Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: Advair Diskus (fluticasone and salmeterol oral inhaler) and other medications of its kind are frequently prescribed for both adult and pediatric patients. We report the case of a pediatric patient who developed an acute psychotic episode (DSM-5 criteria) after starting Advair Diskus. The package insert for Advair Diskus describes reports of "behavioral changes."
Brief Psychotic Episode Caused by Advair Diskus in a Pediatric Patient
To the Editor: Advair Diskus (fluticasone and salmeterol oral inhaler) and other medications of its kind are frequently prescribed for both adult and pediatric patients. We report the case of a pediatric patient who developed an acute psychotic episode (DSM-5 criteria) after starting Advair Diskus. The package insert1 for Advair Diskus describes reports of "behavioral changes." However, there is no report of any psychotic episodes associated with this medication.
Case report. A 14-year-old boy presented to the emergency department with his mother because of new-onset visual hallucinations, vague delusions, and bizarre behavior for 5 days’ duration. No significant stressors or substance use were identified. There was no history of trauma, depressive episodes, manic episodes, or any prior psychotic episodes. The patient’s mother reported no significant family history. Vital signs and general laboratory values were within normal limits. The urine drug screen was positive for tricyclic antidepressants. His aspirin, acetaminophen, and blood alcohol levels were all nondetectable. A head computed tomography scan was negative. The positive tricyclic antidepressant result on the urine drug screen was most likely due to the diphenhydramine that the patient was taking for sleep.2
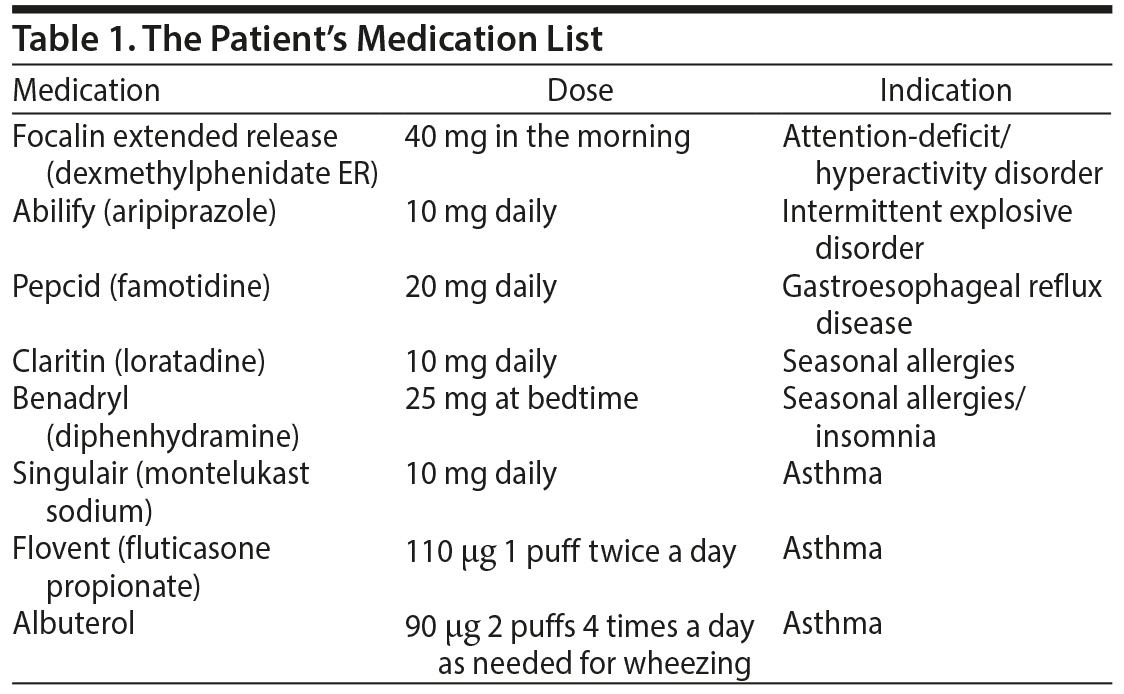
The patient’s past psychiatric diagnoses included attention-deficit/hyperactivity disorder and intermittent explosive disorder (DSM-5 criteria). The patient was currently prescribed the medications listed in Table 1 for 1 year with no changes in dosing. One-and-a-half weeks prior to presentation, the patient was changed from Flovent (fluticasone propionate) to Advair Diskus (100 μg/50 μg twice a day).
While in the hospital, the patient’s atomoxetine (Focalin) was discontinued because of its potential effect of worsening psychotic symptoms.3 Advair Diskus was also switched back to Flovent at admission. The workup of this psychotic episode included an electroencephalograph and magnetic resonance image, results of which showed no abnormalities. The patient endorsed no mood symptoms and denied suicidal and homicidal ideation. During the 6-day hospital stay, the patient reported no psychotic symptoms, and none were witnessed by staff.
The patient had an acute onset of psychotic symptoms of 5 days’ duration. The differential diagnosis included brief psychotic disorder, seizure disorder, organic brain disorder, and substance-induced psychotic disorder. The patient’s electroencephalograph and magnetic resonance imaging scans showed no abnormalities, and the patient denied substance use. The only change in the medication regimen was the addition of Advair Diskus. Because of the brevity of this psychotic episode, the quick remittance, and the negative test results obtained during his hospital stay, we believe that the potential cause of this episode was Advair Diskus.
A literature search yielded an article4 that evaluated asthma medications and associated adverse drug reactions. The results showed that psychiatric disorders accounted for a total of 13% of the reported adverse reactions. The article4 further clarified that in the Advair group, there were 10 reported cases of induced psychiatric disorders. There was no description of what these psychiatric disorders entailed.4 This article4 has opened the door to more research into the different asthma medications and their potential for psychiatric side effects.
It is paramount that prescribers fully understand the potential side effects of the medications utilized and to be on the lookout for these side effects. It is our hope that this case will bring other reports of this nature to light so that we may all be more aware of the potential side effects of Advair Diskus and other medications in this class.
References
1. Advair Diskus [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2014.
2. Moeller KE, Lee KC, Kissack JC. Urine drug screening: practical guide for clinicians. Mayo Clin Proc. 2008;83(1):66-76. PubMed doi:10.4065/83.1.66
3. Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am J Psychiatry. 2006;163(7):1149-1152. PubMed doi:10.1176/ajp.2006.163.7.1149
4. Aagaard L, Hansen EH. Paediatric adverse drug reactions following use of asthma medications in Europe from 2007 to 2011. Int J Clin Pharm. 2014;36(6):1222-1229. PubMed doi:10.1007/s11096-014-0020-0
aPsychiatry and Behavioral Sciences Residency Program, KU School of Medicine, The University of Kansas, Wichita
bDepartment of Psychiatry and Behavioral Services, KU School of Medicine, The University of Kansas, Wichita
Potential conflicts of interest: None.
Funding/support: None.
Disclaimer: All information presented in this case report has been de-identified.
Published online: May 11, 2017.
Prim Care Companion CNS Disord 2017;19(3):16l02052
https://doi.org/10.4088/PCC.16l02052
© Copyright 2017 Physicians Postgraduate Press, Inc.
Please sign in or purchase this PDF for $40.00.
Save
Cite