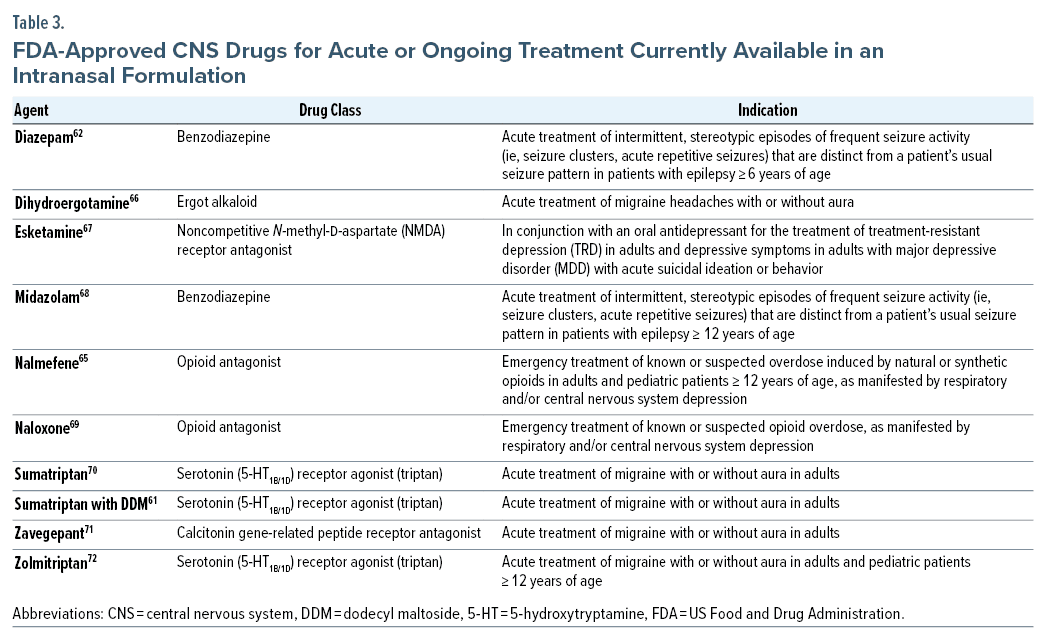
Intranasal administration offers several potential benefits while presenting specific challenges around formulation and pharmacokinetics based on the anatomy and physiology of the nasal cavity (Table 2). FDA-approved intranasal formulations are currently available for several central nervous system (CNS) drugs used as acute or ongoing treatments, including esketamine, nalmefene, naloxone, dihydroergotamine, sumatriptan, zavegepant, zolmitriptan, midazolam, and diazepam (Table 3).73
Nasal Anatomy and Physiology
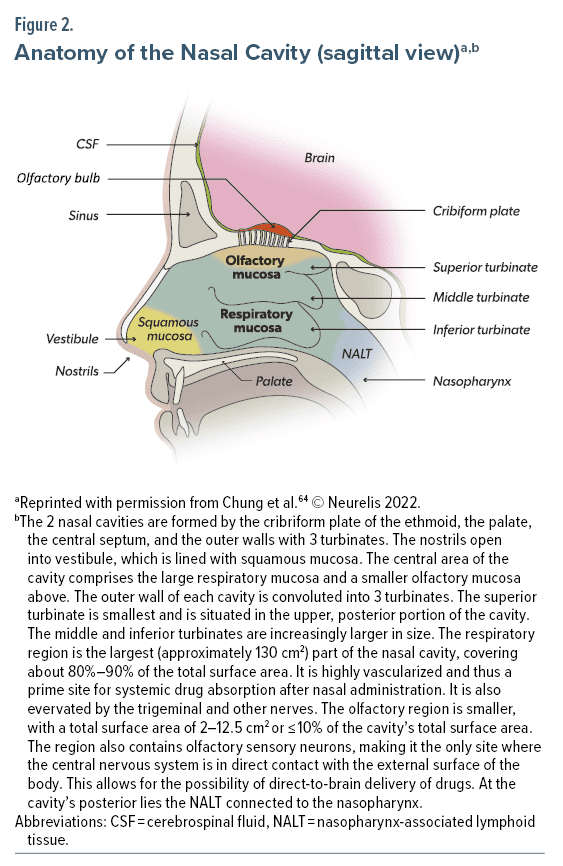
The nose and nasal cavity play a role in heating and humidifying respired air, removing particles and dust, and olfaction.64 The internal structures of the nose include 2 large irregularly shaped nasal cavities (Figure 2). The nasal cavity is subdivided into 4 areas: the vestibule, respiratory region, olfactory region, and nasopharynx-associated lymphatic tissue (NALT). The nostrils open into the vestibule, a small area lined with squamous epithelial cells that are unsuited to extensive drug absorption. At the back of the cavity lies the NALT, a part of the mucosal immune system involved in protection from infection. The large, central portion of the cavity is divided into the respiratory and olfactory regions; these regions are relevant for intranasal drug delivery due to the presence of a rich vasculature, which provides access to the general circulation, and the presence of olfactory and trigeminal nerves, which allow for the possibility of direct-to-brain delivery of drugs.64
The nasal mucosa has several characteristics that are relevant to drug absorption. The epithelium is covered by a biphasic mucus layer with an outer layer of higher viscosity adhesive properties.64 Mucus is continuously moved posteriorly toward the nasopharynx for removal. Due to this mucociliary clearance, turnover time for mucus is 10 to 20 minutes, which has a potential impact on the drug residence time in the nasal cavity.64 The membranes of the respiratory and olfactory regions are composed of ciliated pseudostratified epithelium of columnar and other cells connected by tight junctions, which become increasingly impermeable to molecules larger than 1,000 Da.64,74
Intranasal Drug Delivery and Transport to the Brain
Intranasal formulations are designed specifically to optimize absorption across the nasal mucosa and achieve adequate bioavailability. Excipients may be added to increase solubility and ensure an adequate dose in a small volume or to increase absorption across the mucosal membrane and achieve adequate bioavailability.73 Some excipients, such as chitosan glutamate and DDM, have not been associated with nasal mucosal toxicity, while many others have been shown to have negative impacts on nasal mucosa.59 Novel carriers, such as nanoparticles, are being researched for intranasal delivery. For example, in vitro studies using cell culture have tested the cellular permeation capacity of chitosan-coated cubosomes of paliperidone palmitate with promising results.75 Different drug delivery device combinations are also being developed, as seen in the propellant-powered delivery device for INP105 that is designed to target drug delivery to the upper nasal cavity and has been tested in preclinical and clinical trials.76,77
Compounds may cross the epithelial cell layer using paracellular (via tight junctions) or transcellular processes, then be transported via the general circulation to the brain, passing through the blood-brain barrier.64 Systemic transport results in rapid delivery to the brain, and evidence from animal models suggests that the systemic pathway predominates for the delivery of benzodiazepines to the brain,78 though recent data have also implicated a direct-to-brain pathway.79 A pharmaco-electroencephalogram (EEG) study of intranasal midazolam in patients with status epilepticus showed a physiologic impact, as seen by an increase in β-band power on EEG, in a mean time of 4:07 minutes after administration, with cessation of status epilepticus by a mean of 5:05 minutes among patients who responded to treatment.80
The presence of sensory neurons in olfactory mucosa of the nasal cavity opens the possibility of direct-to-brain transport of some drugs, which may come with benefits in greater CNS therapeutic bioavailability and less systemic adverse effects.73 Studies of intraneuronal transport have reported transport times of 1.5 hours to 6 hours via the olfactory nerve, and thus this pathway is not likely a main mode of drug transport to the brain.81 Extracellular diffusion through perineural spaces has been reported to require 0.7 hours to 2.3 hours to reach the olfactory bulb.73 Other mechanisms, such as bulk flow through perivascular spaces around arteries supplying the olfactory bulb, may also be involved.73 Morphine and carbamazepine have been shown to follow direct olfactory nerve pathways,82,83 while sumatriptan may be transported via both direct olfactory and indirect circulatory pathways to the brain.84
Current oral and IM formulations of antipsychotics reach the brain through systemic pathways, but little is known of the potential for direct-to-brain delivery of antipsychotics in humans. Studies remain at the preclinical stage with various advanced deliveries, such as nanoparticles, dendrimers, and in situ gels, being explored with a number of antipsychotics, including haloperidol, asenapine, risperidone, and olanzapine.85,86
Intranasal Administration of Other CNS Drugs
Several CNS treatments are currently available in intranasal formulations that are used in community settings, and these may be self-administered or administered by a caregiver or care partner. These CNS treatments provide relevant guidance on the potential of intranasal formulations for favorable pharmacokinetics, as well as positive patient-related factors, in the treatment of other CNS conditions, including acute agitation.
Sumatriptan for migraine is available as a subcutaneous injection, an oral tablet, and intranasal formulations for use in the community.87 Early studies showed the subcutaneous injection was the fastest acting of these formulations but was also associated with more adverse events than oral sumatriptan.88 The intranasal formulation had similar efficacy as the oral tablet but with a shorter time to onset of action. The first commercially available early intranasal formulation was limited by low bioavailability and slow absorption.87 A formulation containing the permeation enhancer DDM provided a more rapid absorption profile in healthy volunteers, with a tmax of 0.25 hours after a 10-mg single dose compared with 2.0 hours for a 20-mg dose of the original intranasal formulation (P < .05, paired t test).87 The enhanced intranasal formulation resulted in systemic exposure similar to subcutaneous sumatriptan (4 mg), and a long-term safety study reported adverse events similar in pattern, frequency and severity as those seen in previous research on triptans and intranasal formulations.87,89 Moreover, patients were more satisfied with the intranasal treatment compared with their usual migraine medication.90 A sumatriptan nasal powder is also available; it is delivered via a breath-activated delivery system.91
Benzodiazepines, specifically diazepam and midazolam, are the cornerstone of rescue therapy for seizure clusters in epilepsy.63 Until 2019, diazepam rectal gel was the only rescue therapy available for use in the community, and while effective, it had drawbacks, including low social acceptability, particularly among adults.63 A pharmacokinetic study of a intranasal formulation of diazepam in healthy volunteers reported a similar tmax between intranasal and rectal formulations, with less variability in bioavailability with the intranasal formulation.92 Absolute bioavailability of the intranasal formulations are 97% for diazepam and approximately 44% for midazolam, compared to their respective IV formulations.62,68 Among the benzodiazepine formulations available for use in the community, intranasal administration leads to the quickest onset of action from time of administration compared with rectal, IM, and sublingual routes.93 Additionally, intranasal delivery may lead to shorter total times from seizure onset to cessation, due in part to less time required to administer an intranasal formulation than an IV formulation.94
Safety studies of benzodiazepine nasal spray rescue therapy confirm it to be well tolerated.95,96 Diazepam nasal spray was associated with minimal nasal irritation and no clinically relevant olfactory changes.95 Intranasal benzodiazepine rescue therapy is associated with high levels of patient satisfaction.97,98 Finally, in one clinical trial, a subset of patients have been reported to successfully self-administer diazepam nasal spray.98
Naloxone has been used for decades to treat symptoms associated with opioid overdose,99 and an FDA-approved, over-the-counter intranasal formulation is now available.100 The pharmacokinetic profile of the approved intranasal naloxone formulation (2–8 mg) was compared with IM naloxone (0.4 mg) in healthy volunteers.101 Plasma concentrations and AUC with all doses of intranasal naloxone were equal to or exceeded those with intramuscular naloxone; tmax was similar between formulations. In a separate cohort, 90% or more of participants were able to correctly administer the intranasal formulation without prior training.101
CONCLUSION
An unmet medical need remains for effective and acceptable alternative treatments for acute agitation associated with schizophrenia or bipolar disorder. Several novel routes of administration are possible, including inhaled loxapine, dexmedetomidine sublingual film, and 2 intranasal olanzapine formulations in clinical development. Intranasal administration is currently being used for other CNS conditions, and it offers the potential to provide effective treatment in both community and hospital settings. This intranasal mode of medication delivery may also be more acceptable to patients with acute agitation and the health care providers treating them than currently available interventions.
Article Information
Published Online: January 30, 2024. https://doi.org/10.4088/PCC.23nr03596
© 2024 Physicians Postgraduate Press, Inc.
Submitted: July 6, 2023; accepted November 3, 2023.
To Cite: Citrome L, Correll CU, San L, et al. Alternative approaches for addressing acute agitation in schizophrenia and bipolar disorder. Prim Care Companion CNS Disord. 2024;26(1):23nr03596.
Corresponding Author: Leslie Citrome, MD, MPH, 11 Medical Park Dr, Ste 102, Pomona, NY 10970 ([email protected]).
Author Affiliations: Psychiatry and Behavioral Sciences, New York Medical College, Valhalla, New York (Citrome); Department of Psychiatry and Molecular Medicine, Zucker School of Medicine at Hofstra/Northwell, Uniondale, New York; Department of Psychiatry, Zucker Hillside Hospital, Glen Oaks, New York; Child and Adolescent Psychiatry, Charité Universitätsmedizin, Berlin, Germany (Correll); Parc Sanitari Sant Joan de Déu, Sant Boi de Llobregat, Barcelona, Spain (San); Centro de Investigación Biomédica en Red de Salud Mental (CIBERSAM), Barcelona, Spain (San); Department of Psychiatry, University of California-Riverside School of Medicine, Riverside, California (Zeller); Clinical Development and Medical Affairs, Neurelis, Inc, San Diego, California (Madden, Lopez-Toledano, Carrazana, Rabinowicz); Formerly of Clinical Development and Medical Affairs, Neurelis, Inc, San Diego, California (Guiterrez, Misra); Department of Family Medicine, John A. Burns School of Medicine, University of Hawaii, Honolulu, Hawaii (Carrazana).
Relevant Financial Relationships: Dr Citrome is a consultant for AbbVie/Allergan, Acadia, Adamas, Alkermes, Angelini, Astellas, Avanir, Axsome, BioXcel, Boehringer Ingelheim, Cadent Therapeutics, Cerevel, Clinilabs, COMPASS, Eisai, Enteris BioPharma, HLS Therapeutics, Idorsia, INmune Bio, Impel, Intra-Cellular Therapies, Janssen, Karuna, Lundbeck, Lyndra, Medavante-ProPhase, Marvin, Merck, Mitsubishi-Tanabe Pharma, Neurelis, Inc, Neurocrine, Novartis, Noven, Otsuka, Ovid, Praxis, Recordati, Relmada, Reviva, Sage, Sunovion, Supernus, Teva, and Vanda; has served as speaker for AbbVie/Allergan, Acadia, Alkermes, Angelini, Axsome, BioXcel, Eisai, Idorsia, Intra-Cellular Therapies, Janssen, Lundbeck, Neurocrine, Noven, Otsuka, Recordati, Sage, Sunovion, Takeda, and Teva; holds stocks (small number of shares of common stock) from Bristol-Myers Squibb, Eli Lilly, Johnson & Johnson, Merck, and Pfizer purchased > 10 years ago; has stock options with Reviva; and receives royalties/publishing income from Taylor & Francis, Wiley, UpToDate, Springer Healthcare, and Elsevier.
Dr Correll has been a consultant and/or advisor to or has received honoraria from AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Boehringer-Ingelheim, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Gedeon Richter, Hikma, Holmusk, Intra-Cellular Therapies, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Neurelis, Inc, Newron, Noven, Otsuka, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Seqirus, SK Life Science, Sunovion, Sun Pharma, Supernus, Takeda, Teva, and Viatris; has provided expert testimony for Janssen and Otsuka; has served on a data safety monitoring board for Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva; has received grant support from Janssen and Takeda; has received royalties from UpToDate; and is a stock option holder of Cardio Diagnostics, Mindpax, LB Pharma, and Quantic. Dr San has received grants and served as consultant, advisor, or CME speaker for AstraZeneca, Bristol-Myers Squibb, Ferrer Internacional, GlaxoSmithKline, Janssen, Lilly, Lundbeck, Neurelis, Inc, Otsuka, Pfizer, Roche, Sanofi-Aventis, Servier, Shire, and the Spanish Ministry of Science and Innovation (CIBERSAM). Dr Zeller is a consultant for BioXcel Therapeutics and Neurelis, Inc. Drs Lopez-Toledano and Rabinowicz are employees of and have received stock options from Neurelis, Inc. Drs Madden and Carrazana are employees of and have received stock and stock options from Neurelis, Inc. Drs Gutierrez and Misra were employees of and have received stock options from Neurelis, Inc.
Funding/Support: This review was funded by Neurelis, Inc (San Diego, CA).
Role of the Funders/Sponsors: Neurelis, Inc was involved in the review of the manuscript. The authors retained full control of content of this manuscript and the decision to submit for publication.
Acknowledgments: Medical writing support was provided by David McLay, PhD, of The Curry Rockefeller Group, LLC (Tarrytown, NY), and was funded by Neurelis, Inc (San Diego, CA).
ORCID: Leslie Citrome: https://orcid.org/0000-0002-6098-9266; Christoph U. Correll: https://orcid.org/0000-0002-7254-5646; Luis San: https://orcid.org/0000-0002-6618-3589; Miguel Lopez-Toledano: https://orcid.org/0000-0002-5743-3750; Sunita N Misra: https://orcid.org/0000-0002-7624-8825; Enrique Carrazana: https://orcid.org/0000-0001-8788-0722; Adrian L Rabinowicz: https://orcid.org/0000-0003-1299-0606
Clinical Points
- The treatment of acute agitation in patients with psychosis or bipolar disorder should prioritize noninvasive/noncoercive interventions and place shared decision making at the center of patient care. When verbal de-escalation techniques alone are insufficient, pharmacologic management using antipsychotics may be required.
- While oral and intramuscular formulations of pharmacologic agents continue to be commonly used to treat acute agitation, novel agents with alternative routes of delivery are becoming available, such as inhaled loxapine and dexmedetomidine sublingual film, or are in clinical development, including intranasal olanzapine.
- Intranasal formulations are designed specifically to address the unique nasal anatomy and physiology and can provide several benefits, including reliable, favorable pharmacokinetics, ease of delivery, and its noninvasive administration being consistent with patient-centered factors such as preference and self-administration.
References (101)

- Garriga M, Pacchiarotti I, Kasper S, et al. Assessment and management of agitation in psychiatry: expert consensus. World J Biol Psychiatry. 2016;17(2):86–128. PubMed CrossRef
- Fillit H, Aigbogun MS, Gagnon-Sanschagrin P, et al. Impact of agitation in long-term care residents with dementia in the United States. Int J Geriatr Psychiatry. 2021;36(12):1959–1969. PubMed CrossRef
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, DSM-5. Fifth Edition. Washington, DC: American Psychiatric Publishing; 2013.
- Roberts J, Gracia Canales A, Blanthorn-Hazell S, et al. Characterizing the experience of agitation in patients with bipolar disorder and schizophrenia. BMC Psychiatry. 2018;18(1):104. PubMed CrossRef
- Volavka J. Violence in schizophrenia and bipolar disorder. Psychiatr Danub. 2013;25(1):24–33. PubMed
- Garrote-Cámara ME, Gea-Caballero V, Sufrate-Sorzano T, et al. Clinical and sociodemographic profile of psychomotor agitation in mental health hospitalisation: a multicentre study. Int J Environ Res Public Health. 2022;19(23):15972. PubMed CrossRef
- Correll CU, Yu X, Xiang Y, et al. Biological treatment of acute agitation or aggression with schizophrenia or bipolar disorder in the inpatient setting. Ann Clin Psychiatry. 2017;29(2):92–107. PubMed
- Dhossche DM, Ghani SO. Who brings patients to the psychiatric emergency room? psychosocial and psychiatric correlates. Gen Hosp Psychiatry. 1998;20(4):235–240. PubMed CrossRef
- Nordstrom K, Zun LS, Wilson MP, et al. Medical evaluation and triage of the agitated patient: consensus statement of the American association for emergency psychiatry project Beta medical evaluation workgroup. West J Emerg Med. 2012;13(1):3–10. PubMed CrossRef
- Miner JR, Klein LR, Cole JB, et al. The characteristics and prevalence of agitation in an Urban County emergency department. Ann Emerg Med. 2018;72(4):361–370. PubMed CrossRef
- Lyketsos CG, Steinberg M, Tschanz JT, et al. Mental and behavioral disturbances in dementia: findings from the Cache County Study on memory in aging. Am J Psychiatry. 2000;157(5):708–714. PubMed CrossRef
- Tractenberg RE, Weiner MF, Thal LJ. Estimating the prevalence of agitation in community-dwelling persons with Alzheimer’s disease. J Neuropsychiatry Clin Neurosci. 2002;14(1):11–18. PubMed CrossRef
- Kverno KS, Rabins PV, Blass DM, et al. Prevalence and treatment of neuropsychiatric symptoms in advanced dementia. J Gerontol Nurs. 2008;34(12):8–15, quiz 16–17. PubMed CrossRef
- Holloman GH Jr, Zeller SL. Overview of project BETA: best practices in evaluation and treatment of agitation. West J Emerg Med. 2012;13(1):1–2. PubMed CrossRef
- Baldaçara L, Ismael F, Leite V, et al. Brazilian guidelines for the management of psychomotor agitation, part 1: non-pharmacological approach. Br J Psychiatry. 2019;41(2):153–167. PubMed CrossRef
- Patel MX, Sethi FN, Barnes TR, et al; With co-authors (in alphabetical order). Joint BAP NAPICU evidence-based consensus guidelines for the clinical management of acute disturbance: de-escalation and rapid tranquillisation. J Psychopharmacol. 2018;32(6):601–640. PubMed CrossRef
- Allen MH, Currier GW, Hughes DH, et al; Expert Consensus Panel for Behavioral Emergencies. The Expert Consensus Guideline Series. Treatment of behavioral emergencies. Postgrad Med. 2001;(Spec No):1–88, quiz 89–90. PubMed
- San L, Marksteiner J, Zwanzger P, et al. State of acute agitation at psychiatric emergencies in Europe: the STAGE study. Clin Pract Epidemiol Ment Health. 2016;12(1):75–86. PubMed CrossRef
- Citrome L, Volavka J. Specific anti-hostility effects of atypical antipsychotics in persons with schizophrenia: from clozapine to cariprazine. Harv Rev Psychiatry. 2021;29(1):20–34. PubMed CrossRef
- Martínez-Raga J, Amore M, Di Sciascio G, et al. 1st International Experts’ Meeting on Agitation: conclusions regarding the current and ideal management paradigm of agitation. Front Psychiatry. 2018;9:54. PubMed CrossRef
- Zeller SL, Citrome L. Managing agitation associated with schizophrenia and bipolar disorder in the emergency setting. West J Emerg Med. 2016;17(2):165–172. PubMed CrossRef
- Ward K, Citrome L. The treatment of acute agitation associated with schizophrenia or bipolar disorder: investigational drugs in early stages of their clinical development, and their clinical context and potential place in therapy. Expert Opin Investig Drugs. 2020;29(3):245–257. PubMed CrossRef
- Wilson MP, Pepper D, Currier GW, et al. The psychopharmacology of agitation: consensus statement of the American Association for Emergency Psychiatry Project Beta Psychopharmacology Workgroup. West J Emerg Med. 2012;13(1):26–34. PubMed CrossRef
- Igalmi. Package insert. BioExcel Therapeutics Inc; 2022.
- Barbic D, Andolfatto G, Grunau B, et al. Rapid agitation control with ketamine in the emergency department: a blinded, randomized controlled trial. Ann Emerg Med. 2021;78(6):788–795. PubMed CrossRef
- Scheppke KA, Braghiroli J, Shalaby M, et al. Prehospital use of IM ketamine for sedation of violent and agitated patients. West J Emerg Med. 2014;15(7):736–741. PubMed CrossRef
- Sullivan N, Chen C, Siegel R, et al. Ketamine for emergency sedation of agitated patients: a systematic review and meta-analysis. Am J Emerg Med. 2020;38(3):655–661. PubMed CrossRef
- Beck K, Hindley G, Borgan F, et al. Association of ketamine with psychiatric symptoms and implications for its therapeutic use and for understanding schizophrenia: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(5):e204693. PubMed CrossRef
- Citrome L. Agitation in schizophrenia: origins and evidence-based treatment. Curr Opin Psychiatry. 2021;34(3):216–221. PubMed CrossRef
- Citrome L. Comparison of intramuscular ziprasidone, olanzapine, or aripiprazole for agitation: a quantitative review of efficacy and safety. J Clin Psychiatry. 2007;68(12):1876–1885. PubMed CrossRef
- Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the Use of Antipsychotics to Treat Agitation or Psychosis in Patients With Dementia. Arlington, VA: American Psychiatric Association; 2016.
- Rappaport SA, Marcus RN, Manos G, et al. A randomized, double-blind, placebo-controlled tolerability study of intramuscular aripiprazole in acutely agitated patients with Alzheimer’s, vascular, or mixed dementia. J Am Med Dir Assoc. 2009;10(1):21–27. PubMed CrossRef
- Meehan KM, Wang H, David SR, et al. Comparison of rapidly acting intramuscular olanzapine, lorazepam, and placebo: a double-blind, randomized study in acutely agitated patients with dementia. Neuropsychopharmacology. 2002;26(4):494–504. PubMed CrossRef
- REXULTI Package insert. Lundbeck; 2023.
- Faden J, Citrome L. Examining the safety, efficacy, and patient acceptability of inhaled loxapine for the acute treatment of agitation associated with schizophrenia or bipolar I disorder in adults. Neuropsychiatr Dis Treat. 2019;15:2273–2283. PubMed CrossRef
- Faden J, Musselman M, Citrome L. Sublingual dexmedetomidine: repurposing an anesthetic as an anti-agitation agent. Expert Rev Neurother. 2023;23(2):97–106. PubMed CrossRef
- Ng AT, Zeller S, Rhoades RW. Clinical challenges in the pharmacologic management of agitation. Prim Psychiatry. 2010;17:46–52.
- Preskorn SH, Risinger R, Kakar R. Sublingual dexmedetomidine vs placebo and acute agitation associated with bipolar disorder-reply. JAMA. 2022;328(2):214–215. PubMed CrossRef
- Krieger E, Moritz S, Lincoln TM, et al. Coercion in psychiatry: a cross-sectional study on staff views and emotions. J Psychiatr Ment Health Nurs. 2021;28(2):149–162. PubMed CrossRef
- Chieze M, Hurst S, Kaiser S, et al. Effects of seclusion and restraint in adult psychiatry: a systematic review. Front Psychiatry. 2019;10:491. PubMed CrossRef
- Loots E, Leys J, Proost S, et al. Medication self-management in hospitalised patients with schizophrenia or bipolar disorder: the perceptions of patients and healthcare providers. Int J Environ Res Public Health. 2022;19(8):4835. PubMed CrossRef
- Slade M. Implementing shared decision making in routine mental health care. World Psychiatry. 2017;16(2):146–153. PubMed CrossRef
- Adasuve. Package insert. Alexza Pharmaceuticals Inc; 2022.
- Kwentus J, Riesenberg RA, Marandi M, et al. Rapid acute treatment of agitation in patients with bipolar I disorder: a multicenter, randomized, placebo-controlled clinical trial with inhaled loxapine. Bipolar Disord. 2012;14(1):31–40. PubMed CrossRef
- Lesem MD, Tran-Johnson TK, Riesenberg RA, et al. Rapid acute treatment of agitation in individuals with schizophrenia: multicentre, randomised, placebo-controlled study of inhaled loxapine. Br J Psychiatry. 2011;198(1):51–58. PubMed CrossRef
- Citrome L. Aerosolised antipsychotic assuages agitation: inhaled loxapine for agitation associated with schizophrenia or bipolar disorder. Int J Clin Pract. 2011;65(3):330–340. PubMed CrossRef
- US Food and Drug Administration. Risk Evaluation and Mitigation Strategies (REMS). US Food and Drug Administration. Accessed February 24, 2023. https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems
- Precedex. Package insert. Hospira Inc; 2022.
- Citrome L, Preskorn SH, Lauriello J, et al. Sublingual dexmedetomidine for the treatment of acute agitation in adults with schizophrenia or schizoaffective disorder: a randomized placebo-controlled trial. J Clin Psychiatry. 2022;83(6):22m14447. PubMed CrossRef
- Preskorn SH, Zeller S, Citrome L, et al. Effect of sublingual dexmedetomidine vs placebo on acute agitation associated with bipolar disorder: a randomized clinical trial. JAMA. 2022;327(8):727–736. PubMed CrossRef
- Citrome L, Risinger R, Rajachandran L, et al. Sublingual dexmedetomidine for agitation associated with schizophrenia or bipolar disorder: a post hoc analysis of number needed to treat, number needed to harm, and likelihood to be helped or harmed. Adv Ther. 2022;39(10):4821–4835. PubMed CrossRef
- Saphris. Package insert. Allergan USA Inc; 2021.
- Pratts M, Citrome L, Grant W, et al. A single-dose, randomized, double-blind, placebo-controlled trial of sublingual asenapine for acute agitation. Acta Psychiatr Scand. 2014;130(1):61–68. PubMed CrossRef
- Bak M, Weltens I, Bervoets C, et al. The pharmacological management of agitated and aggressive behavior: a systematic review and meta-analysis. Eur Psychiatry. 2019;57:78–100. PubMed CrossRef
- Zyprexa. Package insert. Lilly; 2021.
- ClinicalTrials.gov. INP105 Proof-of-Concept Study for the Acute Treatment of Agitation in Adolescents and Young Adults With ASD (CALM 201). Accessed Dec 22, 2022. https://clinicaltrials.gov/ct2/show/NCT05163717
- Shrewsbury SB, Hocevar-Trnka J, Satterly KH, et al. The SNAP 101 double-blind, placebo/active-controlled, safety, pharmacokinetic, and pharmacodynamic study of INP105 (nasal olanzapine) in healthy adults. J Clin Psychiatry. 2020;81(4):19m13086. PubMed CrossRef
- Neurelis, Inc. Challenging the Way We Treat Acute Agitation Episodes. Neurelis, Inc. Accessed December 22, 2022. https://neurelis.com/our-pipeline/nrl-4
- Rabinowicz AL, Carrazana E, Maggio ET. Improvement of intranasal drug delivery with Intravail alkylsaccharide excipient as a mucosal absorption enhancer aiding in the treatment of conditions of the central nervous system. Drugs R D. 2021;21(4):361–369. PubMed CrossRef
- Maggio ET, Pillion DJ. High efficiency intranasal drug delivery using Intravail alkylsaccharide absorption enhancers. Drug Deliv Transl Res. 2013;3(1):16–25. PubMed CrossRef
- Tosymra. Package insert. Upsher-Smith Laboratories, LLC; 2021.
- Valtoco. Package insert. Neurelis, Inc; 2023.
- Cloyd J, Haut S, Carrazana E, et al. Overcoming the challenges of developing an intranasal diazepam rescue therapy for the treatment of seizure clusters. Epilepsia. 2021;62(4):846–856. PubMed CrossRef
- Chung S, Peters JM, Detyniecki K, et al. The nose has it: opportunities and challenges for intranasal drug administration for neurologic conditions including seizure clusters. Epilepsy Behav Rep. 2022;21:100581. PubMed CrossRef
- Opvee. Package insert. Opiant Pharmaceuticals, Inc; 2023.
- Migranal. Package insert. Bausch Health US, LLC; 2022.
- Spravato Package insert. Janssen Pharmaceutical Companies; 2020.
- Nayzilam. Package insert. UCB, Inc; 2023.
- Narcan. nasal spray Package insert. Adapt Pharma, Inc; 2020.
- Imitrex. nasal spray Package insert. GlaxoSmithKline; 2017.
- Zavzpret. Package insert. Pfizer Inc; 2023.
- Zomig. Package insert. Amneal Pharmaceuticals; 2019.
- Lofts A, Abu-Hijleh F, Rigg N, et al. Using the intranasal route to administer drugs to treat neurological and psychiatric illnesses: rationale, successes, and future needs. CNS Drugs. 2022;36(7):739–770. PubMed CrossRef
- McMartin C, Hutchinson LE, Hyde R, et al. Analysis of structural requirements for the absorption of drugs and macromolecules from the nasal cavity. J Pharm Sci. 1987;76(7):535–540. PubMed CrossRef
- Deruyver L, Rigaut C, Gomez-Perez A, et al. In vitro evaluation of paliperidone palmitate loaded cubosomes effective for nasal-to-brain delivery. Int J Nanomedicine. 2023;18:1085–1106. PubMed CrossRef
- Shrewsbury S, Davies G, McConnachie L, et al. The pharmacokinetics of drug delivery to the upper nasal space: a review of INP105 development. Med Res Arch. 2022;10(9). CrossRef
- Hoekman J. The Upper Nasal Cavity: Drug Delivery’s Next Frontier. Outsourced Pharma. Accessed March 16, 2023. https://www.outsourcedpharma.com/doc/the-upper-nasal-space-drug-delivery-s-next-frontier-0001
- Kaur P, Kim K. Pharmacokinetics and brain uptake of diazepam after intravenous and intranasal administration in rats and rabbits. Int J Pharm. 2008;364(1):27–35. PubMed CrossRef
- Watanabe K, Kimura S, Hazama Y, et al. Direct nose-to-brain delivery of diazepam via trigeminal nerve contributes to rapid seizure suppression in pentylenetetrazole-induced status epilepticus model rats. J Drug Deliv Ther. 2023;13(1):44–56. CrossRef
- Kay L, Merkel N, von Blomberg A, et al. Intranasal midazolam as first-line in-hospital treatment for status epilepticus: a pharmaco-EEG cohort study. Ann Clin Transl Neurol. 2019;6(12):2413–2425. PubMed CrossRef
- Gänger S, Schindowski K. Tailoring formulations for intranasal nose-to-brain delivery: a review on architecture, physico-chemical characteristics and mucociliary clearance of the nasal olfactory mucosa. Pharmaceutics. 2018;10(3):116. PubMed CrossRef
- Westin U, Piras E, Jansson B, et al. Transfer of morphine along the olfactory pathway to the central nervous system after nasal administration to rodents. Eur J Pharm Sci. 2005;24(5):565–573. PubMed CrossRef
- Barakat NS, Omar SA, Ahmed AA. Carbamazepine uptake into rat brain following intra-olfactory transport. J Pharm Pharmacol. 2006;58(1):63–72. PubMed CrossRef
- Luthringer R, Djupesland PG, Sheldrake CD, et al. Rapid absorption of sumatriptan powder and effects on glyceryl trinitrate model of headache following intranasal delivery using a novel bi-directional device. J Pharm Pharmacol. 2009;61(9):1219–1228. PubMed CrossRef
- Kumar M, Misra A, Babbar AK, et al. Intranasal nanoemulsion based brain targeting drug delivery system of risperidone. Int J Pharm. 2008;358(1-2):285–291. PubMed CrossRef
- Pandey M, Jain N, Kanoujia J, et al. Advances and challenges in intranasal delivery of antipsychotic agents targeting the central nervous system. Front Pharmacol. 2022;13:865590. PubMed CrossRef
- Munjal S, Gautam A, Offman E, et al. A randomized trial comparing the pharmacokinetics, safety, and tolerability of DFN-02, an intranasal sumatriptan spray containing a permeation enhancer, with intranasal and subcutaneous sumatriptan in healthy adults. Headache. 2016;56(9):1455–1465. PubMed CrossRef
- Tfelt-Hansen P. Efficacy and adverse events of subcutaneous, oral, and intranasal sumatriptan used for migraine treatment: a systematic review based on number needed to treat. Cephalalgia. 1998;18(8):532–538. PubMed CrossRef
- Munjal S, Brand-Schieber E, Allenby K, et al. A multicenter, open-label, long-term safety and tolerability study of DFN-02, an intranasal spray of sumatriptan 10 mg plus permeation enhancer DDM, for the acute treatment of episodic migraine. J Headache Pain. 2017;18(1):31. PubMed CrossRef
- Lipton RB, Munjal S, Brand-Schieber E, et al. DFN-02, sumatriptan 10 mg nasal spray with permeation enhancer, for the acute treatment of migraine: a randomized, double-blind, placebo-controlled study assessing functional disability and subject satisfaction with treatment. CNS Drugs. 2019;33(4):375–382. PubMed CrossRef
- Onzetra. Xsail. Package insert. Currax Pharmaceuticals LLC; 2019.
- Hogan RE, Gidal BE, Koplowitz B, et al. Bioavailability and safety of diazepam intranasal solution compared to oral and rectal diazepam in healthy volunteers. Epilepsia. 2020;61(3):455–464. PubMed CrossRef
- Almohaish S, Sandler M, Brophy GM. Time is brain: acute control of repetitive seizures and status epilepticus using alternative routes of administration of benzodiazepines. J Clin Med. 2021;10(8):1754. PubMed CrossRef
- Chhabra R, Gupta R, Gupta LK. Intranasal midazolam versus intravenous/rectal benzodiazepines for acute seizure control in children: a systematic review and meta-analysis. Epilepsy Behav. 2021;125:108390. PubMed CrossRef
- Wheless JW, Miller I, Hogan RE, et al; DIAZ.001.05 Study Group. Final results from a phase 3, long-term, open-label, repeat-dose safety study of diazepam nasal spray for seizure clusters in patients with epilepsy. Epilepsia. 2021;62(10):2485–2495. PubMed CrossRef
- Wheless JW, Meng TC, Van Ess PJ, et al. Safety and efficacy of midazolam nasal spray in the outpatient treatment of patients with seizure clusters: an open-label extension trial. Epilepsia. 2019;60(9):1809–1819. PubMed CrossRef
- Meng TC, Szaflarski JP, Chen L, et al. Psychosocial outcomes of repeated treatment of seizure clusters with midazolam nasal spray: results of a phase 3, open-label extension trial. Epilepsy Behav. 2023;138:108989. PubMed CrossRef
- Penovich P, Wheless JW, Hogan RE, et al. Examining the patient and caregiver experience with diazepam nasal spray for seizure clusters: results from an exit survey of a phase 3, open-label, repeat-dose safety study. Epilepsy Behav. 2021;121(Pt A):108013. PubMed CrossRef
- Wermeling DP. A response to the opioid overdose epidemic: naloxone nasal spray. Drug Deliv Transl Res. 2013;3(1):63–74. PubMed CrossRef
- Food & Drug Administration (FDA). FDA Approves First Over-the-Counter Naloxone Nasal Spray. Food and Drug Administration. Accessed September 11, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-over-counter-naloxone-nasal-spray
- Krieter P, Chiang N, Gyaw S, et al. Pharmacokinetic properties and human use characteristics of an FDA-approved intranasal naloxone product for the treatment of opioid overdose. J Clin Pharmacol. 2016;56(10):1243–1253. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite