Objective: To determine whether physical dependence developed during lisdexamfetamine dimesylate treatment, as evidenced by presence of withdrawal symptoms after treatment cessation in adults with binge-eating disorder (BED) treated for up to 38 weeks.
Methods: Three studies enrolled adults with DSM-IV-TR-defined BED. In two 12-week, randomized, double-blind, placebo-controlled studies conducted from November 2012 to September 2013, participants were treated with placebo or dose-optimized lisdexamfetamine (50 or 70 mg). In a double-blind, placebo-controlled, randomized-withdrawal maintenance-of-efficacy study conducted from January 2014 to April 2015, participants categorized as responders after 12 weeks of open-label lisdexamfetamine (50 or 70 mg) were randomized to continued lisdexamfetamine or placebo for 26 weeks. The Amphetamine Cessation Symptom Assessment (ACSA), a 16-item self-report instrument (total score: 0-64), assessed withdrawal experiences. Mean ± SD ACSA scores and medians are presented for study completers.
Results: In the short-term efficacy studies, mean ± SD ACSA aggregate scores for placebo and lisdexamfetamine (pooled data) were 7.0 ± 7.60 (n = 275) and 4.9 ± 6.41 (n = 271), respectively, on the day of the last dose at week 12/early termination (ET) and 4.8 ± 6.82 (n = 234) and 5.5 ± 7.50 (n = 221) on day 7 after the last dose. In the maintenance-of-efficacy study, mean ± SD ACSA aggregate scores for placebo and lisdexamfetamine were 4.8 ± 6.67 (n = 44) and 4.7 ± 7.78 (n = 85) on the day of the last dose at week 38/ET and 3.9 ± 5.75 (n = 37) and 5.2 ± 7.93 (n = 71) on day 7 after the last dose.
Conclusions: Study results suggest that abrupt lisdexamfetamine termination was not associated with amphetamine withdrawal symptoms at the exposure durations and therapeutic doses analyzed.
Trial Registration: Clinicaltrials.gov identifiers: NCT01718483, NCT01718509, and NCT02009163
Objective: To determine whether physical dependence developed during lisdexamfetamine dimesylate treatment, as evidenced by presence of withdrawal symptoms after treatment cessation in adults with binge-eating disorder (BED) treated for up to 38 weeks.
Methods: Three studies enrolled adults with DSM-IV-TR-defined BED. In two 12-week, randomized, double-blind, placebo-controlled studies conducted from November 2012 to September 2013, participants were treated with placebo or dose-optimized lisdexamfetamine (50 or 70 mg). In a double-blind, placebo-controlled, randomized-withdrawal maintenance-of-efficacy study conducted from January 2014 to April 2015, participants categorized as responders after 12 weeks of open-label lisdexamfetamine (50 or 70 mg) were randomized to continued lisdexamfetamine or placebo for 26 weeks. The Amphetamine Cessation Symptom Assessment (ACSA), a 16-item self-report instrument (total score: 0-64), assessed withdrawal experiences. Mean ± SD ACSA scores and medians are presented for study completers.
Results: In the short-term efficacy studies, mean ± SD ACSA aggregate scores for placebo and lisdexamfetamine (pooled data) were 7.0 ± 7.60 (n = 275) and 4.9 ± 6.41 (n = 271), respectively, on the day of the last dose at week 12/early termination (ET) and 4.8 ± 6.82 (n = 234) and 5.5 ± 7.50 (n = 221) on day 7 after the last dose. In the maintenance-of-efficacy study, mean ± SD ACSA aggregate scores for placebo and lisdexamfetamine were 4.8 ± 6.67 (n = 44) and 4.7 ± 7.78 (n = 85) on the day of the last dose at week 38/ET and 3.9 ± 5.75 (n = 37) and 5.2 ± 7.93 (n = 71) on day 7 after the last dose.
Conclusions: Study results suggest that abrupt lisdexamfetamine termination was not associated with amphetamine withdrawal symptoms at the exposure durations and therapeutic doses analyzed.
Trial Registration: Clinicaltrials.gov identifiers: NCT01718483, NCT01718509, and NCT02009163
Prim Care Companion CNS Disord 2020;22(2):19m02540
To cite: Robertson B, Wu J, Fant RV, et al. Assessment of amphetamine withdrawal symptoms of lisdexamfetamine dimesylate treatment for adults with binge-eating disorder. Prim Care Companion CNS Disord. 2020;22(2):19m02540.
To share: https://doi.org/10.4088/PCC.19m02540
© Copyright 2020 Physicians Postgraduate Press, Inc.
aGlobal Clinical Development, Shire, a member of the Takeda group of companies, Lexington, Massachusetts, at the time this research was conducted; currently employed by Yumanity Therapeutics Inc, Cambridge, Massachusetts
bBiostatistics, Shire, a member of the Takeda group of companies, Lexington, Massachusetts, at the time this research was conducted; currently employed by Ironwood Pharmaceuticals, Boston, Massachusetts
cPinney Associates Inc., Bethesda, Maryland
dLindner Center of HOPE, Mason, Ohio
eDepartment of Psychiatry and Behavioral Neuroscience, University of Cincinnati College of Medicine, Cincinnati, Ohio
*Corresponding author: Susan L. McElroy, MD, Research Institute, Lindner Center of HOPE, 4075 Old Western Row Rd, Mason, OH 45040 ([email protected]).
The DSM-5 recognizes binge-eating disorder (BED) as a distinct eating disorder.1 BED is characterized by consumption of a larger quantity of food than is typical for most people under similar circumstances, a sense of lack of control over eating during the episode, and marked distress about binge eating.1 A BED diagnosis requires that binge-eating episodes occur, on average, at least once a week for 3 months.1
Lisdexamfetamine dimesylate is approved in the United States and other countries for use in children, adolescents, and adults with attention-deficit/hyperactivity disorder (ADHD)2-5 and adults with moderate to severe BED.3,4 In short-term efficacy studies, lisdexamfetamine produced significantly greater reductions in binge-eating days per week than placebo in adults with moderate to severe BED.6,7 In a 38-week maintenance-of-efficacy study,8 lisdexamfetamine demonstrated superiority over placebo for time to relapse in adults with BED.
In these BED studies,6-8 the overall safety and tolerability of lisdexamfetamine in terms of adverse events and cardiovascular effects were generally consistent with its established profile.3 Across the short-term efficacy studies,6,7 treatment-emergent adverse events reported by ≥ 5% of participants at > 2 times the rate of placebo were dry mouth, insomnia, decreased appetite, feeling jittery, and constipation. During the maintenance-of-efficacy study,8 treatment-emergent adverse events reported by > 5% of participants at twice the placebo rate during the randomized-withdrawal phase were upper respiratory tract infection and dry mouth. Across the phase 3 BED studies,7,8 mean increases from baseline in blood pressure and heart rate were consistent with the established safety profile of lisdexamfetamine,3 and with that of psychostimulants,9 in ADHD.
The cessation of psychostimulant use can be associated with withdrawal symptoms, including hypersomnia, increased appetite, and depressed mood.10 In 1 study11 of 56 individuals meeting diagnostic criteria for methamphetamine dependence, cessation of methamphetamine use was associated with red/itchy eyes, increased appetite, lack of motivation, decreased energy, and sleep difficulties. Therefore, it is important to know if the abrupt cessation of lisdexamfetamine treatment is associated with withdrawal symptoms in adults with BED.
The Amphetamine Cessation Symptom Assessment (ACSA) scale12 is a self-administered instrument that assesses the subjective symptoms of amphetamine withdrawal. The ACSA includes 16 items that can be summed to generate an aggregate score. In addition, 3 subscale scores can be generated by summing different item groupings (anxiety and mood subscale [based on 11 items], fatigue subscale [based on 3 items], and craving subscale [based on 2 items]).12 The ACSA, which was psychometrically evaluated in treatment-seeking amphetamine-dependent individuals,12 exhibited satisfactory reliability. Across a reported amphetamine use range of 0.1 to 6.5 g/d (median: 0.5 g) over the past month, there was a positive relationship between ACSA scores and the amount of amphetamines used (r = 0.24, P < .01),12 with the use of greater amounts of amphetamine being associated with higher ACSA scores (ie, greater withdrawal symptoms).
In the phase 3 lisdexamfetamine clinical program in BED, the ACSA was included as a secondary safety endpoint to measure potential amphetamine withdrawal symptoms following treatment cessation.7,8 There was no evidence of withdrawal following lisdexamfetamine treatment cessation in the phase 3 studies,7,8 as measured by ACSA aggregate scores at 7 days posttreatment. The objective of this post hoc analysis was to describe the time course of changes in ACSA aggregate and subscale scores following the cessation of lisdexamfetamine treatment in adults who participated in the phase 3 BED studies.7,8
METHODS
The designs and methods of the studies included in these analyses have previously been described in detail.7,8
Study Design and Treatment
Each study was conducted in accordance with the International Council for Harmonization Good Clinical Practice guidelines, the Declaration of Helsinki, and other applicable local ethical and legal requirements. The study protocols, amendments, final approved informed consent document, and all relevant supporting documentation were submitted to and approved by ethics committees and regulatory agencies (as appropriate) before study initiation. Written informed consent was required before study participation.

- The cessation of psychostimulant use can be associated with withdrawal symptoms.
- In clinical studies, adults with binge-eating disorder have been treated with lisdexamfetamine for up to 38 weeks.
- Evidence of amphetamine withdrawal symptoms following the cessation of lisdexamfetamine treatment in adults with binge-eating disorder was not observed in multiple clinical studies.
The short-term efficacy studies were randomized, double-blind, placebo-controlled, parallel-group, 12-week studies conducted from November 2012 to September 2013 (NCT01718483, NCT01718509).7 The short-term efficacy studies were identically designed; each included a 2-week screening phase, a 12-week double-blind phase (dose optimization, 4 weeks; dose maintenance, 8 weeks), and a follow-up phase of at least 1 week (Supplementary Figure 1A). Participants were treated daily with lisdexamfetamine (30 mg during week 1, 50 mg during week 2, and dose-optimized [50 or 70 mg] during weeks 3 and 4) or placebo. During weeks 5 through 12, the optimized lisdexamfetamine dose was maintained. If a dose reduction occurred during dose optimization, additional reductions were not allowed during the maintenance phase; participants requiring another dose reduction were discontinued.
The maintenance-of-efficacy study8 was a 38-week, double-blind, placebo-controlled, randomized-withdrawal study conducted from January 2014 to April 2015 (NCT02009163). It included a 12-week open-label phase (dose optimization, 4 weeks; dose maintenance, 8 weeks); a 26-week, double-blind, randomized-withdrawal phase; and a 1-week follow-up phase (Supplementary Figure 1B). During the open-label phase, participants were treated daily with lisdexamfetamine (30 mg during week 1, 50 mg during week 2, and 70 mg during week 3 [as clinically indicated and tolerated]). Down-titration to 50 mg lisdexamfetamine was allowed if 70 mg was not tolerated. Once a dose reduction took place, further changes were not allowed. No dose changes were permitted after week 3. If lisdexamfetamine 50 mg was not tolerated, the participant was discontinued. At the end of the open-label phase, lisdexamfetamine responders (individuals reporting ≤ 1 binge-eating day/wk for 4 consecutive weeks and having a Clinical Global Impressions-Severity [CGI-S] rating ≤ 2 at randomization) were randomized 1:1 to placebo or continued treatment with the optimized dose of lisdexamfetamine (50 or 70 mg) established during the open-label phase.
Participants
Men and nonpregnant women (aged 18-55 years) were eligible to participate. Key inclusion criteria across all 3 studies were meeting DSM-IV-TR criteria for BED (confirmed by the eating disorders module of the Structured Clinical Interview for DSM-IV-TR Axis I Disorders13 and the Eating Disorder Examination Questionnaire14), having protocol-defined moderate to severe BED (≥ 3 binge-eating days/wk during the 14 days before baseline and a CGI-S15 rating ≥ 4 at screening and baseline), and having a body mass index ≥ 18 and ≤ 45 kg/m2. Key exclusion criteria across all 3 studies included current anorexia nervosa or bulimia nervosa diagnosis; comorbid psychiatric disorder controlled with prohibited medications or uncontrolled and associated with significant symptoms; considered a suicide risk, previously made a suicide attempt, or currently demonstrating active suicidal ideation; history of symptomatic cardiovascular issues; moderate or severe hypertension; resting average sitting systolic blood pressure > 139 mm Hg or diastolic blood pressure > 89 mm Hg at screening or baseline; lifetime history of amphetamine or psychostimulant abuse or recent history of substance abuse or dependence; and intolerance or hypersensitivity to lisdexamfetamine or related compounds.
Endpoints
Findings for the key prespecified endpoints have been reported.7,8 This report describes data from the ACSA, which was a prespecified secondary safety endpoint in all 3 studies. The ACSA12 contains 16 items that are rated on 5-point scales (0 [not at all] to 4 [extremely]). Individual items are summed to generate an aggregate score (score range, 0-64; higher scores indicate greater withdrawal symptom severity) and 3 subscale scores (anxiety and mood [11 items; range, 0-44], fatigue [3 items; range, 0-12], and craving [2 items; range, 0-8]). The ACSA was administered at baseline and daily from the week 12/early termination (ET) visit through the follow-up visit in the short-term efficacy studies and at baseline, week 12, and daily from the week 38/ET visit through the follow-up visit in the maintenance-of-efficacy study.
Data Presentation and Statistical Analyses
Descriptive statistics are presented for ACSA scores at baseline (randomized-withdrawal baseline for the maintenance-of-efficacy study), the day of the last study drug dose, and for 7 days after the last study drug dose. In the short-term studies, pooled data from study completers from the safety analysis set (participants who took ≥ 1 study drug dose and had ≥ 1 safety assessment) were used. In the maintenance-of-efficacy study, data from completers from the randomized safety analysis set (participants who took ≥ 1 study drug dose during the open-label phase, had ≥ 1 postbaseline safety assessment, and took ≥ 1 study drug dose during the double-blind randomized-withdrawal phase) were used. Inferential statistics are not reported because the studies were not powered for assessment of this secondary safety endpoint.
RESULTS
Participant Disposition and Demographics
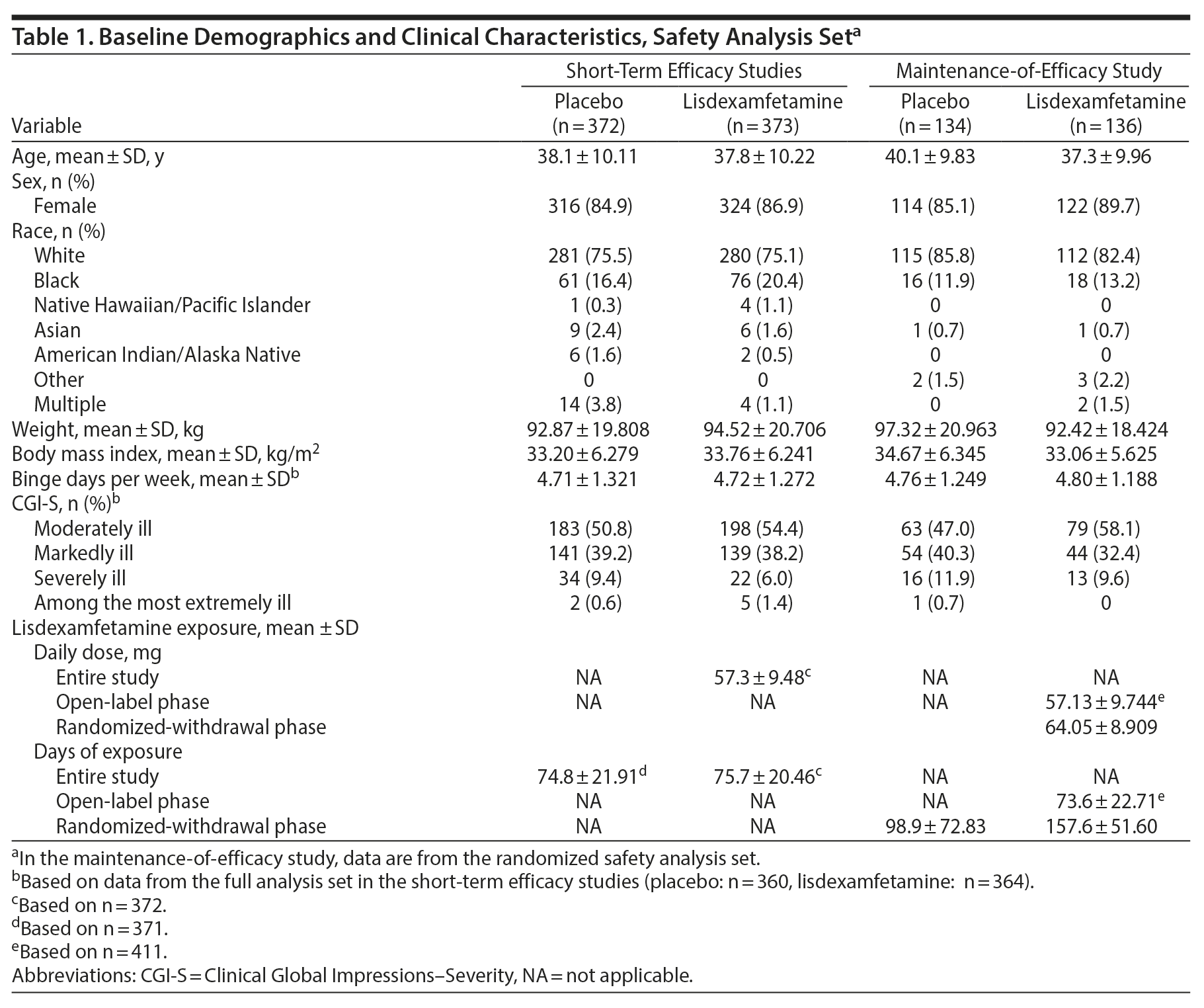
In the short-term efficacy studies, the pooled safety analysis set and pooled completer set, respectively, included 372 and 304 placebo participants and 373 and 305 lisdexamfetamine participants. In the maintenance-of-efficacy study, the randomized safety analysis set and randomized-withdrawal phase completer set, respectively, included 134 and 50 placebo participants and 136 and 102 lisdexamfetamine participants. Participant characteristics are summarized in Table 1.
ACSA Aggregate Scores
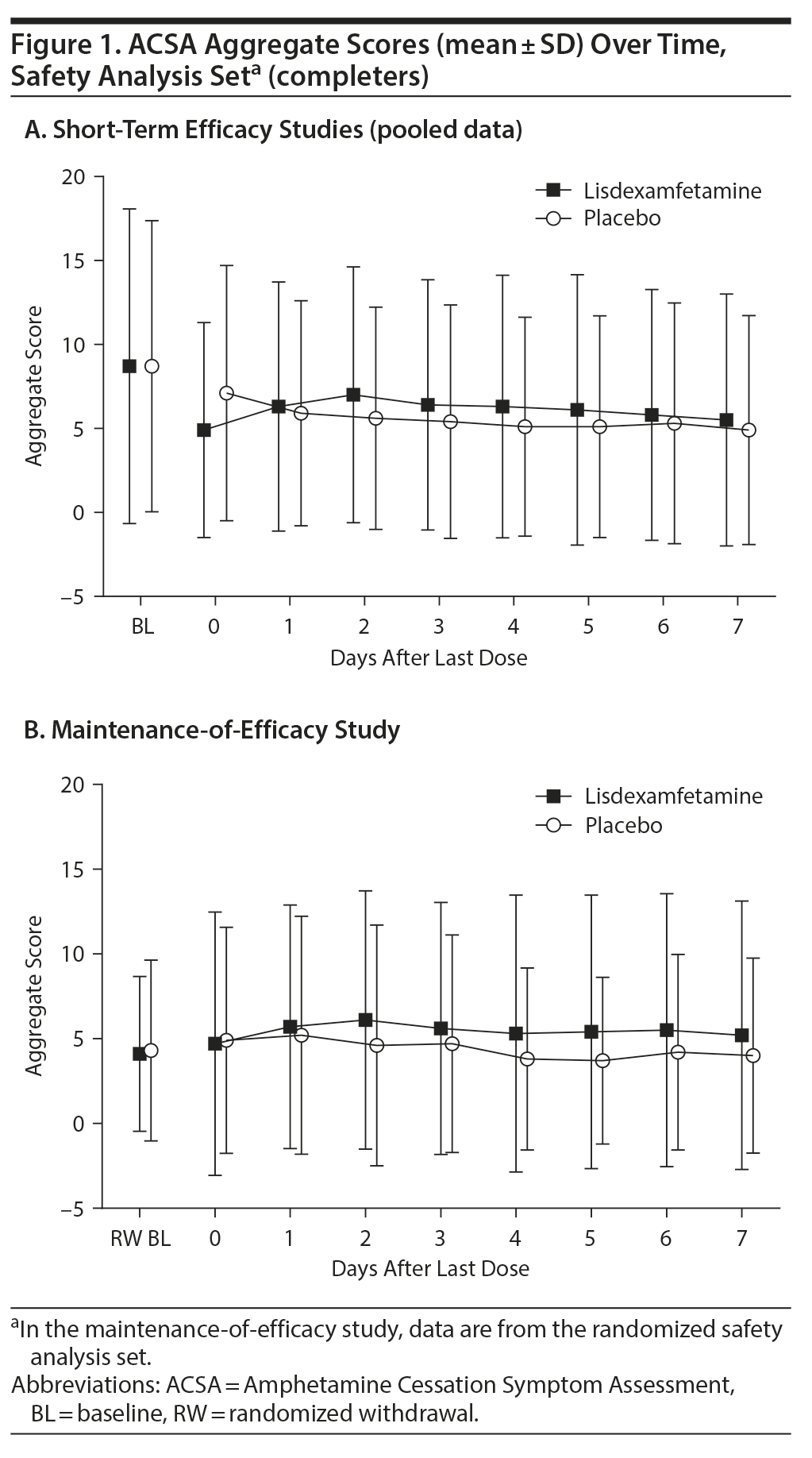
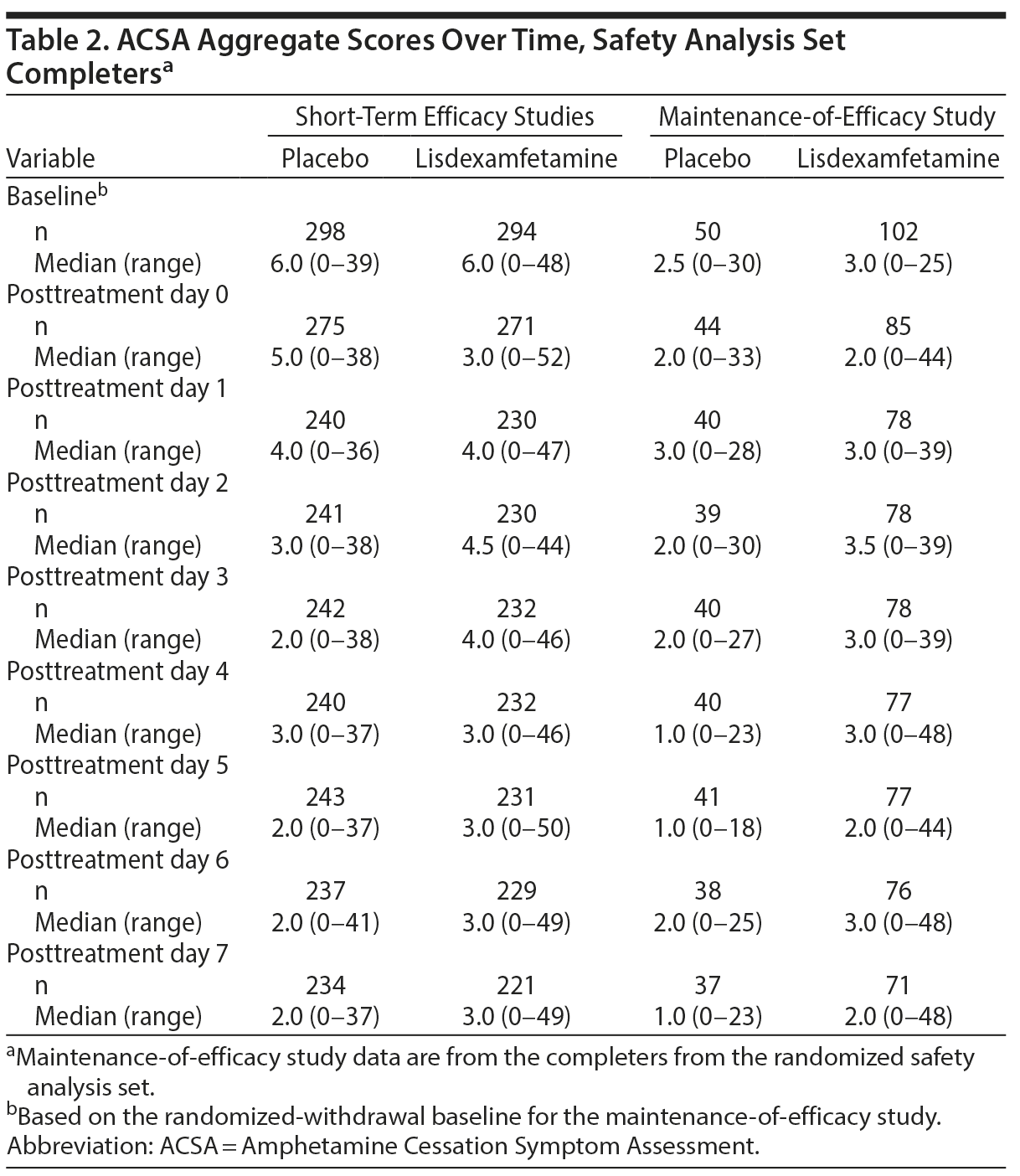
In the short-term efficacy studies, mean ± SD ACSA aggregate scores were 7.0 ± 7.60 with placebo and 4.9 ± 6.41 with lisdexamfetamine on day 0 (the last day of treatment). Mean (Figure 1A) and median (Table 2) ACSA aggregate scores were lower over the 7 days following the last placebo dose than on day 0. Mean ± SD scores for placebo and lisdexamfetamine (pooled data), respectively, were 4.8 ± 6.82 and 5.5 ± 7.50 on day 7 after the last dose. The maximum ACSA aggregate score at day 0 was only exceeded on post-placebo treatment day 6 (Table 2). With, mean ± SD ACSA aggregate scores were increased on all post-lisdexamfetamine treatment days relative to day 0; ACSA aggregate scores peaked on post-lisdexamfetamine treatment day 2 and then decreased through post-lisdexamfetamine treatment day 7 (Figure 1A). Median ACSA aggregate scores on post-lisdexamfetamine treatment days 1, 2, and 3 exceeded the median ACSA aggregate score on day 0; the maximum ACSA aggregate score on day 0 was not exceeded on any post-lisdexamfetamine treatment day (Table 2).
In the maintenance-of-efficacy study, mean ± SD ACSA aggregate scores were 4.8 ± 6.67 with placebo and 4.7 ± 7.78 with lisdexamfetamine on day 0. The mean and median ACSA aggregate score increased on post-placebo treatment day 1 (5.1 ± 7.02 and 3.0), but mean and median scores were equal to or lower than day 0 score from post-placebo treatment days 2 through 7 (Figure 1B, Table 2). Mean ± SD aggregate scores for placebo and lisdexamfetamine, respectively, were 3.9 ± 5.75 and 5.2 ± 7.93 on day 7 after the last dose. The maximum ACSA aggregate score at day 0 was not exceeded on any post-placebo treatment day (Table 2). With lisdexamfetamine, mean ± SD ACSA aggregate scores increased on all days after day 0, with scores peaking on post-lisdexamfetamine treatment day 2 (Figure 1B); median scores were increased relative to day 0 on post-lisdexamfetamine treatment days 1-4 and 6 (Table 2). The maximum ACSA aggregate score at day 0 was exceeded on post-lisdexamfetamine treatment days 4, 6, and 7 (Table 2).
ACSA Subscale Scores
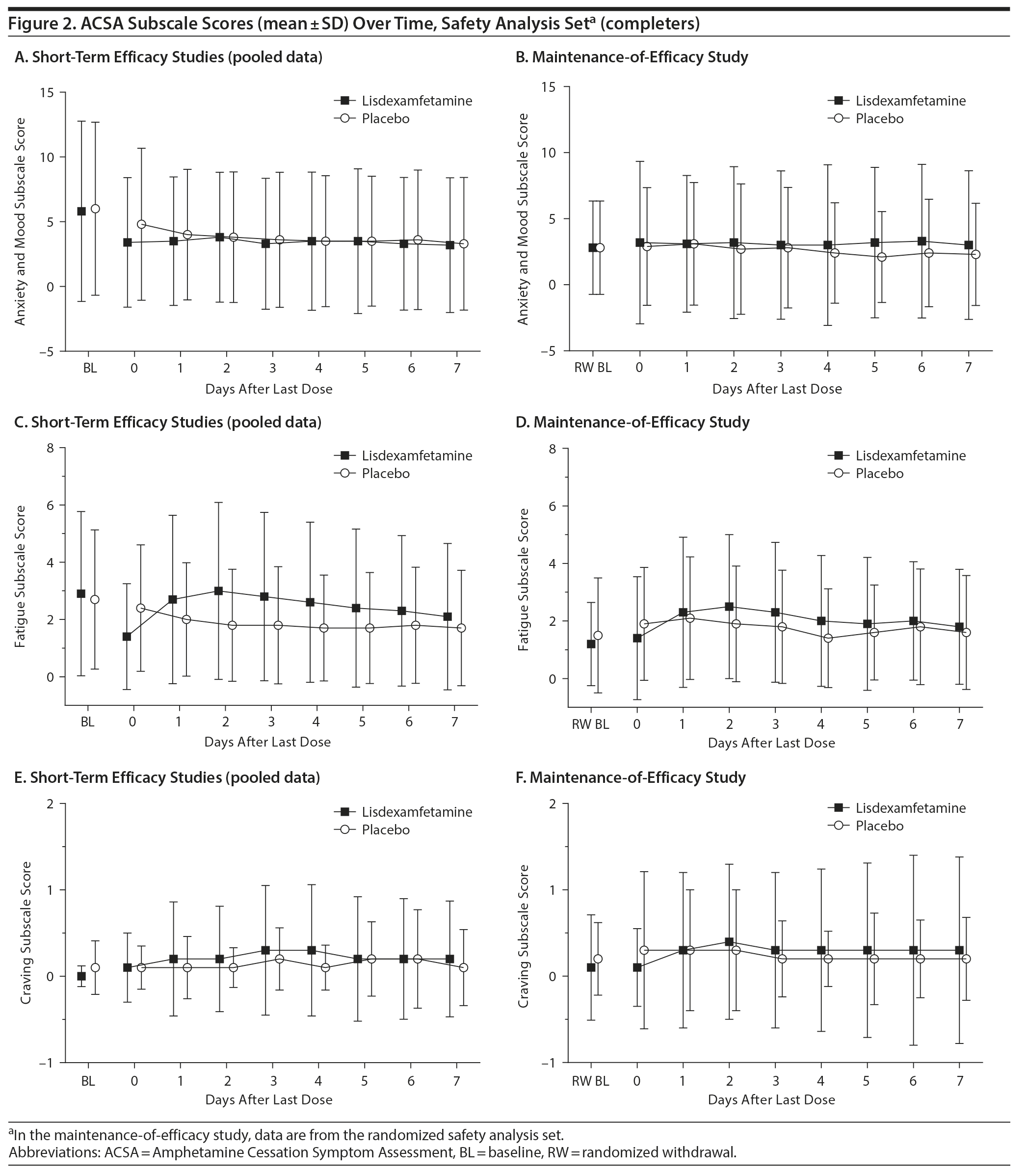
ACSA subscale scores over time in the short-term efficacy and maintenance-of-efficacy studies are presented in Figures 2A-2F. In the short-term efficacy studies, mean ± SD ACSA subscale scores (placebo and lisdexamfetamine, respectively) on day 0 were 4.7 ± 5.87 and 3.4 ± 5.01 for anxiety and mood, 2.3 ± 2.21 and 1.4 ± 1.85 for fatigue, and 0.0 ± 0.25 and 0.1 ± 0.40 for craving (Figures 2A, 2C, and 2E). Mean and median ACSA subscale scores over all post-placebo dose days were less than or equal to scores on day 0 for the anxiety and mood (Figure 2A, Table 3) and fatigue (Figure 2C, Table 3) subscales. Mean and median scores on the craving subscale were at or near 0 for all assessments (Figure 2E, Table 3). Maximum values exceeded day 0 on post-placebo treatment days 2 and 3 for the anxiety and mood subscale, none of the post-placebo treatment days on the fatigue subscale, and on post-placebo treatment days 1 and 5-7 on the craving subscale (Table 3). With lisdexamfetamine, mean ± SD ACSA subscale scores were increased compared with day 0 at post-lisdexamfetamine treatment days 1, 2, 4, and 5 for the anxiety and mood subscale (Figure 2A) and on all post-lisdexamfetamine treatment days for the fatigue (Figure 2C) and craving (Figure 2E) subscales, with scores peaking on post-lisdexamfetamine treatment day 2 for the anxiety and mood and fatigue subscales and on post-lisdexamfetamine treatment days 3 and 4 for the craving subscale. Median values were numerically greater on post-lisdexamfetamine treatment days 1 and 2 compared with day 0 on the anxiety and mood subscale and on post-lisdexamfetamine treatment days 1-6 compared with day 0 on the fatigue subscale; median values on the craving subscale did not differ across assessment days (Table 3). Maximum values exceeded the day 0 value on none of the post-lisdexamfetamine treatment days for the anxiety and mood subscale and on all post-lisdexamfetamine treatment days for the fatigue and craving subscales (Table 3).
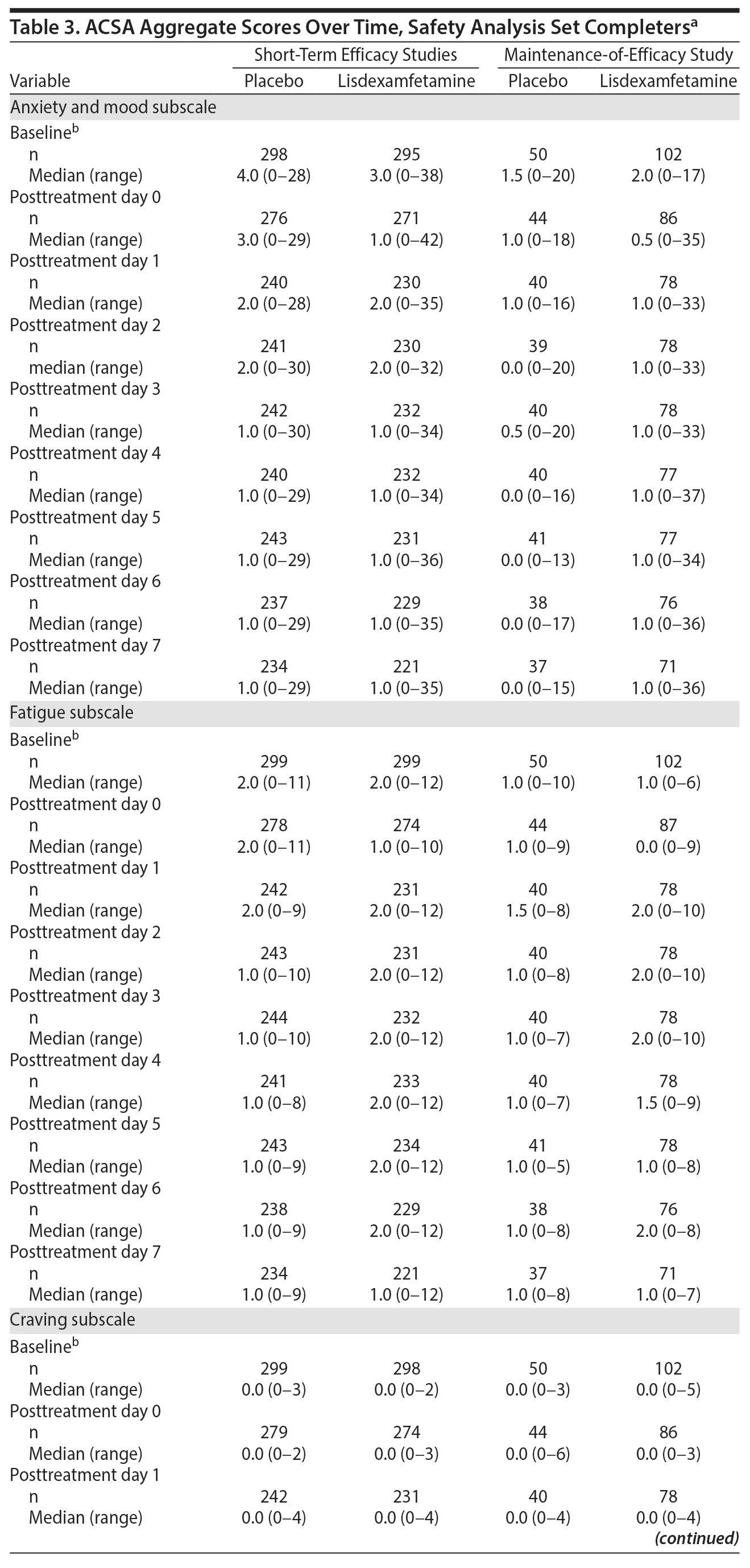
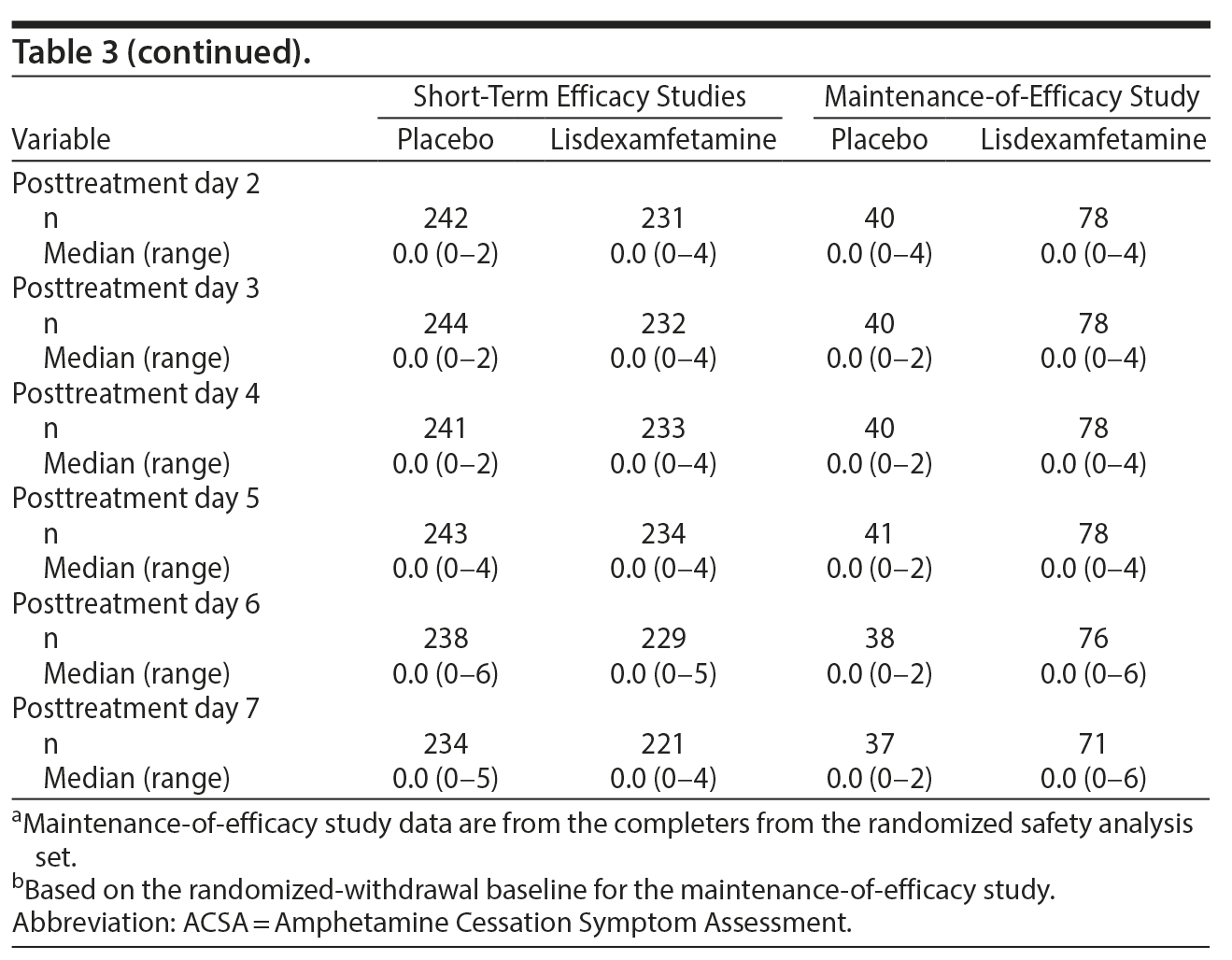
In the maintenance-of-efficacy studies, mean ± SD ACSA subscale scores (placebo and lisdexamfetamine, respectively) were 2.8 ± 4.46 and 3.2 ± 6.15 for anxiety and mood, 1.8 ± 1.96 and 1.4 ± 2.14 for fatigue, and 0.2 ± 0.91 and 0.1 ± 0.45 for craving on day 0. Mean ± SD ACSA subscale scores were increased compared with day 0 on post-placebo treatment day 1 for the anxiety and mood (Figure 2B) and the fatigue (Figure 2D) subscales. Across all post-placebo treatment days, craving subscale scores were equal to or less than the day 0 score (Figure 2F). Post-placebo treatment day median values did not exceed the day 0 value on any day for the anxiety and mood or craving subscales and only exceeded day 0 on post-placebo treatment day 1 on the fatigue subscale (Table 3). Maximum values exceeded day 0 on post-placebo treatment days 2 and 3 on the anxiety and mood subscale, but not on any post-placebo treatment day on the fatigue and craving subscales (Table 3). With the lisdexamfetamine group, mean scores on the anxiety and mood subscale were increased on post-lisdexamfetamine treatment day 6 compared with day 0 and across all post-lisdexamfetamine treatment days on the fatigue (Figure 2D) and craving (Figure 2F) subscales. Median values exceeded the day 0 value on all post-lisdexamfetamine treatment days on the anxiety and mood and fatigue subscales, but not on any post-lisdexamfetamine treatment day on the craving subscale (Table 3). Maximum values exceeded the day 0 value on post-lisdexamfetamine treatment days 4, 6, and 7 on the anxiety and mood subscale, days 1-3 on the fatigue subscale, and on all post-lisdexamfetamine treatment days on the craving subscale (Table 3).
DISCUSSION
In the short-term efficacy studies and 38-week maintenance-of-efficacy study, mean ACSA aggregate and subscale scores were numerically comparable in placebo and lisdexamfetamine participants at baseline, the day of the last dose, and over the 7 days following the cessation of treatment. Although the mean ACSA aggregate score on day 0 with placebo exceeded the mean aggregate score with lisdexamfetamine in the short-term efficacy studies, score variability was high for both groups on day 0 (SDs of 7.60 with placebo and 6.41 with lisdexamfetamine). Furthermore, day 0 aggregate scores were low relative to the maximum possible aggregate score of 64. Therefore, these differences in mean aggregate score are unlikely to have clinical significance. Consistent with the mean data, median values were also similar between treatments over time, and maximum values during post-treatment days infrequently exceeded baseline or day 0 values. These findings suggest that abrupt lisdexamfetamine cessation after as long as 38 weeks of treatment was not associated with clinically relevant amphetamine withdrawal symptoms during a 1-week posttreatment assessment period.
Although mean and median ACSA scores did not approach the maximum values allowed by the scales in either treatment group, trends toward increases in mean and median scores relative to day 0 were observed more frequently with lisdexamfetamine treatment than placebo treatment. However, these increases were small in magnitude, and separation from placebo was not observed. This finding could indicate that either there were no differences in amphetamine withdrawal symptomatology between treatment groups or that the ACSA was not sensitive enough to assess differences based on the study sample sizes. It should also be noted that increases in maximum aggregate and subscale scores were observed on posttreatment days compared with day 0, and these increases were observed more frequently with lisdexamfetamine than placebo. The maximum values reported should be considered cautiously because they may represent outliers. Detailed patient-level analyses are required to more definitively examine this issue.
Although it has been suggested that abrupt cessation of psychostimulant medication may result in withdrawal effects,16 data assessing withdrawal in individuals with ADHD following cessation of psychostimulant treatment are limited. A 2006 literature review17 found no studies examining psychostimulant withdrawal in adults with ADHD. Only a few small studies and case reports were identified in children with ADHD, and the authors17 reported that, apart from a return of hyperactivity, withdrawal effects were uncommon in children. Furthermore, in a randomized-withdrawal study18 of osmotic-release oral system methylphenidate, adults with ADHD treated for at least 52 weeks exhibited no adverse events indicative of withdrawal after being switched to placebo. It is also worth noting that in clinical studies of lisdexamfetamine in schizophrenia19 and major depressive disorder,20,21 cessation of lisdexamfetamine treatment was also not associated with symptoms of amphetamine withdrawal as measured by ACSA scores.
Amphetamine withdrawal is associated with physical symptoms (eg, headache, constipation, diarrhea, irregular/pounding heartbeat, red/itchy eyes, muscle or joint pain) and a variety of emotional (eg, depression, decreased motivation) and functional (eg, increased appetite, sleep difficulties) symptoms.11 In the clinical experience of the authors, amphetamine withdrawal symptoms are generally observed in individuals chronically using supratherapeutic doses of amphetamine. For example, in 1 published study12of amphetamine withdrawal, study participants were amphetamine-dependent individuals who used a median of 0.5 g/d amphetamine over the past month and used amphetamine for more than 8 years. This duration and level of amphetamine exposure far exceeds the amphetamine exposure levels that occurred during the course of the lisdexamfetamine studies included in these analyses.
These data should be interpreted in light of several limitations. First, these descriptive data are based on a secondary endpoint for which the studies were not powered, so statistical inferences were not made. Second, study attrition could have influenced the results observed during the randomized-withdrawal phase of the maintenance-of-efficacy study. Third, participants were predominantly women and white, met criteria for obesity, and had no current psychiatric comorbidities, so it is unknown how these data would generalize to a more diverse BED population. Also, vital sign measurements were not included as part of these analyses, so it is unknown if these physical symptoms would have provided evidence supportive of amphetamine withdrawal. Additionally, drug accountability was assessed using pill counts (to determine unused study product). Therefore, treatment compliance, which may have affected the results, cannot be confirmed. Lastly, the 7-day follow-up phase used in these studies would be considered the acute phase of amphetamine withdrawal.12,22 Thus, it may be of value to examine longer posttreatment periods to assess withdrawal symptoms that might persist over longer periods.
CONCLUSIONS
Across all studies, mean and median ACSA scores did not approach the maximum scores allowed by the scale and were similar in the placebo and lisdexamfetamine groups at baseline, the day of the last dose, and over the 7 days following the last study drug dose. These study results suggest that, on average, abrupt lisdexamfetamine termination was not associated with amphetamine withdrawal symptoms as measured by mean aggregate and subscale scores on the ACSA after up to 38 weeks of once-daily treatment at therapeutic doses.
Submitted: September 5, 2019; accepted December 17, 2019.
Published online: March 26, 2020.
Potential conflicts of interest: Dr Robertson was an employee of Shire, a member of the Takeda group of companies, at the time this research was conducted and holds Takeda stock; she is currently employed by Yumanity Therapeutics Inc (Cambridge, Massachusetts). Dr Wu was an employee of Shire, a member of the Takeda group of companies, at the time this research was conducted and holds Takeda stock; he is currently employed by Ironwood Pharmaceuticals (Boston, Massachusetts). Drs Fant and Schnoll are employees of Pinney Associates Inc, which received funding from Shire, a member of the Takeda group of companies, to provide consultation services regarding these data. Dr McElroy is a consultant to and has received grant support from Shire; has been or is a consultant to or member of the scientific advisory boards of Alkermes, Avanir, Bracket, Corcept, F. Hoffmann-LaRoche Ltd, MedAvante, Mitsubishi Tanabe, Myriad, Naurex, Novo Nordisk, Opiant, Otsuka, Sunovion, and Teva; received grant support from the Agency for Healthcare Research & Quality, Alkermes, AstraZeneca, Azevan, Brainsway, Cephalon (now Teva), Forest, Lilly, Marriott Foundation, Medibio, National Institute of Mental Health, Naurex, Neurocrine, Novo Nordisk, Orexigen, Pfizer, Sunovion, Takeda, and Transcept; is inventor on United States patent no. 6,323,236 B2 (Use of Sulfamate Derivatives for Treating Impulse Control Disorders) and, along with the patent’s assignee (University of Cincinnati, Cincinnati, Ohio), has received payments from Johnson & Johnson, which has exclusive rights under the patent.
Funding/support: This clinical research was funded by Shire Development LLC, a member of the Takeda group of companies, Lexington, Massachusetts. Shire Development LLC, a member of the Takeda group of companies, provided funding to Complete Healthcare Communications, LLC (CHC; North Wales, Pennsylvania), a CHC group company, for support in writing and editing this manuscript.
Role of the sponsor: The sponsor was involved in the design and conduct of these studies; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript.
Previous presentations: These data were presented at the Annual Meetings of the American Society of Clinical Psychopharmacology; May 30-June 3, 2016; Scottsdale, Arizona, and Eating Disorders Research Society; October 27-29, 2016; New York, New York.
Acknowledgments: Under the direction of the authors, Shelly Lim, PhD, and Craig Slawecki, PhD, employees of CHC, provided writing and formatting assistance for this manuscript. Drs Lim and Slawecki have no conflicts of interest to disclose related to the subject of this article.
Supplementary material: See accompanying pages.
REFERENCES
1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
2.Vyvanse Product Monograph [packge insert]. Saint-Laurent, Québec: Shire Pharma Canada ULC; 2016.
3.Vyvanse (lisdexamfetamine dimesylate) [packge insert]. Lexington, MA: Shire US Inc; 2017.
4.Venvanse (dimesilato de lisdexanfetamina) [packge insert]. São Paulo, Brazil: Shire Farmacêutica Brasil Ltda; 2019.
5.Elvanse (lisdexamfetamine dimesylate) [packge insert]. London, UK: Shire Pharmaceutical Contracts Ld; 2019.
6.McElroy SL, Hudson JI, Mitchell JE, et al. Efficacy and safety of lisdexamfetamine for treatment of adults with moderate to severe binge-eating disorder: a randomized clinical trial. JAMA Psychiatry. 2015;72(3):235-246. PubMed CrossRef
7.McElroy SL, Hudson J, Ferreira-Cornwell MC, et al. Lisdexamfetamine dimesylate for adults with moderate to severe binge eating disorder: Results of two pivotal phase 3 randomized controlled trials. Neuropsychopharmacology. 2016;41(5):1251-1260. PubMed CrossRef
8.Hudson JI, McElroy SL, Ferreira-Cornwell MC, et al. Efficacy of lisdexamfetamine in adults with moderate to severe binge-eating disorder: a randomized clinical trial. JAMA Psychiatry. 2017;74(9):903-910. PubMed CrossRef
9.Duong S, Chung K, Wigal SB. Metabolic, toxicological, and safety considerations for drugs used to treat ADHD. Expert Opin Drug Metab Toxicol. 2012;8(5):543-552. PubMed CrossRef
10.Phillips KA, Epstein DH, Preston KL. Psychostimulant addiction treatment. Neuropharmacology. 2014;87:150-160. PubMed CrossRef
11.Zorick T, Nestor L, Miotto K, et al. Withdrawal symptoms in abstinent methamphetamine-dependent subjects. Addiction. 2010;105(10):1809-1818. PubMed CrossRef
12.McGregor C, Srisurapanont M, Mitchell A, et al. Psychometric evaluation of the Amphetamine Cessation Symptom Assessment. J Subst Abuse Treat. 2008;34(4):443-449. PubMed CrossRef
13.First M, Spitzer R, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I), Clinician Version. Arlington, VA: American Psychiatric Publishing; 1997.
14.Fairburn CG, Beglin SJ. Assessment of eating disorders: interview or self-report questionnaire? Int J Eat Disord. 1994;16(4):363-370. PubMed
15.Guy W. Clinical Global Impression (CGI). Rockville, MD: US Department of Health, Education, and Welfare; 1976.
16.Kutcher S, Aman M, Brooks SJ, et al. International consensus statement on attention-deficit/hyperactivity disorder (ADHD) and disruptive behaviour disorders (DBDs): clinical implications and treatment practice suggestions. Eur Neuropsychopharmacol. 2004;14(1):11-28. PubMed CrossRef
17.Ashton H, Gallagher P, Moore B. The adult psychiatrist’s dilemma: psychostimulant use in attention deficit/hyperactivity disorder. J Psychopharmacol. 2006;20(5):602-610. PubMed CrossRef
18.Buitelaar JK, Trott GE, Hofecker M, et al. Long-term efficacy and safety outcomes with OROS-MPH in adults with ADHD. Int J Neuropsychopharmacol. 2012;15(1):1-13. PubMed CrossRef
19.Lasser RA, Dirks B, Nasrallah H, et al. Adjunctive lisdexamfetamine dimesylate therapy in adult outpatients with predominant negative symptoms of schizophrenia: open-label and randomized-withdrawal phases. Neuropsychopharmacology. 2013;38(11):2140-2149. PubMed CrossRef
20.Richards C, Iosifescu DV, Mago R, et al. A 12-month open-label extension study of the safety and tolerability of lisdexamfetamine dimesylate for major depressive disorder in adults. J Clin Psychopharmacol. 2018;38(4):336-343. PubMed CrossRef
21.Richards C, McIntyre RS, Weisler R, et al. Lisdexamfetamine dimesylate augmentation for adults with major depressive disorder and inadequate response to antidepressant monotherapy: Results from 2 phase 3, multicenter, randomized, double-blind, placebo-controlled studies. J Affect Disord. 2016;206:151-160. PubMed CrossRef
22.McGregor C, Srisurapanont M, Jittiwutikarn J, et al. The nature, time course and severity of methamphetamine withdrawal. Addiction. 2005;100(9):1320-1329. PubMed CrossRef
Please sign in or purchase this PDF for $40.00.
Save
Cite