Prim Care Companion CNS Disord 2022;24(5):21cr03207
To cite: Shiba S, Fukuchi J, Hirachi-Murakawa T, et al. Auditory hallucinations after microvascular decompression for intractable trigeminal neuralgia. Prim Care Companion CNS Disord. 2022;24(5):21cr03207.
To share: https://doi.org/10.4088/PCC.21cr03207
© 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Faculty of Medicine, Saga University, Saga City, Japan
*Corresponding author: Akira Monji, MD, Saga University, Nabeshima 5-1-1, Saga, Japan 840-8502 ([email protected]).
Microvascular decompression for refractory trigeminal neuralgia is often performed, but hallucinatory experiences as an adverse effect are rare. Most hallucinations reported after microvascular decompression for trigeminal neuralgia are visual.1–5 Hallucinations are reported to be partly attributable to withdrawal reaction of carbamazepine.4 Here, we report the case of a female patient with auditory hallucinations after microvascular decompression for intractable trigeminal neuralgia.
Case Report
A 41-year-old woman had experienced left trigeminal neuralgia for 6 years. She had also suffered from depression for a couple of years and was treated with escitalopram 20 mg/d and trazodone 12.5 mg/d. Her mother had a history of depression, and her cousin committed suicide. Her symptoms of trigeminal neuralgia were well controlled with carbamazepine; however, they gradually became difficult to control with the medication. She thus underwent microvascular decompression for left trigeminal neuralgia. Auditory hallucinations such as hearing men and women talking to her appeared on the day after the operation. She was suspected to have delirium after surgery and was thus consulted by the department of psychiatry. The psychiatrists observed no disturbance of consciousness, which was supported by normal electroencephalogram findings. The patient had insight into her psychiatric symptoms and agreed to treatment. She was tentatively diagnosed with acute transient psychotic disorder or schizophrenia according to the DSM-5. Aripiprazole 12 mg/d and quetiapine 25 mg/d in addition to carbamazepine were prescribed for her psychiatric symptoms.
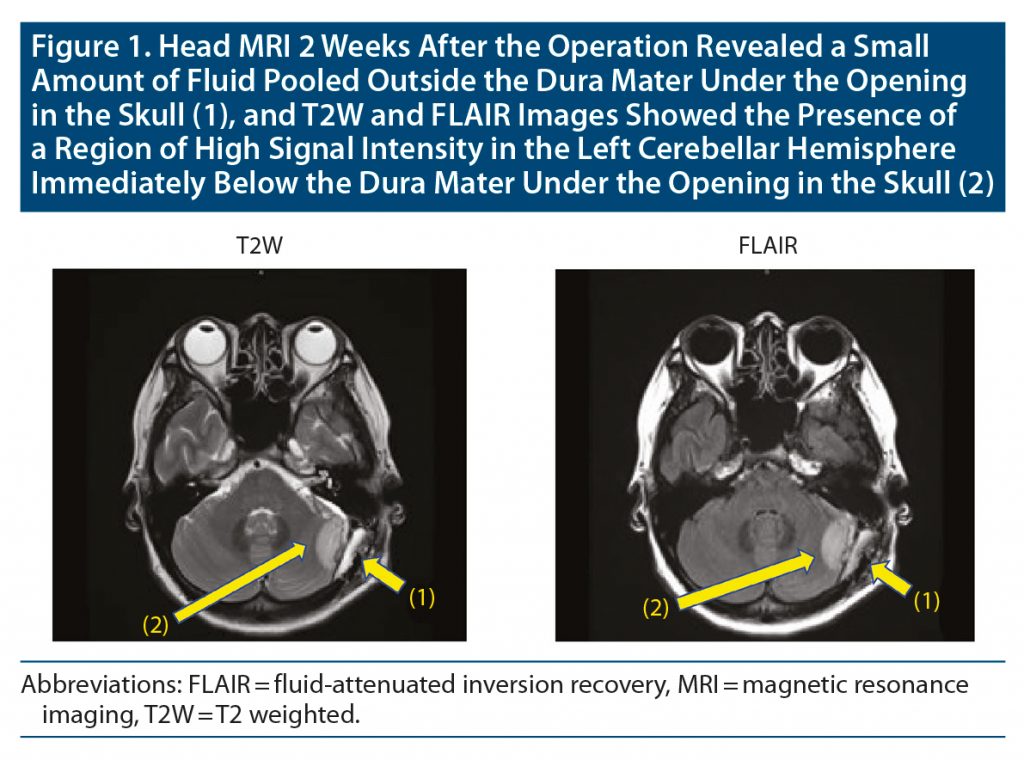
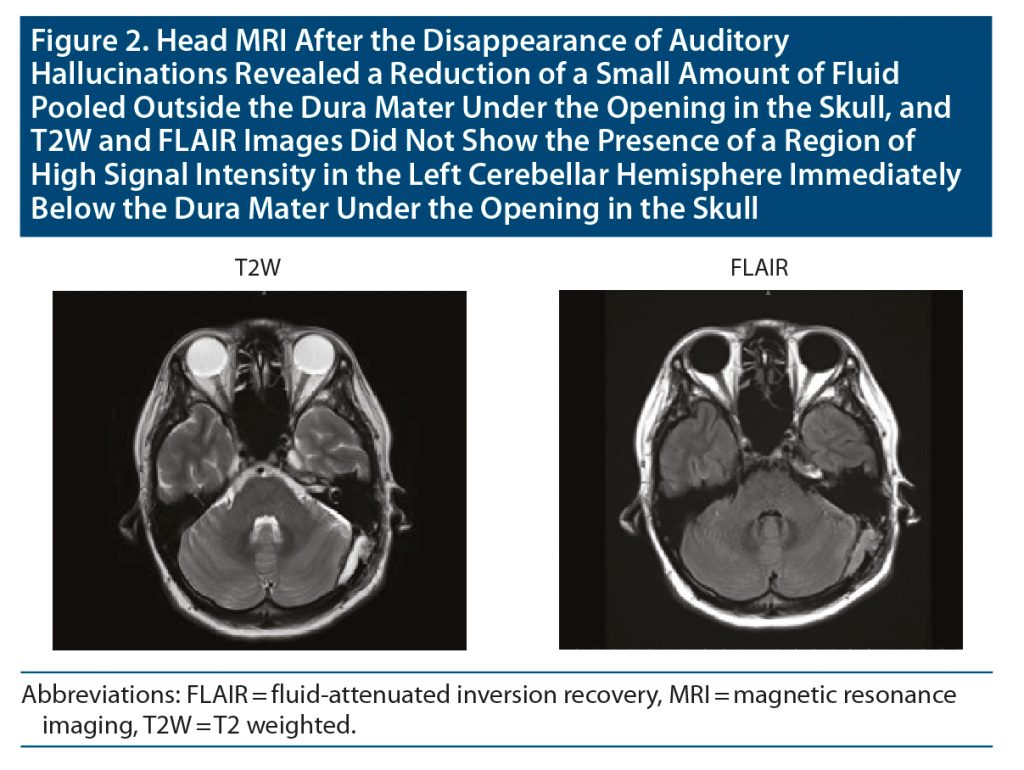
Four days later, she was admitted to the hospital’s psychiatry department. After admission, the dose of aripiprazole was increased to 24 mg/d, but the hallucinations continued. Head magnetic resonance imaging (MRI) 2 weeks after the operation revealed a small amount of fluid pooled outside the dura mater under the opening in the skull, and T2-weighted and fluid-attenuated inversion recovery images showed the presence of a region of high signal intensity in the left cerebellar hemisphere immediately below the dura mater under the opening in the skull (Figure 1). Since akathisia symptoms were observed, aripiprazole 24 mg/d was changed to brexpiprazole 2 mg/d. Thereafter, her auditory hallucinations gradually ameliorated, and the auditory hallucinations disappeared about 2 weeks after admission to the psychiatry department. The previously mentioned abnormal MRI findings were also improved at that time (Figure 2). Ten days later, she was discharged from the hospital. There has been no relapse of psychiatric symptoms with the prescription of brexpiprazole 2 mg/d for 4 months at the time of this writing.
Discussion
In the present case, auditory hallucinations suddenly appeared after microvascular decompression for left trigeminal neuralgia. Initially, acute transient psychotic disorder or schizophrenia was suspected, but abnormal lesions were observed on the postoperative follow-up head MRI. The same MRI lesions had almost disappeared at the time that her psychiatric symptoms improved. Therefore, her auditory hallucinations may have been induced by the abnormal lesions detected by the MRI, which may have been caused by microvascular decompression.
There have been only a few reports that described hallucinations after microvascular decompression. Tsukamoto et al1 reported a case of a 63-year-old woman with visual and auditory hallucinations. Her visual hallucinations due to brain stem lesions were consistent with the original definition of peduncular hallucinosis.1 Chen and Lui2 reported a case of a 62-year-old woman with visual hallucinations diagnosed as peduncular hallucinosis following microvascular decompression. Koerbel et al3 also reported the case of a 50-year-old man with visual hallucinations diagnosed as peduncular hallucinosis following microvascular decompression.
The 3 cases1–3 of peduncular hallucinosis were reported to have appeared after obliteration of veins of the petrosal complex for trigeminal neuralgia. Chen and Lui2 investigated the withdrawal effect of carbamazepine-associated reaction (CAWR) after neurovascular decompression for trigeminal neuralgia. Their study2 demonstrated that, among 90 patients, 26 (28.9%) suffered from CAWR for 3 days after neurovascular decompression for trigeminal neuralgia and that the symptoms of CAWR included 3 cases with hallucinations.4 Although the 3 case reports1–3 describing hallucinations following microvascular decompression did not clearly describe whether carbamazepine was discontinued, carbamazepine was not stopped after neurovascular decompression for trigeminal neuralgia in the present case.
Miyazawa et al5 reported a case of a 68-year-old woman with visual hallucinations diagnosed as peduncular hallucinosis following microvascular decompression without direct brain stem injury. CT and T2-weighted MRI in their case revealed a high-intensity lesion on the surface of the left hemisphere suspected to represent ischemic edema due to retraction. These neuroimaging findings are quite similar to those of the present case. Miyazawa et al5 suggested that the mechanism of visual hallucinations may be similar to cerebellar cognitive affective syndrome.6 Cerebellar feedback pathways through the thalamus to the cerebral cortex may contribute to peduncular hallucinosis.7 A report8 suggested that microglia-related cerebellar inflammation may be related to psychiatric symptoms. Strong connectivity between the VIIb and IX vermis areas and the visual network has been noted, and this area is also known to have reduced blood flow in schizophrenia patients, which in turn could be a factor in visual hallucinations experience by the patient. The same can be said with hemispheric areas VI, VIIb, and VIII, which show connectivity with the auditory network and could explain auditory hallucinations present in some schizophrenia patients.9,10 It is thus possible that the patient in the present case had a lesion in a different part of the cerebellum than those patients in previous case reports. The patient’s psychiatric and family history may also be related to the emergence of auditory hallucinations in the present case. However, it is unclear as to why no visual hallucinations were observed, and this should be clarified in detail in future studies.
Published online: October 13, 2022.
Relevant financial relationships: None.
Funding/support: None.
Additional information: Information has been de-identified to protect patient anonymity.
References (10)

- Tsukamoto H, Matsushima T, Fujiwara S, et al. Peduncular hallucinosis following microvascular decompression for trigeminal neuralgia: case report. Surg Neurol. 1993;40(1):31–34. PubMed CrossRef
- Chen HJ, Lui CC. Peduncular hallucinosis following microvascular decompression for trigeminal neuralgia: report of a case. J Formos Med Assoc. 1995;94(8):503–505. PubMed
- Koerbel A, Wolf SA, Kiss A. Peduncular hallucinosis after sacrifice of veins of the petrosal venous complex for trigeminal neuralgia. Acta Neurochir (Wien). 2007;149(8):831–832, discussion 832–833. PubMed CrossRef
- Chen MJ, Zhang WJ, Guo ZL, et al. Withdrawal reaction of carbamazepine after neurovascular decompression for trigeminal neuralgia: a preliminary study. J Neurol Sci. 2014;338(1–2):43–45. PubMed CrossRef
- Miyazawa T, Ito M, Yasumoto Y. Peduncular hallucinosis following microvascular decompression for trigeminal neuralgia without direct brainstem injury: case report. Acta Neurochir (Wien). 2009;151(3):285–286. PubMed CrossRef
- Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121(Pt 4):561–579. PubMed CrossRef
- Manford M, Andermann F. Complex visual hallucinations: clinical and neurobiological insights. Brain. 1998;121(Pt 10):1819–1840. PubMed CrossRef
- Yamamoto M, Kim M, Imai H, et al. Microglia-triggered plasticity of intrinsic excitability modulates psychomotor behaviors in acute cerebellar inflammation. Cell Rep. 2019;28(11):2923–2938.e8. PubMed CrossRef
- Phillips JR, Hewedi DH, Eissa AM, et al. The cerebellum and psychiatric disorders. Front Public Health. 2015;3:66. PubMed CrossRef
- Sang L, Qin W, Liu Y, et al. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. Neuroimage. 2012;61(4):1213–1225. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite