Prim Care Companion CNS Disord 2022;24(3):21br03084
To cite: Naguy A, Alamiri B. Antidepressants–a misnomer? clinical impressionism or scientific empiricism? Prim Care Companion CNS Disord. 2022;24(3):21br03084.
To share: https://doi.org/10.4088/PCC.21br03084
© 2022 Physicians Postgraduate Press, Inc.
aAl-Manara CAP Centre, Kuwait Centre for Mental Health, Shuwaikh, Kuwait
*Corresponding author: Ahmed Naguy, MBBch, MSc, Al-Manara CAP Centre, Kuwait Centre for Mental Health, Jamal Abdul-Nassir St, Shuwaikh, Sulibikhat 21315, Kuwait ([email protected]).
Use of antidepressants on clinical grounds is overinflated both on- and off-label. Apart from depression, the clinical indications of the so-called antidepressants are legion. This is at times confusing to patients who, to their surprise, find themselves prescribed an antidepressant for their bulimia. The neuroscience-based nomenclature was a promising step to designate psychotropic agents by mode of action rather than by indication given the protean uses of these agents in real practice. This endeavor was unfortunately stymied given that monoaminergic mechanisms are now believed to be oversimplistic and reductionistic. Barring composite mechanisms, many antidepressants do possess “secondary pharmacodynamic actions” that contribute to clinical efficacy and are yet to be fully elucidated. Moreover, for a therapeutic response, too many factors are at play, including a patient’s genetics/epigenetics, heterogeneity of clinical phenotypes, and crosstalk between different neurocircuits and pathways (inflammatory, oxidative/nitrosative, hypothalamic-pituitary-adrenal axis, glucose, bioenergetics, one-carbon cycle, opioid, cholinergic, neutrophin signaling).1,2
We coined the term broad-spectrum psychotropic agents in lieu, akin to the “broad-spectrum antibiotics” (Ahmed Naguy, MBBch, MSc, personal communication, 2022). But, reading the history of psychiatry (eg, as in the case of the term hysteria), we do know some terms die hard and tend to outlive their obituaries.
Let us examine this contention with a closer look at the multitude of indications of antidepressants that render the alternative designation of broad-spectrum psychotropic agents, which we view to be more apt and evocative. That said, it should be kept in mind that the clinical indications discussed here are largely off-label, and only a modicum of the evidence base supports their use after exhausting more established options with a higher evidence base. Examination of the extant evidence is beyond the scope of this report.
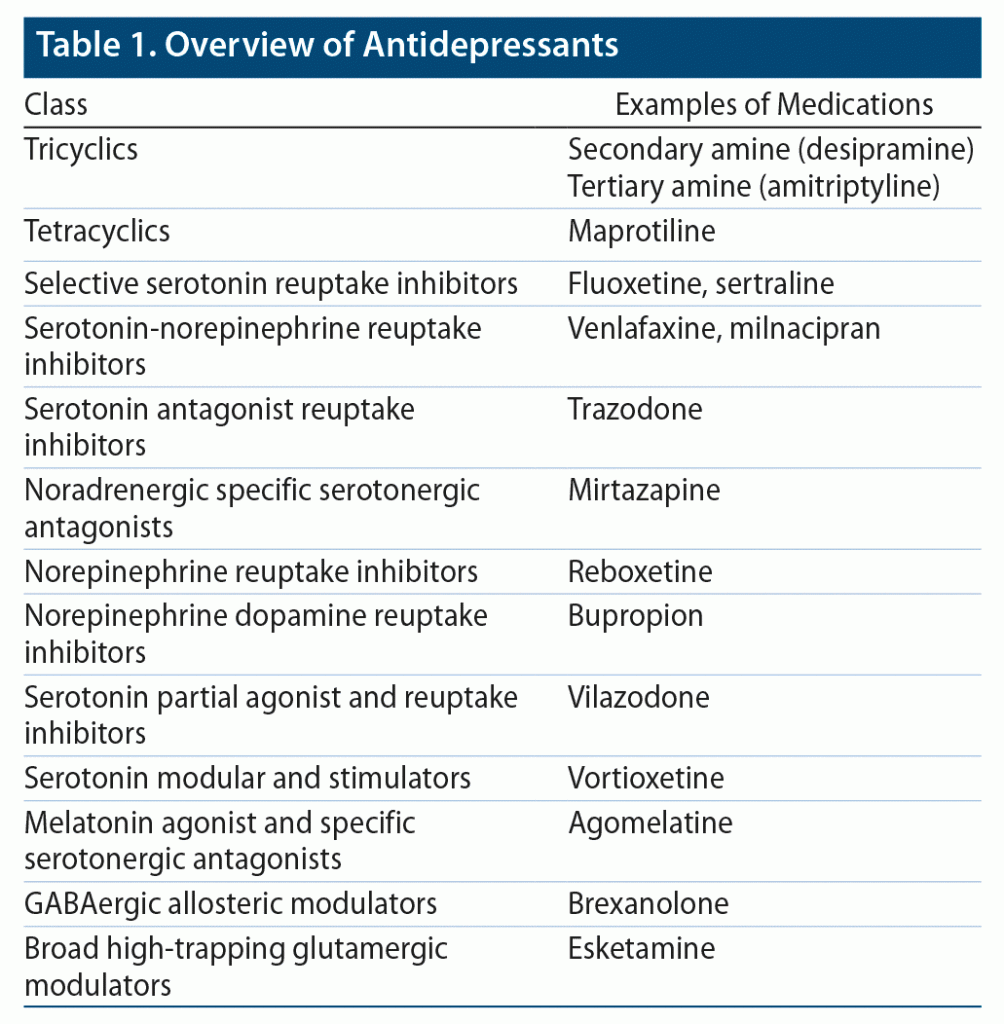
Since various regulatory bodies and agencies do differ on licensed indications and posology, readers are strongly advised to refer to their corresponding formularies lest inconsistencies should arise. An overview of antidepressants currently on the market is depicted in Table 1 following traditional nosology.
Antidepressants show a wide range of efficacy in the treatment of depressive disorders across different age groups. These disorders span unipolar major depressive disorder, bipolar depression, dysthymia, adjustment disorder with depressive symptoms, and the newly introduced disruptive mood dysregulation disorder. Although antidepressants are generally considered ineffective and potentially harmful and can trigger manic shifts and increase cyclicity in bipolar depression, some data support their use in bipolar II depression or only use in conjunction with a thymoleptic (best exemplified with the fixed fluoxetine-olanzapine combination). Bupropion is generally favored in these settings.3
Antidepressants, especially selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs), remain a first-line treatment for anxiety and trauma/stressor-related disorders including generalized anxiety disorder, panic disorder, specific phobias, and social anxiety disorder. Serotonergic antidepressants (SSRIs and clomipramine) are the mainstay of treatment in obsessive-compulsive disorder, especially at higher, at times supramaximal, doses and for longer duration.4
Fluoxetine at a high-dose, typically 60 mg/d, is indicated for bulimia nervosa. Sedating antidepressants at low doses (eg, mirtazapine, trazodone, doxepin) can help with insomnia. Mirtazapine at higher doses is more noradrenergic and hence less sedative. Trazodone5 at antidepressant doses is typically anxiogenic.
SSRIs are generally effective for parasomnias (eg, nightmare disorder) due to rapid eye movement–suppressant actions. SSRIs cause anorgasmia, and thus clinicians have used them to treat premature ejaculation. Dapoxetine is the only US Food and Drug Administration (FDA)–approved SSRI for this indication. Paroxetine is typically used.6
Some antidepressants (eg, amitriptyline) have been successfully used for (ciguatera) fish poisoning.7
Fluoxetine has been reported of use in recurrent syncope. This use should be balanced against rare reports of probable QTc prolongation.8
Bupropion9 has nACh antagonistic actions, hence the use for smoking cessation (branded as Zyban). Given that it boosts dopamine (DA) tone in the ventral tegmental area (Brodmann area 10), bupropion has been used in dual diagnoses as well where DA is linked to hedonism.
Bupropion, a norepinephrine-dopamine reuptake inhibitor (NDRI), has been used as third- or fourth-line treatment in attention-deficit/hyperactivity disorder (ADHD), especially with comorbid depression, by boosting DA and NE tone in prefrontal cortex for vigilance and attention. SNRIs have similarly been used to address ADHD.10 Imipramine, as a monoaminergic reuptake inhibitor, has also been used in the past for ADHD.
Antidepressants with procognitive actions have been used for executive dysfunction (eg, vortioxetine).11 Escitalopram has demonstrated procognitive actions and has been proposed preemptively for poststroke depression.12
Venlafaxine has been long used for cataplexy in narcolepsy. SSRIs have traditionally been used for impulse dyscontrol, eg, in cluster B personality disorders.13
Doxepin, a tricyclic antidepressant (TCA), has a potent antihistaminergic action and comes in a topical gel form (sold as Zonalon) for pruritis and allergy.14 TCAs (eg, amitriptyline) can be used for neuropathic pain and migraine prophylaxis. Antinociceptive actions may be due to facilitating analgesia by enhancing central monoaminergic transmission (SNRI) as well as facilitating the descending pain modulation system (decreasing afferent input through the spinothalamic tract). Central skeletal muscle relaxant action (depression polysynaptic reflexes causing muscle spasm/tension) could be contributory.15
Tianeptine, a unique selective serotonin reuptake enhancer,16 has been demonstrated to help with asthma and carcinoid syndrome. TCAs by virtue of anticholinergic actions can help with major depressive disorder (MDD) comorbid with Parkinson disease with improved motoric agility. This is tempered by TCA-inherent anticognitive downsides. Bupropion, an NDRI, can, at least in theory, mechanistically fit in Parkinson disease treatment to boost DA tone.17
Mirtazapine, along with β-blockers, is now first-line treatment for akathisia by virtue of 5-HT2A blockade, akin to cyproheptadine.18 Similarly, the TCA amitriptyline, due to M1 blockade, has been anecdotally used to safeguard against conventional antipsychotic-induced extrapyramidal symptoms.
SNRIs (eg, venlafaxine) have generally been used successfully to address vasomotor changes in menopause. Interestingly, low-dose paroxetine (branded as Brisdelle 7.5 mg) is FDA approved for this indication. Similarly, pulse use of SSRIs (eg, fluoxetine marketed as Sarafem) is indicated for premenstrual dysphoric disorder. Brexanolone infusion is FDA approved for postpartum depression.19
Amitriptyline has been previously trialed for bothersome clozapine-related sialorrhea. This combination is now discouraged, as it can increase risk of ileus. In the same vein, bupropion, albeit sounds psychotomimetic, has been reported to be helpful.
Imipramine is FDA approved for nocturnal enuresis.20 It can help through anticholinergic actions, impacting sleep structure and antidiuretic hormone.
Mirtazapine, through 5-HT3 blockade akin to setrons, has antiemetic actions.21 It has been reported to address MDD comorbid with hyperemesis gravidarum, morning sickness, and chemotherapy side effects.
SSRIs, due to anti-inflammatory actions, have been repurposed as antimicrobial. Fluvoxamine, possibly due to actions on sigma-1 receptors (with reduction in platelet aggregation, decreased mast cell degranulation, interference with endolysosomal viral trafficking, regulation of inositol-requiring enzyme 1α-driven inflammation, and increased melatonin levels), has demonstrated antiviral activity for COVID-19.22 Mirtazapine, similarly, has been used for progressive multifocal leukoencephalopathy.
SNRIs (eg, duloxetine, milnacipran) are approved for fibromyalgia, chronic fatigue syndrome, peripheral neuropathy, and urinary stress incontinence. Due to resultant increase in noradrenergic (NE) tone, stimulation of the α-adrenergic receptors promotes urinary continence via contraction of the bladder trigone and internal sphincter, while stimulation of β-receptors in the bladder results in smooth muscle relaxation of the bladder wall. Net effect would be urinary continence.23
Mirtazapine might be used to address stimulant-related appetite suppression in ADHD without compromising therapeutic response (compare to cyproheptadine). Mirtazapine stimulates appetite via H1 and 5-HT2c antagonism in eating disorders (eg, ARFID).24 Of related interest, duloxetine was found to be promising for binge-eating disorder.25 On the other hand, bupropion causes weight loss; hence, it is combined with naltrexone (Contrave) and zonisamide (Empatic) for obesity.
Prominent negative symptoms affect approximately 40% of patients with schizophrenia, which are pervasive but sometimes underrecognized and especially difficult to treat. Boosting NE drive with subsequent disinhibition of dopaminergic projections to the dorsolateral prefrontal cortex corrects the dopamine hypofrontality underlying the negative symptoms. Moreover, decreased NE in patients with chronic schizophrenia is well-documented in the literature, and psychotropics acting primarily to increase NE (eg, milnacipran) were reported to mitigate negative symptom domain.26
TCAs are traditionally prescribed for diarrhea-dominant irritable bowel syndrome (IBS), while SSRIs are prescribed for constipation-dominant IBS. Mirtazapine has been used successfully to address inappropriate sexual behaviors in autism spectrum disorder. Paroxetine, commonly in combination with atypical antipsychotic risperidone, has been used similarly.27
Antidepressants have antisuicidal actions especially evident for those aged >65 years.28 Neuroprotectant actions by promoting adult neurogenesis in dentate gyrus of hippocampus have also been demonstrated.29 Glutamate-based agents, known as rapidly acting antidepressants, ketamine and esketamine have demonstrated robust antisuicidal actions.30,31
SSRIs have generally been used for agitation in dementia, more so in frontotemporal dementia.32 Likewise, in open-label trials SSRIs were shown to help behavioral dyscontrol in neurodevelopmental disorders (eg, autism). Fluoxetine has long been used by neurologists to address pseudobulbar affect.33
This list is by no means inclusive and in fact keeps expanding, reflective of the wide therapeutic potential of antidepressants (or lack of better alternatives), but gives credence to the notion of broad-spectrum psychotropic agents. Trust, time would tell.
Submitted: July 29, 2021; accepted September 24, 2021.
Published online: June 14, 2022.
Relevant financial relationships: None.
Funding/support: None.
References (33)

- Moodliar S, Naguy A. What’s around the corner in the niche of psychopharmacotherapy of major depressive disorder? Asian J Psychiatr. 2019;46:13–14. PubMed CrossRef
- Naguy A, Pridmore S, Abuzeid MY, et al. Depression: a gut “microbiome” feeling! J Nerv Ment Dis. 2021;209(9):691–692. PubMed CrossRef
- Naguy A. Adolescent depression- pharmacotherapy or psychotherapy? J Dev Behav Pediatr. 2016;37(3):264. PubMed CrossRef
- Naguy A, Alamiri B. Treatment-resistant OCD: a psychopharmacological ‘touche d’art.’ Asian J Psychiatr. 2018;34:98–99. PubMed CrossRef
- Albert U, Lamba P, Stahl SM. Early response to trazodone once-a-day in major depressive disorder: review of the clinical data and putative mechanism for faster onset of action. CNS Spectr. 2021;26(3):232–242. PubMed CrossRef
- Naguy A. Paroxetine: into oblivion? well, guess not. Prim Care Companion CNS Disord. 2019;21(2):18l02369. PubMed CrossRef
- Davis RT, Villar LA. Symptomatic improvement with amitriptyline in ciguatera fish poisoning. N Engl J Med. 1986;315(1):65. PubMed CrossRef
- Naguy A. Psychotropic drugs and prolonged QTc interval: does it really that matter? Indian J Psychol Med. 2016;38(2):165–166. PubMed CrossRef
- Naguy A, Badr BHM. Bupropion- myth-bsting! [published online ahead of print April 12, 2021]. CNS Spectr. 2021:1–2.
- Naguy A, Elsori DH, AlAwadhi DS, et al. Add-on milnacipran boosts methylphenidate response in an adolescent with attention-deficit/hyperactivity disorder with comorbid anxiety and enuresis. Am J Ther. 2019;26(6):e730–e732. PubMed CrossRef
- Blumberg MJ, Vaccarino SR, McInerney SJ. Procognitive effects of antidepressants and other therapeutic agents in major depressive disorder: a systematic review. J Clin Psychiatry. 2020;81(4):4. PubMed CrossRef
- Naguy A, Moodliar-Rensburg S, Alamiri B. Cognitive symptoms domain in major depressive disorder-revisited. Asian J Psychiatr. 2020;53:102216. PubMed CrossRef
- Naguy A. Viewpoint: borderline personality disorder-light at the end of the tunnel! Isr J Psychiatry. 2020;57(1):41–43.
- Naguy A. Transdermal psychotropic agents: “aye” or “nay”? Prim Care Companion CNS Disord. 2021;23(2):2. PubMed CrossRef
- Naguy A. Milnacipran for postcoital cephalgia and premature ejaculation. Am J Ther. 2019;26(5):e662–e664. PubMed CrossRef
- Naguy A, Ali M, Elsori DH, et al. A case of prepubertal low-functioning autism with behavioral decompensation favorably responding to tianeptine. J Clin Psychopharmacol. 2019;39(5):524–525. PubMed CrossRef
- Naguy A. Parkinson disease-associated psychosis: psychopharmacological considerations [published online ahead of print December 26, 2020]. Am J Ther. 2020. PubMed CrossRef
- Naguy A, Moodliar-Rensburg S, Alamiri B. Mirtazapine treatment for azithromycin-associated akathisia. Am J Ther. 2020;28(6):e751–e752. PubMed CrossRef
- Naguy A. Brexanolone and postpartum depression: what does it have to do with GABA? Arch Women Ment Health. 2019;22(6):833–834. PubMed CrossRef
- Naguy A. Imipramine-precipitated status epilepticus. World J Pediatr. 2021;17(2):218–219. PubMed CrossRef
- Naguy A. Mirtazapine for major depression developed after hyperemesis gravidarum. Am J Ther. 2019;26(5):e661–e662. PubMed CrossRef
- Sukhatme VP, Reiersen AM, Vayttaden SJ, et al. Fluvoxamine: a review of its mechanism of action and its role in COVID-19. Front Pharmacol. 2021;12:652688. PubMed CrossRef
- Naguy A. Atomoxetine successfully addresses double-sphincteric incontinence in child with attention-deficit/hyperactivity disorder. J Clin Psychopharmacol. 2016;36(6):743. PubMed CrossRef
- Naguy A, Roshdy R, Al-Mutairi A, et al. Mirtazapine improved eating patterns in avoidant/restrictive food intake disorder [published online ahead of print January 20, 2021]. Am J Ther. 2021. PubMed CrossRef
- Guerdjikova AI, McElroy SL, Winstanley EL, et al. Duloxetine in the treatment of binge eating disorder with depressive disorders: a placebo-controlled trial. Int J Eat Disord. 2012;45(2):281–289. PubMed CrossRef
- Naguy A. Negative symptom domain schizophrenia: a psychopharmacologic impasse? Prim Care Companion CNS Disord. 2022. In press.
- Naguy A, Alrashidi F, AlShalabi SR. Mirtazapine for inappropriate sexual behaviors in autism. Am J Ther. 2019;26(6):e751–e753. PubMed CrossRef
- Naguy A, AlAwadhi D. Psychopharmacology of suicide. Asian J Psychiatr. 2018;36:100–101. PubMed CrossRef
- Naguy A, Elbadry H, Salem H. suicide: a précis! J Family Med Prim Care. 2020;9(8):4009–4015. PubMed CrossRef
- Naguy A. RAADs (rapidly acting anti-depressants)- a quantum leap? Asian J Psychiatr. 2019;44:156–157. PubMed CrossRef
- Naguy A, Alamiri B. 2019 FDA approved psychotherapeutic medications. Asian J Psychiatr. 2020;49:101976. PubMed CrossRef
- Manoochehri M, Huey ED. Diagnosis and management of behavioral issues in frontotemporal dementia. Curr Neurol Neurosci Rep. 2012;12(5):528–536. PubMed CrossRef
- McGrane I, VandenBerg A, Munjal R. Treatment of pseudobulbar affect with fluoxetine and dextromethorphan in a woman with multiple sclerosis. Ann Pharmacother. 2017;51(11):1035–1036. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite