Prim Care Companion CNS Disord 2022;24(3):21cr03163
To cite: Jegede O, Parida S, De Aquino JP. Buprenorphine treatment of fentanyl-related opioid use disorder. Prim Care Companion CNS Disord. 2022;24(3):21cr03163.
To share: https://doi.org/10.4088/PCC.21cr03163
© Copyright 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Yale University School of Medicine, New Haven, Connecticut
bConnecticut Mental Health Center, New Haven, Connecticut
cVA Connecticut Healthcare System, West Haven, Connecticut
*Corresponding author: Oluwole Jegede, MD, MPH, Yale University School of Medicine, 34 Park St, New Haven, CT 06519 ([email protected]).
The widespread availability of fentanyl has been implicated in the rise of opioid overdose deaths across North America, leading clinicians and researchers to question the efficacy of medications for opioid use disorder (MOUD).1
Fentanyl is a full μ-opioid receptor (MOR) agonist that is 50 to 100 times more potent than morphine.2 Following its intake, fentanyl is widely distributed in plasma, rapidly crossing the blood-brain barrier.3 Afterward, fentanyl is quickly redistributed into muscle and fat tissue and slowly released back to the circulation.4 The half-lives of these first and the second distributions are of approximately 10–15 minutes and 3–4 hours, respectively.4,5 As a result, fentanyl’s pharmacologic profile enhances this agent’s potential for addiction and overdose deaths.
Unlike fentanyl, buprenorphine is a partial agonist with low efficacy at the MOR, with much lower abuse potential and risk of respiratory depression.6 Even though buprenorphine is currently recommended as a first-line MOUD, the rise of ultrapotent fentanyl derivatives has led to some controversy regarding buprenorphine’s effectiveness.6
Case Report
A 69-year-old man maintained on buprenorphine/naloxone 24 mg/6 mg sublingual (SL) daily for opioid use disorder (OUD) returned to using 6 bags of inhaled heroin twice a week. In view of the patient’s persistent cravings, the buprenorphine/naloxone dose was increased to 32 mg/8 mg SL daily. Still, the patient continued to use heroin and to experience craving and sedation. Since a chromatography test was positive for fentanyl, the possibility of fentanyl’s displacing buprenorphine from brain MORs was entertained to explain the persistent craving and worsening sedation. Hence, switching from the partial MOR agonist buprenorphine to the full MOR agonist methadone was temporarily considered.
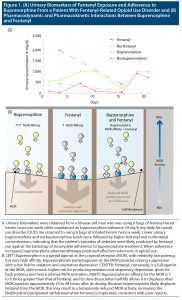
Further urine drug studies revealed a low norbuprenorphine-to-buprenorphine ratio, suggesting incomplete adherence to buprenorphine. As shown in Figure 1A, lower buprenorphine and norbuprenorphine levels were followed by higher fentanyl and norfentanyl levels. These findings contradicted the hypothesis that fentanyl was displacing buprenorphine. Moreover, subsequent adherence to treatment resulted in an abatement of illicit opioid use and an improvement in overall functioning.
Discussion
We have described a case of a patient treated with buprenorphine in the context of illicit fentanyl exposure. In this case, there was a temporary concern that fentanyl was undermining buprenorphine’s efficacy—even at high doses of buprenorphine (32 mg). However, careful laboratory monitoring provided evidence of incomplete adherence to buprenorphine, likely explaining the persistent opioid craving, sedation, and recurrent fentanyl use.
Buprenorphine has an extremely high affinity to the MOR—estimated to be 5.4 and 6.2 times greater than that of fentanyl.2,7 Furthermore, buprenorphine’s slow dissociation half-life from the MOR—166 minutes, in contrast to 7 minutes for fentanyl—allows it to displace MOR agonists even 24 to 48 hours after its dosing.2 Although these pharmacokinetic properties can precipitate withdrawal during induction onto buprenorphine (Figure 1B)—potentially impacting early treatment outcomes—altogether, they provide robust protection against illicit opioids thereafter.8,9 Preliminary evidence shows that buprenorphine may be equally effective as naloxone in reversing the fentanyl-induced respiratory depression, but the interaction between buprenorphine and fentanyl has yet to be systematically examined in humans.10
Rather than assuming inefficacy of MOUD, clinicians should test individuals using fentanyl for both fentanyl and buprenorphine exposure, as well as exposure to their respective metabolites. The growing availability of fentanyl underscores treatment adherence as an important aspect of assessing the efficacy of OUD pharmacotherapies.11
In sum, although novel clinical guidelines are needed to enhance treatment outcomes, convergent evidence indicates that buprenorphine remains an essential component of OUD pharmacotherapy. Especially in the era of illicit fentanyl, increasing medication adherence is a vital part of recovery.
Published online: April 26, 2022.
Relevant financial relationships: None.
Funding/support: This work was funded in part by the State of Connecticut, Department of Mental Health and Addiction Services.
Role of the sponsor: The State of Connecticut, Department of Mental Health and Addiction Services had no role in the conduct, interpretation, or reporting of this research.
Disclaimer: This publication does not express the views of the Department of Mental Health and Addiction Services or the State of Connecticut. The views and opinions expressed are those of the authors.
Patient consent: Consent was received from the patient to publish the case report, and information has been de-identified to protect anonymity.
References (11)

- Zoorob M. Fentanyl shock: the changing geography of overdose in the United States. Int J Drug Policy. 2019;70:40–46. PubMed CrossRef
- Comer SD, Cahill CM. Fentanyl: receptor pharmacology, abuse potential, and implications for treatment. Neurosci Biobehav Rev. 2019;106:49–57. PubMed
- Hug CC Jr, Murphy MR. Fentanyl disposition in cerebrospinal fluid and plasma and its relationship to ventilatory depression in the dog. Anesthesiology. 1979;50(4):342–349. PubMed CrossRef
- Scholz J, Steinfath M, Schulz M. Clinical pharmacokinetics of alfentanil, fentanyl and sufentanil: an update. Clin Pharmacokinet. 1996;31(4):275–292. PubMed CrossRef
- McClain DA, Hug CC Jr. Intravenous fentanyl kinetics. Clin Pharmacol Ther. 1980;28(1):106–114. PubMed CrossRef
- Parida S, Carroll KM, Petrakis IL, et al. Buprenorphine treatment for opioid use disorder: recent progress. Expert Rev Clin Pharmacol. 2019;12(8):791–803. PubMed CrossRef
- Yassen A, Kan J, Olofsen E, et al. Mechanism-based pharmacokinetic-pharmacodynamic modeling of the respiratory-depressant effect of buprenorphine and fentanyl in rats. J Pharmacol Exp Ther. 2006;319(2):682–692. PubMed CrossRef
- Boas RA, Villiger JW. Clinical actions of fentanyl and buprenorphine: the significance of receptor binding. Br J Anaesth. 1985;57(2):192–196. PubMed CrossRef
- Hardman JG, Limbird LE, Gilman AG, eds. .Goodman and Gilman’s The Pharmacological Basis of Therapeutics. McGraw Hill, New York: McGraw Hill; 2001.
- Boysen K, Hertel S, Chraemmer-Jørgensen B, et al. Buprenorphine antagonism of ventilatory depression following fentanyl anaesthesia. Acta Anaesthesiol Scand. 1988;32(6):490–492. PubMed CrossRef
- Donroe JH, Holt SR, O’Connor PG, et al. Interpreting quantitative urine buprenorphine and norbuprenorphine levels in office-based clinical practice. Drug Alcohol Depend. 2017;180:46–51. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite