;
;
Antioxidants for the Treatment of Tardive Dyskinesia:
AIMing to Find the Best Treatment Option
Tardive dyskinesia (TD), a neurologic disorder of involuntary movements, most commonly occurs in 15%-30% of patients taking antipsychotic drugs for many years; however, in some cases, TD may occur after a brief exposure to the offending agent.1 The Abnormal Involuntary Movement Scale (AIMS)2 can detect TD and measure the severity of dyskinesias over time. An AIMS score of 2 in ≥ 2 movement categories or a score ≥ 3 in a single movement category is considered a positive AIMS score.2
The cause of TD is not entirely known; however, multiple theories exist. Currently, the 2 most accepted hypotheses are upregulation of postsynaptic dopamine receptor responsiveness and oxidative stress.1 This report will mainly focus on the oxidative stress theory. TD has been suggested to occur as a result of the blockage of dopamine receptors by antipsychotic drugs, leading to increased dopamine metabolism and turnover and resulting in free radicals causing neuronal damage.1,3,4
Recently, new pharmacologic discoveries for the treatment of TD have occurred, which include deutetrabenazine and valbenazine. Both agents are vesicular monoamine transporter 2 inhibitors.3 While clinical studies5-7 have found these medications to cause a statistically significant decrease in a patient’s AIMS score, their use comes with a large price tag. The wholesale acquisition cost of deutetrabenazine and valbenazine is $90,071 and $75,789, respectively.5 While wholesale acquisition costs are quite large for these medications, assistance programs are provided through Shared Solutions8 for deutetrabenazine and INBRACE9 for valbenazine that may reduce copays for both patients and practitioners if qualifications are met. However, for mild cases of TD, clinicians may want to seek out more cost-effective options that may also treat or prevent TD. It has been postulated that antioxidants serve a role in the prevention and treatment of TD due to the oxidative stress hypothesis.10 Antioxidants are stable molecules capable of donating an electron to a free radical, which results in its neutralization and thus decreases its ability to cause damage.10 Studies have analyzed the use of high doses of melatonin, omega-3, and vitamin B6 and their possible role in the prevention or treatment of TD. All 3 of these agents have shown beneficial effects in possibly reversing the symptoms of TD.11-14
Case Report
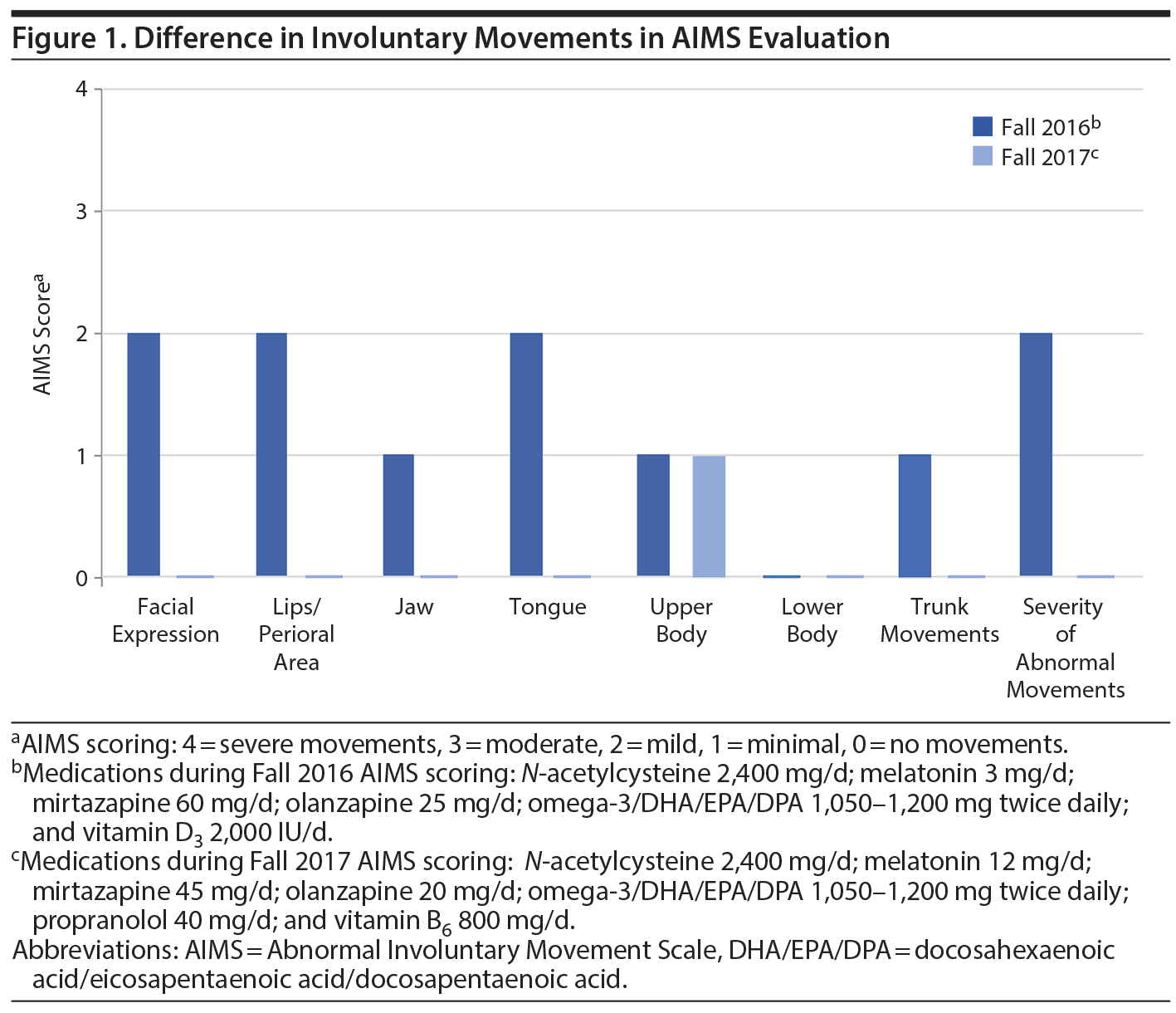
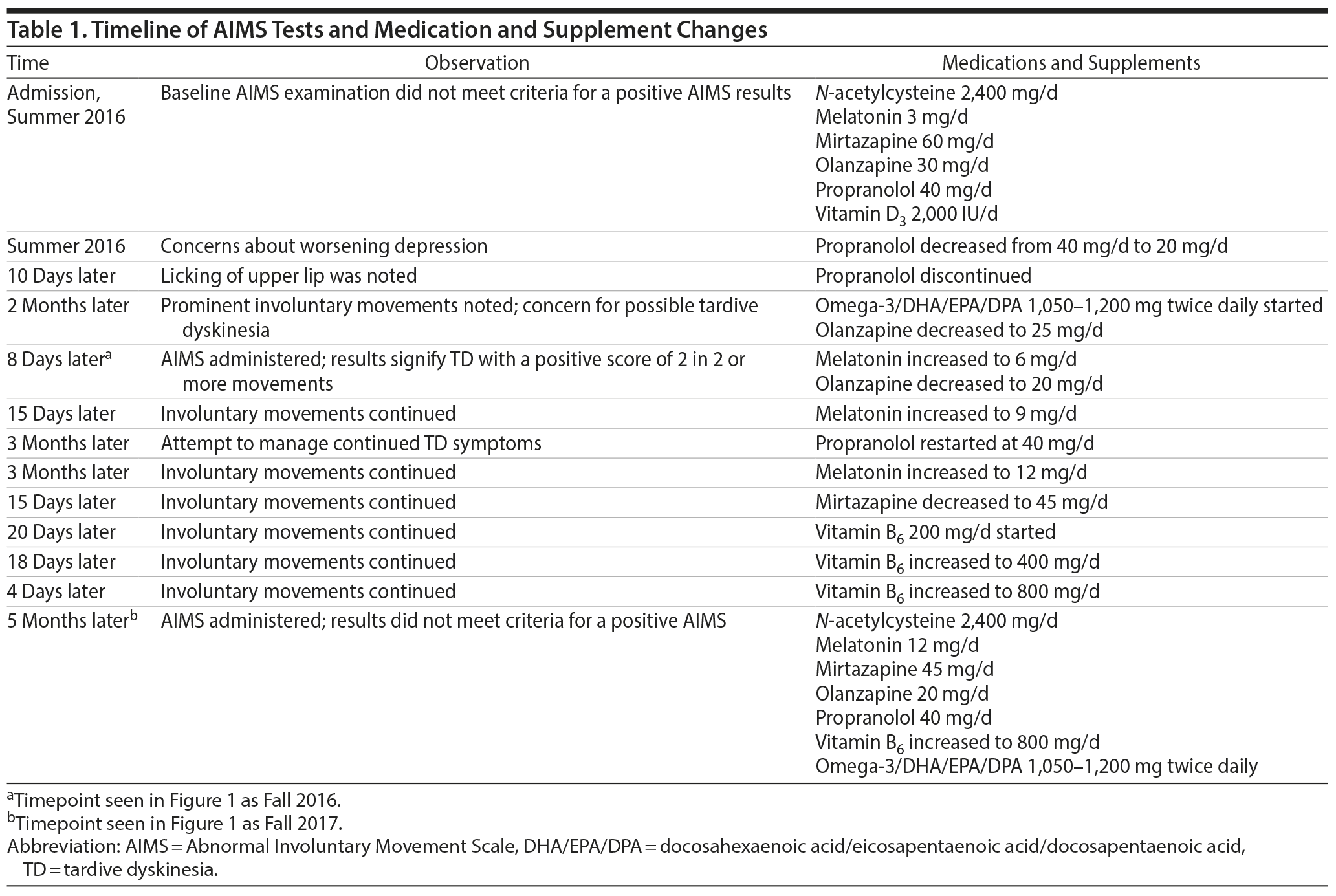
Mr A is a man in his 30s with a diagnosis of schizoaffective disorder, bipolar type residing at a long-term state psychiatric facility. In the summer of 2016 during a monthly evaluation, the psychiatrist noted possible involuntary movements of the patient’s tongue. Current medications at the time were N-acetylcysteine 2,400 mg/d, melatonin 3 mg/d, mirtazapine 60 mg/d, olanzapine 30 mg/d, propranolol 20 mg/d (recently decreased from 40 mg/d), and vitamin D3 2,000 IU/d. Laboratory results at the time were unremarkable. Due to concern for possible TD, Mr A’s olanzapine dose was decreased to 25 mg on the same day and omega-3/DHA/EPA/DPA 1,050-1,200 mg twice daily was initiated. Eight days later, the psychiatrist performed a formal AIMS examination, which was positive with a score of 2 in at least 2 different movement categories (Figure 1). The psychiatrist was concerned with mild TD in those 2 categories, and treatment was optimized to prevent worsening of the TD and improve symptomatology. Over the course of 6 months, omega-3/DHA/EPA/DPA 1,050-1,200 mg twice daily was continued and other antioxidants were added including vitamin B6 800 mg/d; melatonin was increased to 12 mg/d. Propranolol 40 mg/d was also reintroduced in an attempt to further reduce symptoms and then was discontinued.
Over the next 12 months, observance of involuntary movements of the tongue, lips, and truncal area had decreased as changes in medications including increases in antioxidant dosages were made. Certain increases in antioxidant dosages may have been higher than normally anticipated due to drug-drug interactions. Specifically, the reintroduction of propranolol may have caused a higher dosage of melatonin to be used due to propranolol’s dose-dependent ability to intrinsically deplete melatonin levels.15 A second AIMS evaluation was performed in the Fall of 2017, and results did not meet criteria for a positive AIMS score, with only minimal involuntary movement in the upper limbs observed with a score of 1 (Figure 1). See Table 1 for a timeline of medication changes and AIMS scores.
Discussion
This case report illustrates the importance of the utilization of antioxidants for treatment of TD. While initial agents did not provide complete improvement of involuntary movements, combining antioxidants had a beneficial effect in lowering symptom severity. This report also suggests that propranolol may have a role in the treatment of TD. Since movements only became apparent after the propranolol dose was lowered, this may indicate that, above a certain threshold, propranolol may also provide beneficial effect in masking, but not worsening, symptoms of TD or the adrenergic system affects modulation of the dopaminergic/cholinergic balance in the striatum.16-18 On the basis of the observations of this case, alternative treatment options may be considered before use of more expensive US Food and Drug Administration-approved agents for mild cases of TD.
Published online: May 9, 2019.
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: Consent was received from the patient to publish the case report, and information has been de-identified to protect anonymity.
REFERENCES
1. Cornett EM, Novitch M, Kaye AD, et al. Medication-induced tardive dyskinesia: a review and update. Ochsner J. 2017;17(2):162-174. PubMed
2. Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: US Department of Health, Education and Welfare; 1976:534-537.
3. Elkashef AM, Wyatt RJ. Tardive dyskinesia: possible involvement of free radicals and treatment with vitamin E. Schizophr Bull. 1999;25(4):731-740. PubMed CrossRef
4. Deardorff OG, Burns NA, Hunter DR. Tardive dyskinesia: is vitamin E singing the prostate blues? Prim Care Companion CNS Disord. 2018;20(2):17l02170. PubMed CrossRef
5. A Look at VMAT2 Inhibitors for Tardive Dyskinesia. Institute for Clinical and Economic Review website. icer-review.org/wp-content/uploads/2017/12/icer_tardive-dyskinesia_RAAG-122217.pdf. Accessed April 9, 2019.
6. Hauser RA, Factor SA, Marder SR, et al. KINECT 3: a phase 3 randomized, double-blind, placebo-controlled trial of valbenazine for tardive dyskinesia. Am J Psychiatry. 2017;174(5):476-484. PubMed CrossRef
7. Fernandez HH, Factor SA, Hauser RA, et al. Randomized controlled trial of deutetrabenazine for tardive dyskinesia: the ARM-TD study. Neurology. 2017;88(21):2003-2010. PubMed CrossRef
8. Overview of Shared Solutions: Your Partner for Patient Support. Austedo for Healthcare Professionals. https://www.austedo.com/hcp/shared-solutions-overview. Accessed April 9, 2019.
9. Neurocrine Biosciences. INBRACE Support Program. INGREZZA (valbenazine) capsules. https://inbracesupportprogram.com. Accessed April 9, 2019.
10. Lobo V, Patil A, Phatak A, et al. Free radicals, antioxidants and functional foods: impact on human health. Pharmacogn Rev. 2010;4(8):118-126. PubMed CrossRef
11. Lerner V, Miodownik C, Kaptsan A, et al. Vitamin B(6) in the treatment of tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Am J Psychiatry. 2001;158(9):1511-1514. PubMed CrossRef
12. Lerner V, Miodownik C, Kaptsan A, et al. Vitamin B6 treatment for tardive dyskinesia: a randomized, double-blind, placebo-controlled, crossover study. J Clin Psychiatry. 2007;68(11):1648-1654. PubMed CrossRef
13. Shamir E, Barak Y, Shalman I, et al. Melatonin treatment for tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Arch Gen Psychiatry. 2001;58(11):1049-1052. PubMed CrossRef
14. Éthier I, Kagechika H, Shudo K, et al. Docosahexaenoic acid reduces haloperidol-induced dyskinesias in mice: involvement of Nur77 and retinoid receptors. Biol Psychiatry. 2004;56(7):522-526. PubMed CrossRef
15. Mayeda A, Mannon S, Hofstetter J, et al. Effects of indirect light and propranolol on melatonin levels in normal human subjects. Psychiatry Res. 1998;81(1):9-17. PubMed CrossRef
16. Bacher NM, Lewis HA. Low-dose propranolol in tardive dyskinesia. Am J Psychiatry. 1980;137(4):495-497. PubMed
17. Hatcher-Martin JM, Armstrong KA, Scorr LM, et al. Propranolol therapy for tardive dyskinesia: a retrospective examination. Parkinsonism Relat Disord. 2016;32:124-126. PubMed CrossRef
18. Wilbur R, Kulik FA. Propranolol (Inderal) for tardive dyskinesia and extrapyramidal side effects from neuroleptics: possible involvement of beta-adrenergic mechanisms. Prog Neuropsychopharmacol. 1980;4(6):627-632. PubMed CrossRef
aFulton State Hospital Pharmacy, Fulton, Missouri
*Corresponding author: O. Greg Deardorff, PharmD, BCPP, Fulton State Hospital, 600 East 5th St, Fulton, MO 65251 ([email protected]).
Prim Care Companion CNS Disord 2019;21(3):18l02375
To cite: Deardorff OG, Ehrhard KA, Cafer J, et al. Antioxidants for the treatment of tardive dyskinesia: AIMing to find the best treatment option. Prim Care Companion CNS Disord. 2019;21(3):18l02375.
To share: https://doi.org/10.4088/PCC.18l02375
© Copyright 2019 Physicians Postgraduate Press, Inc.
Please sign in or purchase this PDF for $40.00.
Save
Cite