ABSTRACT
Objective: To assess the humanistic and economic burden of attention-deficit/hyperactivity disorder (ADHD) among adult patients treated with immediate-release (IR) only or extended-release (ER) only stimulants and those unmedicated versus treated with ER + IR stimulants.
Methods: This study analyzed linked data from National Health and Wellness Survey and claims to assess the differences in patient characteristics and outcomes, including health-related quality of life (HRQoL), work productivity and activity impairment, and health care resource utilization (HRU) and associated costs by comparing ADHD patients treated with either IR or ER and those unmedicated for ADHD versus ER + IR.
Results: The burden of ADHD was compared among adults on stimulant medications with different duration of effect (DoE) (ER + IR: n = 34, ER: n = 184, IR: n = 149) and the unmedicated group (n = 114). Bivariate analysis showed the IR (P = .047) and unmedicated groups (P = .01) had significantly lower Medical Outcomes Study 36-item Short Form physical component summary scores versus ER + IR. The unmedicated group had higher HRU and associated costs versus other groups. Multivariable analysis revealed that the unmedicated group had twice as many outpatient visits (P = .001) and higher total annual direct costs than those on ER + IR (risk ratio = 2.20, P = .016). Patients with mental health comorbidities had significantly poorer HRQoL mental component summary scores and higher activity impairment versus those without mental health comorbidities (P = .001 and P < .001, respectively).
Conclusions: Patients with ADHD treated with longer DoE formulations had substantially better economic outcomes versus shorter DoE formulation or unmedicated groups, offering potential cost savings to the health care system and the patient. Furthermore, it is important to consider the effect of mental health comorbidities in the overall management of ADHD.
Prim Care Companion CNS Disord 2023;25(2):22m03348
To cite: Lee L, Arunajadai S, Mikl J, et al. The burden of attention-deficit/hyperactivity disorder in adults: a real-world linked data study. Prim Care Companion CNS Disord. 2023;25(2):22m03348.
To share: https://doi.org/10.4088/PCC.22m03348
© 2023 Physicians Postgraduate Press, Inc.
aCerner Enviza, Malvern, Pennsylvania
bPurdue Pharma L.P., Stamford, Connecticut
cJohns Hopkins University School of Medicine, Baltimore, Maryland
*Corresponding author: Lulu K. Lee, PhD, Cerner Enviza, 51 Valley Stream Pkwy, Malvern, PA 19355 ([email protected]).
Attention-deficit/hyperactivity disorder (ADHD) is a common childhood-onset neurobiological disorder characterized by inattention and/or hyperactivity and impulsivity with difficulties that frequently persist into adulthood.1–4 According to a meta-analysis of 175 studies, the overall pooled prevalence of ADHD among children and adolescents was 7.2% globally.5 In recent years, studies6,7 have shown an increase in prevalence of ADHD among children and adolescents and adults in the United States.
ADHD imposes a substantial humanistic and economic burden on affected individuals and society, especially in adults, even after remission of some symptoms.2,8,9 Children and adults with ADHD have lower physical, social, mental, and emotional functioning and thus show impaired health-related quality of life (HRQoL).10–14 The annual costs attributed to adult ADHD in the United States have been estimated at $105 to $194 billion, with ADHD-related work productivity and income losses accounting for the largest share of the economic burden.9
Psychostimulants are the most effective and preferred treatment for moderate to severe ADHD,15 while nonstimulant medications are used in case of lack of efficacy, side effect intolerance, medical contraindications, specific clinical issues (ie, anorexia or risk of substance abuse), or intolerance to stimulant formulations.15,16 The duration of effect (DoE) for stimulants can have immediate-release (IR) or extended-release (ER) properties based on the formulation. ERs were developed to allow for less frequent dosing, improved tolerability, increased effectiveness, and better treatment adherence and compliance.17–20 ER stimulants are considered the first-line treatment for adult ADHD.21,22 ER formulations are well tolerated and more effective,19,20,23–25 improve QoL,23,26 and decrease health care resource utilization (HRU)18 versus IR formulations or placebo in adults with ADHD. Previous studies18,19 have demonstrated increased treatment adherence and compliance among adults on ER formulations versus IR formulations. ER formulations may be supplemented with another ADHD medication later in the day to extend the duration of coverage to control symptoms throughout the day and into the evening.27 Studies28–32 suggest that combining ER stimulants with other ADHD medications lead to greater improvements in clinical and/or cognitive outcomes versus monotherapies in children and adolescents with ADHD.
However, there is a lack of information on assessing the relationship between medication DoE and the burden of ADHD in adult patients. Previous studies used limited data from either only claims databases33 or only patient surveys.12 Linking of a data source that is rich in patient-reported outcomes with claims data has not been previously applied to the ADHD population. The primary objective of this study was to assess the impact of ADHD on HRQoL, work productivity and activity impairment (WPAI), and HRU and associated costs using linked data sources among adults treated with either IR or ER and those unmedicated for ADHD versus those medicated with ER + IR stimulants.
METHODS
Study Design and Data Source
This study analyzed linked data (between January 1, 2015, and December 31, 2018) from 2 distinct data sources, National Health and Wellness Survey (NHWS) and Komodo Health’s medical and pharmacy claims, to assess the study questions in patients with ADHD.
US NHWS (2015–2018)
The NHWS is a self-administered internet-based online survey that includes annually collected data of ~75,000 respondents (≥ 18 years) representative of the general population in the United States.34–36 Respondents are recruited through a web-based consumer panel that includes opt-in e-mails, co-registration with panel partners, e-newsletter campaigns, banner placements, and affiliate networks. The NHWS information contains data on demographics, behaviors, and outcomes for participating subjects. The NHWS is reviewed and granted exemption status by the Pearl Institutional Review Board.
Medical and Pharmacy Claims
Komodo Health, a health care technology company, provided information on deidentified claims (> 65 billion clinical, pharmacy, and laboratory encounters) for > 320 million patients in the United States between 2012 and 2020.
Medical Claims
The present study included payor-completed data (insurance eligibility and insurer-reported costs) and open medical claims data collected from claims clearing houses (includes patient location, admission/discharge dates of inpatient, diagnosis and procedure codes, and costs of health care visit).
Pharmacy Claims
Pharmacy Benefits Manager provided pharmacy claim data on the patient’s enrollment start/end date, age, gender, fill date/number, pharmacy location and national provider identifier and specialty, national drug code number, diagnoses and/or procedures related to the prescription, authorized fills/prescription, units of measure, days’ supply, drug costs (covered and copay), and concomitant medications for comorbidities.
Linkage of Databases
The datasets of respondents to NHWS were linked to their corresponding medical/pharmacy claims using Datavant’s Health Insurance Portability and Accountability Act certified deidentified linking software. This software uses a proprietary probabilistic matching algorithm (reported to have a 0.2% false positive rate and a 2% false negative rate [Datavant, unpublished data, 2019]) on personally identifiable information (PII) from the NHWS and PII from the claims databases to find matches in the datasets.
Study Eligibility Criteria
Patients were included if they were aged ≥ 18 years with a self-reported physician diagnosis of ADHD in NHWS and linked to either medical or pharmacy claims data or had a billable/specific code or ADHD prescription in the claims databases.
Outcome Measures
All outcome measurements and their sources are provided in Supplementary Table 1.37–40
Statistical Analysis
Individual (or all) outcomes (Supplementary Table 1) were assessed by comparing demographics and clinical characteristics between patients in each treatment group based on DoE (IR stimulants, ER stimulants, and ER+IR). The Wilcoxon rank sum test and χ2 tests were used for continuous variables and categorical variables, respectively. A statistical significance threshold of α = 0.05 was used.
Additionally, to control for confounding and between-group differences, multivariable modeling was performed. Each outcome was modeled individually using generalized linear models as a function of treatment (ER, IR, unmedicated vs ER+IR) controlling for covariates. The regression models controlled for the presence of mental health comorbidities (with vs without), which are common in ADHD (ie, depression, anxiety, generalized anxiety disorder, bipolar disorder, obsessive-compulsive disorder, panic disorder, social anxiety disorder, posttraumatic stress disorder, phobias, and schizophrenia).41 Other covariates included sociodemographic and health characteristics (age, gender, ethnicity, marital status, education, income, insurance, US census region, Charlson comorbidity index, alcohol consumption, body mass index [BMI], exercise, headache, nicotine dependence, and substance abuse disorder).
RESULTS
Among the 8,432 patients who had a self-reported ADHD diagnosis, 538 patients were linked to claims for analysis. Using the linked cohort, the burden of ADHD was compared with those treated with a longer versus shorter DoE (ER + IR [n = 34], ER [n = 184], IR [n = 149]) and those who were unmedicated (n = 114).
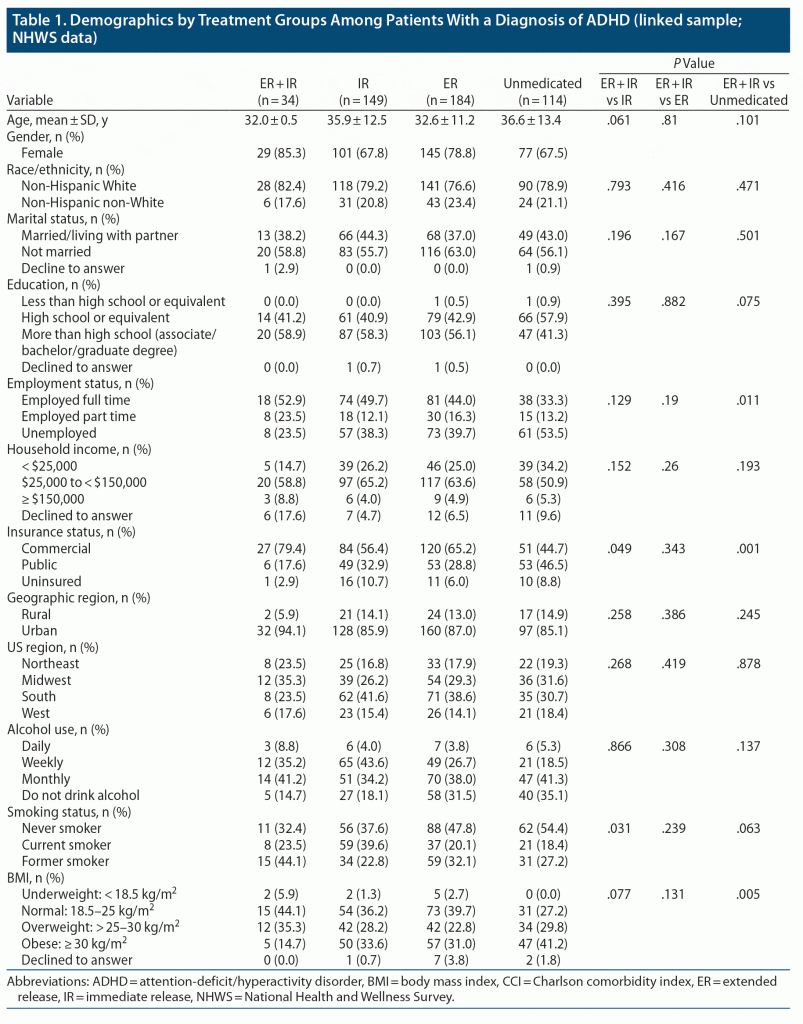
In general, except for gender, employment status, health insurance, BMI, and current smoking status, the demographics between the patient groups were similar (Table 1). A higher proportion of patients treated with longer DoE formulations (ER + IR or ER) were female (85.3% and 78.8%, respectively) compared to patients treated with shorter DoE formulations (IR: 67.8%) or those who were unmedicated (67.5%) (Table 1). Compared with the ER + IR group, a lower proportion of those in the unmedicated group were employed full time (33.3% vs 52.9%, P = .011), and a lower proportion of individuals had commercial insurance in the IR (56.4% vs 79.4%, P = .049) and unmedicated groups (44.7% vs 79.4%, P = .001). Moreover, patients who received treatment with a stimulant (ER, IR, or ER + IR) had attained higher levels of education (associate/bachelor/graduate degree) than the unmedicated group (56.1%, 58.3%, 58.9% vs 41.3%).
HRQoL Outcomes (SF-36v2 in NHWS Data)
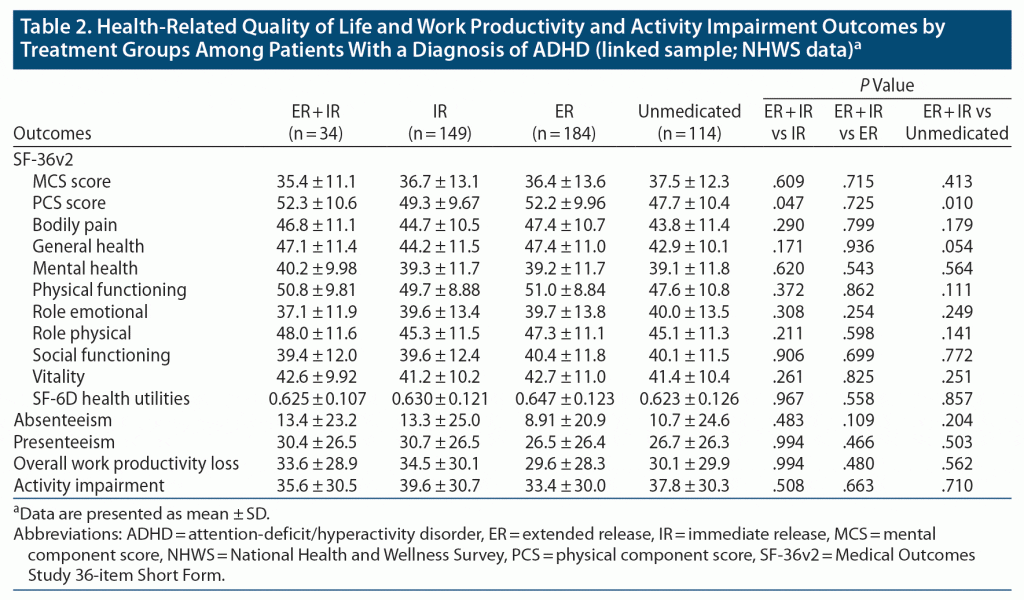
In general, the entire study population scored below the standardized norm score for the US population for most of the domains in the Medical Outcomes Study 36-item Short Form (SF-36v2), and higher scores were observed among patients in the longer DoE group compared with those in the shorter DoE or unmedicated group. There were no significant differences on mental component summary (MCS) scores among all groups; however, the mean MCS scores were below the standardized US population norm of 50 in all groups (35.4–37.5), indicating poorer mental health. Compared with the ER + IR group, significantly lower physical component summary (PCS) scores were observed in the IR group (mean: 49.3 vs 52.3, P = .047), indicating a poorer physical QoL; differences in the PCS scores also met the minimal important difference of 3 points. The unmedicated group also had significantly lower PCS scores than the ER + IR group (mean: 47.7 vs 52.3, P = .01) (Table 2). Additionally, in the ER + IR group, PCS scores were observed to be higher than the standardized US population norm.
WPAI
There were no significant differences across all groups on all WPAI metrics (Table 2). However, across the groups, the overall work productivity loss was ~30% of the time (ER + IR group: 33.6, IR: 34.5, ER: 29.6, unmedicated: 30.1), and general activity impairment was reported by > 30% of all subjects included in the study. In contrast, among the NHWS-only respondents who did not have a diagnosis of ADHD, overall work impairment was lower (~20%). In patients with ADHD, all WPAI metrics were higher across the groups, demonstrating greater impairment when compared with the non-ADHD population (Supplementary Table 2).
HRU and Direct Costs
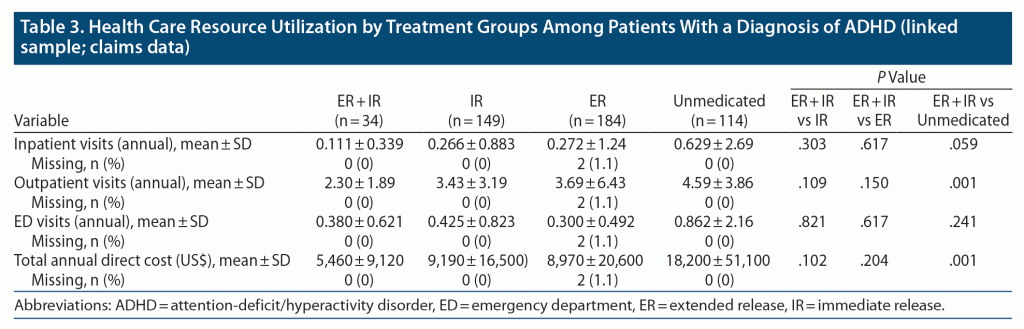
The unmedicated group experienced numerically higher annual inpatient visits versus those treated with stimulants (unmedicated: 0.629, ER+IR: 0.111, ER: 0.272, IR: 0.266). The ER + IR group had the lowest number of inpatient visits versus other groups (Table 3). The unmedicated group had significantly higher annual outpatient visits versus the ER + IR group (4.59 vs 2.30, P = .001). Moreover, the ER + IR group (2.3) had numerically lower annual outpatient visits versus the IR (3.43) and ER (3.69) groups. Compared with the ER + IR group, the total annual direct costs were significantly higher in the unmedicated group (US $5,460 vs $18,200, P = .001, Table 3). Of note, the ER + IR group had the lowest total annual direct costs (US $5,460), followed by the ER ($8,970), IR ($9,190), and unmedicated ($18,200) groups. In general, all HRU and associated costs were higher among unmedicated versus the treated groups; inpatient (0.629 vs 0.111, P = .06), outpatient (4.59 vs 2.30, P = .001), emergency department visits (0.862 vs 0.380, P = .241), and total annual direct cost ($18,200 vs $5460, P = .001) were higher among the unmedicated versus ER + IR groups.
Multivariable Analysis: HRQoL and WPAI
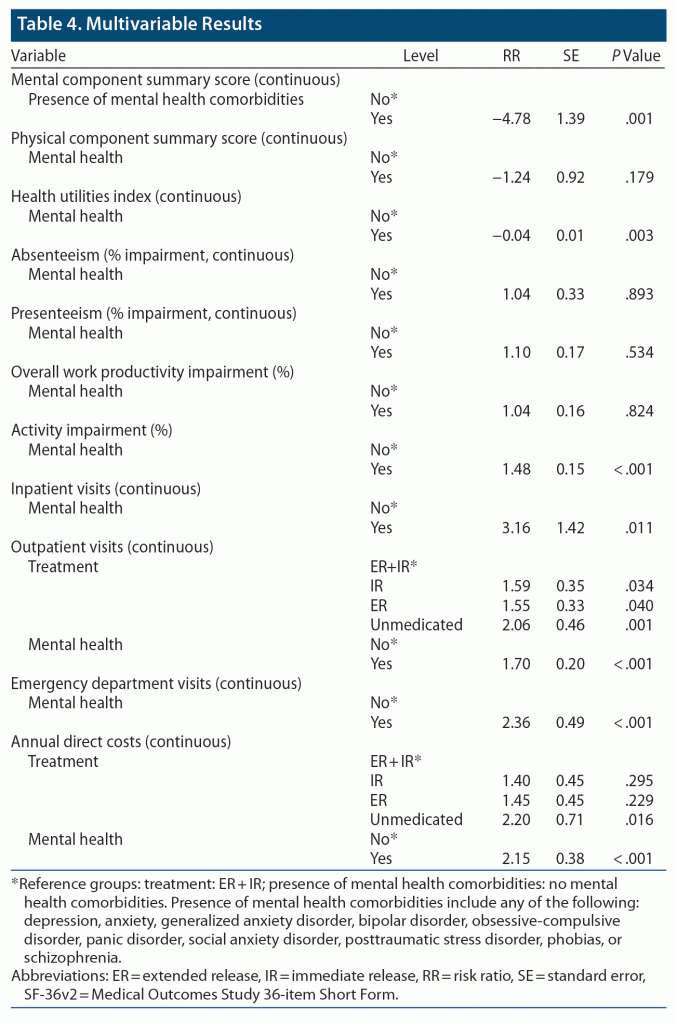
No significant differences were observed by ADHD treatment on the SF-36v2. However, significant differences were observed for presence of mental health comorbidity. Specifically, those with mental health comorbidities scored 5 points lower on the MCS versus those without (P = .001), indicating poorer QoL (Table 4). Additionally, other health domains, including general health, mental health, physical functioning, role emotional, role physical, social functioning, and vitality were also significantly lower among those with mental health comorbidities than those without (all P < .05) (data not shown in the table). Further, those with mental health comorbidities had about 50% higher activity impairment (P < .001) than those without (Table 4).
HRU and Cost
Patients with mental health comorbidities had 3.16 times more inpatient visits than those without (P = .011) (Table 4). Although nonsignificant, the size of the rate ratios for inpatient visits was highest among the unmedicated (risk ratio [RR] = 2.14), followed by ER (RR = 1.41) and IR (RR = 1.14) versus ER + IR. A significant difference was observed among treatment regimen for outpatient visits. Patients unmedicated for ADHD had twice as many outpatient visits than those on ER + IR (P = .001). Furthermore, compared with those on ER + IR, those on IR (P = .034) and ER (P = .040) had 59% and 55% higher outpatient visits, respectively. Those with mental health comorbidities had 70% higher outpatient visits and more than twice the number of emergency department visits compared to those without mental health comorbidities (P < .001 for both, Table 4). Those patients who were unmedicated had higher total annual direct costs than those who were on ER + IR (RR = 2.20, P = .016). Total annual direct costs were observed to be 2.15 times higher in those with mental health comorbidities compared to those without. Although statistically nonsignificant, ER and IR had 40%–45% higher costs versus the ER + IR group (Table 4). Thus, presence of mental health comorbidities increases most HRU (inpatient, outpatient, and emergency department) and associated costs.
DISCUSSION
This study compared the demographics, health characteristics, and humanistic and economic burden of patients with ADHD who were unmedicated and those who were treated with IR, ER, and ER + IR stimulants. This study allowed for simultaneous examination of NHWS self-reported data and medical/pharmacy claims data for the same individuals.
A retrospective analysis of claims database data demonstrated that early intervention of methylphenidate-ER was associated with lower emergency department visits versus methylphenidate-IR.18 In our study, we also observed trends of less HRU for patients with ADHD treated with longer DoE (ER or ER + IR). Specifically, those on ER + IR had fewer outpatient visits than those on IR or who were unmedicated, possibly indicating better symptom management with stable treatment with longer DoE.
Studies12,42 have reported greater work difficulties in adults with ADHD versus those without ADHD. While we found no significant differences across treatment groups in any of the WPAI metrics by ADHD treatment, individuals with ADHD were experiencing work productivity loss at least 30% of the time. In contrast, among NHWS respondents (unlinked) who did not have a diagnosis of ADHD, reported work productivity loss was 20%, thus supporting the existing literature (Supplementary Table 2). To assess potential improvement in work productivity, the Permanent Product Measure of Performance (PERMP) total scores (which assess sustained attention and ability to initiate and stay on task and do effortful work throughout the simulated adult workplace environment [AWE] session) have been used as a proxy for work productivity. Several simulated AWE studies as well as adult laboratory classroom studies using the PERMP have demonstrated efficacy up to 16 hours with different long-acting stimulant medications versus placebo.43–47 Although these measures cannot be directly correlated with WPAI scales, better work productivity indicated by better PERMP scores after treatment with ER medications may be considered.
In the current study, we found no significant differences in HRQoL between groups except for the PCS domain of the SF-36v2 (IR or unmedicated vs ER + IR), yet high scores close to the standardized norm score of the US population in the majority of SF-36v2 parameters was indicative of overall better QoL in patients treated with longer DoE formulations. Previous research examining the burden of illness and impact on HRQoL in older adults with ADHD reported that HRQoL suffered from the cumulative negative impact of ADHD that impairs many aspects of daily life and overall well-being.48
In a previous study by Goodman et al,49 adherence to ADHD medications was increased with once-daily dosing of long-acting stimulant formulations, such as mixed amphetamine salts-ER and modified-release methylphenidate. The study49 also reported significant improvements in ADHD symptoms and QoL with the use of mixed amphetamine salts-ER following 10 weeks of treatment.49 Regardless of treatment regimen, we did not observe significant differences in all domains of HRQoL measures most likely due to the study design. Specifically, HRQoL was only measured as one point in time, thus we do not know baseline measures of these patients, which may differ by disease duration and severity. It may be that patients who were on longer DoEs have more severe symptoms, higher Charlson comorbidity index scores, and a lower HRQoL at baseline than those who were on shorter DoEs.
Multivariable analyses indicated that those with mental health comorbidities had significantly lower scores on the SF-36v2 MCS and other health domains than those without such comorbidities. Furthermore, mental health comorbidities were associated with higher HRU and costs. Likewise, previously published studies have also indicated that ADHD patients with physical or mental health comorbidities had significantly higher outpatient and inpatient costs, drug costs, and total medical costs versus non-ADHD cohorts50 or ADHD patients with no additional health concerns.51–53 A retrospective study50 of a large claims database reported that ADHD adults with comorbidities had 2.5 times higher inpatient costs and 2 times higher outpatient and total medical costs versus non-ADHD patients. Hence, it is important to consider management of adult mental health comorbidities in the overall management of ADHD. In this regard, focus should be on providing consistent care over the lifespan of the patient to prevent comorbidities with progressing age. Although neurodevelopmental comorbidities and associated impairments have been commonly observed in routine clinical practice, only a few studies12,53 have evaluated the impact of ADHD in adults. Furthermore, identifying concurrent comorbidities would help create a diagnostic prioritization for treatment and the development of a pharmacologic/psychotherapeutic algorithm.
Strengths and Limitations
Linking a data source that is rich in patient-reported outcomes with claims data was not previously applied to the ADHD population. Our study has several strengths; linkage of NHWS and claims complement each other and resulted in a more robust dataset. NHWS (representative of the US general population) provides real-world data on patient characteristics, patient-reported outcomes, HRU, and treatment, which may not be captured through controlled clinical trials or pharmacy claims. The linkage allowed more comprehensive understanding of the burden, treatment, and health care costs all within one study for patients with ADHD.
Our study also has some limitations. Self-reported data may introduce bias, inconsistencies, or inaccuracies.54,55 Moreover, NHWS may not be truly representative of the ADHD population at large. Survey data may have selection bias due to disorder-related treatment. For example, treated patients might have better symptom control and thus completed the survey, whereas diagnosed but unmedicated patients or patients with severe levels of attention deficit might not be able to complete the survey. Additionally, the patient-reported outcomes from NHWS were only collected at one point in time. Thus, comparing HRQoL or WPAI by treatment groups may be challenging, as patients on these treatments could have different baseline levels for these metrics. Although, we have not considered the likely effect of executive dysfunction on the daily functioning of adults with ADHD, its presence cannot be ruled out as one of the contributing factors, and further research on this aspect is warranted. Importantly, pharmacy claims data reflect only fill patterns and assume that patients take the medication as prescribed; however, that may not always be the case.56
CONCLUSION
Based on this linked study, the DoE was found to be a significant predictor of HRU. In the overall management of ADHD, it is important to consider negative impact on HRQoL activity impairment and a higher HRU in patients with mental health comorbidities. Further, patients with ADHD treated with longer DoE formulations had substantially better economic outcomes versus shorter DoE formulation or unmedicated groups, offering potential cost savings to the patient and the health care system. Thus, as reflected by economic indicators (HRU and costs), treatment compared to no treatment is beneficial. Furthermore, comorbidities, especially mental health, present a challenge when treating these patients.
Submitted: June 27, 2022; accepted August 23, 2022.
Published online: March 14, 2023.
Funding/support: This study was funded by Purdue Pharma L.P., Stamford, Connecticut.
Role of the sponsor: Purdue Pharma L.P. funded the study and was involved in the design, as well as review and approval of the manuscript.
Acknowledgments: Medical writing support was provided by Ashwini Atre, PhD, and Sulekha Shafeeq, PharmD (Indegene Pvt. Ltd, Bengaluru, India). The authors would also like to thank Marc Cataldo, PharmD (Purdue Pharma L.P. Medical Affairs) for his reviews of the manuscript. The acknowledged individuals report no conflicts of interest related to the study.
Supplementary material: See accompanying pages.
CLINICAL POINTS
- Longer duration of effect formulations may provide better economic outcomes for adults with attention-deficit/hyperactivity disorder (ADHD).
- The effect of mental health comorbidities should be considered in the overall management of ADHD.
References (56)

- Faraone SV, Asherson P, Banaschewski T, et al. Attention-deficit/hyperactivity disorder. Nat Rev Dis Primers. 2015;1(1):15020. PubMed CrossRef
- Thapar A, Cooper M. Attention deficit hyperactivity disorder. Lancet. 2016;387(10024):1240–1250. PubMed CrossRef
- Biederman J, Petty CR, Evans M, et al. How persistent is ADHD? a controlled 10-year follow-up study of boys with ADHD. Psychiatry Res. 2010;177(3):299–304. PubMed CrossRef
- Sibley MH, Arnold LE, Swanson JM, et al. Variable patterns of remission from ADHD in the multimodal treatment study of ADHD. Am J Psychiatry. 2022;179(2):142–151. PubMed CrossRef
- Thomas R, Sanders S, Doust J, et al. Prevalence of attention-deficit/hyperactivity disorder: a systematic review and meta-analysis. Pediatrics. 2015;135(4):e994–e1001. PubMed CrossRef
- Xu G, Strathearn L, Liu B, et al. Twenty-year trends in diagnosed attention-deficit/hyperactivity disorder among US children and adolescents, 1997–2016. JAMA Netw Open. 2018;1(4):e181471. PubMed CrossRef
- Chung W, Jiang SF, Paksarian D, et al. Trends in the prevalence and incidence of attention-deficit/hyperactivity disorder among adults and children of different racial and ethnic groups. JAMA Netw Open. 2019;2(11):e1914344. PubMed CrossRef
- Volkow ND, Swanson JM. Adult attention deficit–hyperactivity disorder. 2016;369(20):1935–1944.
- Doshi JA, Hodgkins P, Kahle J, et al. Economic impact of childhood and adult attention-deficit/hyperactivity disorder in the United States. J Am Acad Child Adolesc Psychiatry. 2012;51(10):990–1002.e2. PubMed CrossRef
- Lee YC, Yang HJ, Chen VC, et al. Meta-analysis of quality of life in children and adolescents with ADHD: by both parent proxy-report and child self-report using PedsQL. Res Dev Disabil. 2016;51-52(110):160–172. PubMed CrossRef
- Bernardi S, Faraone SV, Cortese S, et al. The lifetime impact of attention deficit hyperactivity disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC). Psychol Med. 2012;42(4):875–887. PubMed CrossRef
- Pitts M, Mangle L, Asherson P. Impairments, diagnosis and treatments associated with attention-deficit/hyperactivity disorder (ADHD) in UK adults: results from the lifetime impairment survey. Arch Psychiatr Nurs. 2015;29(1):56–63. PubMed CrossRef
- Danckaerts M, Sonuga-Barke EJS, Banaschewski T, et al. The quality of life of children with attention deficit/hyperactivity disorder: a systematic review. Eur Child Adolesc Psychiatry. 2010;19(2):83–105. PubMed CrossRef
- Agarwal R, Goldenberg M, Perry R, et al. The quality of life of adults with attention deficit hyperactivity disorder: a systematic review. Innov Clin Neurosci. 2012;9(5-6):10–21. PubMed
- Caye A, Swanson JM, Coghill D, et al. Treatment strategies for ADHD: an evidence-based guide to select optimal treatment. Mol Psychiatry. 2019;24(3):390–408. PubMed CrossRef
- Posner J, Polanczyk GV, Sonuga-Barke E. Attention-deficit hyperactivity disorder. Lancet. 2020;395(10222):450–462. PubMed
- Childress A. The safety of extended-release drug formulations for the treatment of ADHD. Expert Opin Drug Saf. 2017;16(5):603–615. PubMed CrossRef
- Kemner JE, Lage MJ. Effect of methylphenidate formulation on treatment patterns and use of emergency room services. Am J Health Syst Pharm. 2006;63(4):317–322. PubMed CrossRef
- Ramos-Quiroga JA, Bosch R, Castells X, et al. Effect of switching drug formulations from immediate-release to extended-release OROS methylphenidate: a chart review of Spanish adults with attention-deficit hyperactivity disorder. CNS Drugs. 2008;22(7):603–611. PubMed CrossRef
- Spencer TJ, Adler LA, McGough JJ, et al; Adult ADHD Research Group. Efficacy and safety of dexmethylphenidate extended-release capsules in adults with attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61(12):1380–1387. PubMed CrossRef
- Kooij JJS, Bijlenga D, Salerno L, et al. Updated European Consensus Statement on diagnosis and treatment of adult ADHD. Eur Psychiatry. 2019;56(1):14–34. PubMed CrossRef
- Canadian ADHD Resource Alliance (CADDRA). Canadian ADHD Practice Guidelines - Fourth Edition. Published 2018. Accessed June 13, 2022. https://www.caddra.ca/wp-content/uploads/CADDRA-Guidelines-4th-Edition_-Feb2018.pdf
- Manor I, Ben-Hayun R, Aharon-Peretz J, et al. A randomized, double-blind, placebo-controlled, multicenter study evaluating the efficacy, safety, and tolerability of extended-release metadoxine in adults with attention-deficit/hyperactivity disorder. J Clin Psychiatry. 2012;73(12):1517–1523. PubMed CrossRef
- Weisler RH, Greenbaum M, Arnold V, et al. Efficacy and safety of SHP465 mixed amphetamine salts in the treatment of attention-deficit/hyperactivity disorder in adults: results of a randomized, double-blind, placebo-controlled, forced-dose clinical study. CNS Drugs. 2017;31(8):685–697. PubMed CrossRef
- Huss M, Ginsberg Y, Tvedten T, et al. Methylphenidate hydrochloride modified-release in adults with attention deficit hyperactivity disorder: a randomized double-blind placebo-controlled trial. Adv Ther. 2014;31(1):44–65. PubMed CrossRef
- Iwanami A, Saito K, Fujiwara M, et al. Safety and efficacy of guanfacine extended-release in adults with attention-deficit/hyperactivity disorder: an open-label, long-term, phase 3 extension study. BMC Psychiatry. 2020;20(1):485. PubMed CrossRef
- Banaschewski T, Coghill D, Santosh P, et al. Long-acting medications for the hyperkinetic disorders: a systematic review and European treatment guideline. Eur Child Adolesc Psychiatry. 2006;15(8):476–495. PubMed CrossRef
- Bilder RM, Loo SK, McGough JJ, et al. Cognitive effects of stimulant, guanfacine, and combined treatment in child and adolescent attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(8):667–673. PubMed CrossRef
- Loo SK, Bilder RM, Cho AL, et al. Effects of d-methylphenidate, guanfacine, and their combination on electroencephalogram resting state spectral power in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2016;55(8):674–682.e1. PubMed CrossRef
- McCracken JT, McGough JJ, Loo SK, et al. Combined stimulant and guanfacine administration in attention-deficit/hyperactivity disorder: a controlled, comparative study. J Am Acad Child Adolesc Psychiatry. 2016;55(8):657–666.e1. PubMed CrossRef
- López FA, Leroux JR. Long-acting stimulants for treatment of attention-deficit/hyperactivity disorder: a focus on extended-release formulations and the prodrug lisdexamfetamine dimesylate to address continuing clinical challenges. Atten Defic Hyperact Disord. 2013;5(3):249–265. PubMed CrossRef
- Feldman M, Bélanger S. Extended-release medications for children and adolescents with attention-deficit hyperactivity disorder. Paediatr Child Health. 2009;14(9):593–602. PubMed CrossRef
- Zhang Q, Zhao Y, Keshishian A, et al. Assessing the economic burden and health care utilization of attention deficit/hyperactivity disorder among medicaid patients in the United States. Value Health. 2016;19(3):A187. CrossRef
- Bolge SC, Doan JF, Kannan H, et al. Association of insomnia with quality of life, work productivity, and activity impairment. Qual Life Res. 2009;18(4):415–422. PubMed CrossRef
- DiBonaventura MD, Wagner JS, Yuan Y, et al. Humanistic and economic impacts of hepatitis C infection in the United States. J Med Econ. 2010;13(4):709–718. PubMed CrossRef
- Finkelstein EA, Allaire BT, DiBonaventura MD, et al. Direct and indirect costs and potential cost savings of laparoscopic adjustable gastric banding among obese patients with diabetes. J Occup Environ Med. 2011;53(9):1025–1029. PubMed CrossRef
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. PubMed CrossRef
- Centers for Disease Control and Prevention. Healthy weight: about adult BMI. Updated June 03, 2022. Accessed June 13, 2022. https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html
- Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey Manual and Interpretation Guide. Boston, MA: The Health Institute, New England Medical Center; 2000.
- Reilly MC, Zbrozek AS, Dukes EM. The validity and reproducibility of a work productivity and activity impairment instrument. Pharmacoeconomics. 1993;4(5):353–365. PubMed CrossRef
- Katzman MA, Bilkey TS, Chokka PR, et al. Adult ADHD and comorbid disorders: clinical implications of a dimensional approach. BMC Psychiatry. 2017;17(1):302. PubMed CrossRef
- Joseph A, Kosmas CE, Patel C, et al. Health-related quality of life and work productivity of adults with ADHD: A U.K. web-based cross-sectional survey. J Atten Disord. 2018;23(13):1610–1623. PubMed CrossRef
- Wigal SB, Wigal TL. The laboratory school protocol: its origin, use, and new applications. J Atten Disord. 2006;10(1):92–111. PubMed CrossRef
- Wigal T, Childress A, Frick G, et al. Effects of SHP465 mixed amphetamine salts in adults with ADHD in a simulated adult workplace environment. Postgrad Med. 2018;130(1):111–121. PubMed CrossRef
- Wigal T, Brams M, Gasior M, et al; 316 Study Group. Randomized, double-blind, placebo-controlled, crossover study of the efficacy and safety of lisdexamfetamine dimesylate in adults with attention-deficit/hyperactivity disorder: novel findings using a simulated adult workplace environment design. Behav Brain Funct. 2010;6(1):34. PubMed CrossRef
- Wigal SB, Wigal T, Childress A, et al. The time course of effect of multilayer-release methylphenidate hydrochloride capsules: a randomized, double-blind study of adults with ADHD in a simulated adult workplace environment. J Atten Disord. 2020;24(3):373–383. PubMed CrossRef
- Childress A, Cutler AJ, Marraffino AH, et al. Randomized, double-blind, placebo-controlled, parallel-group, adult laboratory classroom study of the efficacy and safety of PRC-063 (extended-release methylphenidate) for the treatment of ADHD. J Atten Disord. 2021;26(6):857–869. PubMed
- Brod M, Schmitt E, Goodwin M, et al. ADHD burden of illness in older adults: a life course perspective. Qual Life Res. 2012;21(5):795–799. PubMed CrossRef
- Goodman DW, Ginsberg L, Weisler RH, et al. An interim analysis of the Quality of Life, Effectiveness, Safety, and Tolerability (QU.E.S.T.) evaluation of mixed amphetamine salts extended release in adults with ADHD. CNS Spectr. 2005;10(suppl 20):26–34. PubMed CrossRef
- Secnik K, Swensen A, Lage MJ. Comorbidities and costs of adult patients diagnosed with attention-deficit hyperactivity disorder. Pharmacoeconomics. 2005;23(1):93–102. PubMed CrossRef
- Hodgkins P, Sasané R, Christensen L, et al. Treatment outcomes with methylphenidate formulations among patients with ADHD: retrospective claims analysis of a managed care population. Curr Med Res Opin. 2011;27(suppl 2):53–62. PubMed CrossRef
- Libutzki B, Ludwig S, May M, et al. Direct medical costs of ADHD and its comorbid conditions on basis of a claims data analysis. Eur Psychiatry. 2019;58:38–44. PubMed CrossRef
- Kawatkar AA, Knight TK, Moss RA, et al. Impact of mental health comorbidities on health care utilization and expenditure in a large US managed care adult population with ADHD. Value Health. 2014;17(6):661–668. PubMed CrossRef
- Ezzati M, Martin H, Skjold S, et al. Trends in national and state-level obesity in the USA after correction for self-report bias: analysis of health surveys. J R Soc Med. 2006;99(5):250–257. PubMed CrossRef
- Thomas III J, Paulet M, Rajpura JR. Consistency between self-reported and recorded values for clinical measures. Cardiol Res Pract. 2016;2016:4364761.
- Funk MJ, Landi SN. Misclassification in administrative claims data: quantifying the impact on treatment effect estimates. Curr Epidemiol Rep. 2014;1(4):175–185. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite