Prim Care Companion CNS Disord 2022;24(3):21cr03233
To cite: Spiegel DR, Kisteneff A, Fidelis W, et al. A case and proposed mechanism of catatonia during the post-acute phase of severe acute respiratory syndrome coronavirus 2 infection. Prim Care Companion CNS Disord. 2022;24(3):21cr03233.
To share: https://doi.org/10.4088/PCC.21cr03233
© Copyright 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry and Behavioral Sciences, Eastern Virginia Medical School, Norfolk, Virginia
*Corresponding author: David R. Spiegel, MD, Department of Psychiatry and Behavioral Sciences, Eastern Virginia Medical School, 825 Fairfax Ave, Norfolk, VA 23507 ([email protected]).
Catatonia is a psychomotor syndrome associated with several psychiatric and medical conditions, with the latter responsible for approximately 25% of cases.1 Toward this end, coronavirus disease 2019 (COVID-19) is a highly infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). There is a scant but increasing number of reports depicting catatonic syndrome due to SARS-CoV-2.2,3 We present the case of a patient who developed catatonia post-acute SARS-CoV-2 infection, as well as a possible mechanism of SARS-CoV-2 infection affecting neurotransmitters posited to be causal in catatonia.
Case Report
Our patient was a 59-year-old white man with no significant past medical or psychiatric history who presented to the emergency department (ED) with cough and shortness of breath. Four days earlier, he tested positive for SARS-CoV-2, but with chest x-ray reporting no acute findings, he was discharged with azithromycin. The patient had no known chronic medical conditions such as diabetes, cardiovascular disease, cancer, or chronic-obstructive pulmonary disease that would place him at elevated risk for complications from COVID-19.
Three days later, he returned with worsening shortness of breath. Chest x-ray showed bilateral, interstitial airway opacities consistent with COVID-19 pneumonia. On oxygen 2 L, arterial blood gas was normal. The patient’s D-dimer was 1.3 mg/L and ferritin was 523 ng/mL, but complete blood count, complete metabolic profile, blood alcohol/culture, and urine drug screen/culture results were unremarkable.
The patient was admitted to the hospital and started on ceftriaxone and methylprednisolone 40 mg/d. The patient maintained normal oxygen saturation (room air) throughout the admission. Seven days later, following cessation of respiratory symptoms, ceftriaxone was discontinued, and methylprednisolone taper was completed. Three days later, the psychiatry department was consulted due the patient’s “change in behavior.”
Upon our evaluation, the patient was noted to have poor eye contact with staring, decreased blinking, and paucity of verbal output to questioning but without fluctuations in mentation. Although the patient was a limited historian on interview, there were no recently reported or observed history of any anxiety, depressive, manic, or psychotic symptoms prior to the onset of or accompanying the catatonia. He scored 24 on the Bush-Francis Catatonia Rating Scale (BFCRS).4
Further evaluation included an unremarkable electroencephalogram (EEG), with no epileptiform discharges. Magnetic resonance imaging (MRI) of the head showed patent arteries, with no cytotoxic/vasogenic edema, thrombosis, or other acute findings. His vital signs were normal throughout our treatment. The patient’s interleukin-6 (IL-6), C-reactive protein, and procalcitonin levels were all elevated. The lorazepam (1 mg intravenous) challenge test was positive, and our patient started lorazepam 1 mg 3 times/d.
On day 2 of lorazepam treatment, his BFCRS score was 20. Lorazepam was titrated, and by day 5 equaled 2 mg 4 times daily. By day 6 of treatment, his BFCRS score decreased to 2. On day 7 of treatment, his BFCRS score was 0, and the lorazepam dose was decreased to 1 mg 3 times/d with instructions to taper on discharge.
Discussion
As per our review of the evidence, the patient was the 15th case of catatonia due to SARS-CoV-2 infection.1,2,3–15 While several hypotheses have emerged to explain how SARS-CoV-2 penetrates the central nervous system (CNS),16 it is suspected that neuropsychiatric symptoms induced by SARS-CoV-2 are due to direct CNS infiltration, cytokine network dysregulation, and peripheral immune cell transmigration.17 Additionally, direct endothelial cell damage can lead to thrombi and direct neural injury.18 While our patient had elevated proinflammatory cytokines, including IL-6, MRI of the head demonstrated no acute vascular/neural injury.
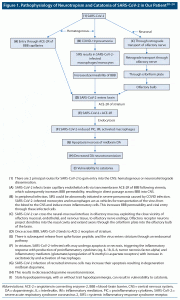
Interestingly, akinetic mutism, a similar phenotype of catatonia, has been reported in patients with COVID-19 manifesting decreased responsiveness. While distinct combinations of etiologic mechanisms likely contribute to akinetic mutism in SARS-CoV-2 infection, disruption of frontal-subcortical circuitry, vis-a-vis massive inflammatory cytokine release, has been proposed.19 The phenotypic/genotypic similarities of akinetic mutism and catatonia make this theory more intriguing to elucidate how SARS-CoV-2 infection could induce catatonia. Figure 1 provides a proposed pathophysiology of SARS-CoV-2–induced catatonia.20–24
In closing, we propose that our patient’s catatonia was precipitated by SARS-CoV-2 infection. As symptoms did not reverse after pneumonia resolved and mentation did not wax/wane, we felt delirium was less likely. Additionally, with a normal EEG, nonconvulsive status epilepticus was ruled out.25 As the COVID-19 pandemic continues, we advise clinicians to evaluate for SARS-CoV-2–induced neuropsychiatric manifestations, including catatonia.
Submitted: December 29, 2021.
Published online: June 14, 2022.
Relevant financial relationships: Dr Spiegel is in the speaker’s bureau for Allergen, Alkermes, Otsuka, and IntraCellular but has no conflict of interest related to this report. Drs Kisteneff and Mclean; Mss Fidelis, Frawley, Crofton, Dacyczyn, Ayzman, Lund, and Rave; and Mssr Connelly, Lopacinski, Conlin, and Rickelmann report no conflicts of interest related to the subject of this report.
Funding/support: None.
Patient consent: Consent was verbally received from the patient to publish the case report, and information has been de-identified to protect anonymity.
References (25)

- Gouse BM, Spears WE, Nieves Archibald A, et al. Catatonia in a hospitalized patient with COVID-19 and proposed immune-mediated mechanism. Brain Behav Immun. 2020;89:529–530. PubMed CrossRef
- Scheiner NS, Smith AK, Wohlleber M, et al. COVID-19 and catatonia: a case series and systematic review of existing literature. J Acad Consult Liaison Psychiatry. 2021;62(6):645–656. PubMed CrossRef
- Varatharaj A, Thomas N, Ellul MA, et al; CoroNerve Study Group. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. (Published correction appears in Lancet Psychiatry. July 14, 2020.) Lancet Psychiatry. 2020;7(10):875–882. PubMed CrossRef
- Bush G, Fink M, Petrides G, et al. Catatonia, I: rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129–136. PubMed CrossRef
- Caan MP, Lim CT, Howard M. A case of catatonia in a man with COVID-19. Psychosomatics. 2020;61(5):556–560. PubMed CrossRef
- Deocleciano de Araujo C, Schlittler LXC, Sguario RM, et al. Life-threatening catatonia associated with coronavirus disease 2019. J Acad Consult Liaison Psychiatry. 2021;62(2):256–257. PubMed CrossRef
- Vazquez-Guevara D, Badial-Ochoa S, Caceres-Rajo KM, et al. Catatonic syndrome as the presentation of encephalitis in association with COVID-19. BMJ Case Rep. 2021;14(6):e240550. PubMed CrossRef
- Fiaschè F, Adriani B, Mancinelli I, et al. Treatment of catatonia with asenapine in a patient with schizotypal personality disorder, psychotic depression and septic shock from SARS-CoV-2: a case report. CNS Neurol Disord Drug Targets. 2021;20(5):473–477. PubMed CrossRef
- Zain SM, Muthukanagaraj P, Rahman N. Excited catatonia: a delayed neuropsychiatric complication of COVID-19 infection. Cureus. 2021;13(3):e13891. PubMed CrossRef
- Torrico T, Kiong T, D’Assumpcao C, et al. Postinfectious COVID-19 catatonia: a report of two cases. Front Psychiatry. 2021;12:696347. PubMed CrossRef
- Mulder J, Feresiadou A, Fällmar D, et al. Autoimmune encephalitis presenting with malignant catatonia in a 40-year-old male patient with COVID-19. Am J Psychiatry. 2021;178(6):485–489. PubMed CrossRef
- Zandifar A, Badrfam R. Exacerbation of psychosis accompanied by seizure and catatonia in a patient with COVID-19: a case report. Psychiatry Clin Neurosci. 2021;75(2):63–64. PubMed CrossRef
- Amouri J, Andrews PS, Heckers S, et al. A case of concurrent delirium and catatonia in a woman with coronavirus disease 2019. J Acad Consult Liaison Psychiatry. 2021;62(1):109–114. PubMed CrossRef
- Austgen G, Meyers MS, Gordon M, et al. The use of electroconvulsive therapy in neuropsychiatric complications of coronavirus disease 2019: a systematic literature review and case report. J Acad Consult Liaison Psychiatry. 2022;63(1):86–93. PubMed CrossRef
- Kwon HJ, Patel KH, Ramirez M, et al. A case of fatal catatonia in a COVID-19 patient. Cureus. 2021;13(7):e16529. PubMed CrossRef
- Wu Y, Xu X, Chen Z, et al. Nervous system involvement after infection with COVID-19 and other coronaviruses. Brain Behav Immun. 2020;87:18–22. PubMed CrossRef
- Nalleballe K, Reddy Onteddu S, Sharma R, et al. Spectrum of neuropsychiatric manifestations in COVID-19. Brain Behav Immun. 2020;88:71–74. PubMed CrossRef
- Nezgovorova V, Ferretti CJ, Pallanti S, et al. Modulating neuroinflammation in COVID-19 patients with obsessive-compulsive disorder. J Psychiatr Res. 2022;149:367–373. PubMed CrossRef
- Fusunyan M, Praschan N, Fricchione G, et al. Akinetic mutism and coronavirus disease 2019: a narrative review. J Acad Consult Liaison Psychiatry. 2021;62(6):625–633. PubMed CrossRef
- Zhou Z, Kang H, Li S, et al. Understanding the neurotropic characteristics of SARS-CoV-2: from neurological manifestations of COVID-19 to potential neurotropic mechanisms. J Neurol. 2020;267(8):2179–2184. PubMed CrossRef
- Boldrini M, Canoll PD, Klein RS. How COVID-19 affects the brain. JAMA Psychiatry. 2021;78(6):682–683. PubMed CrossRef
- Gubernatorova EO, Gorshkova EA, Polinova AI, et al. IL-6: relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020;53:13–24. PubMed CrossRef
- Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24(2):168–175. PubMed CrossRef
- Rustad JK, Landsman HS, Ivkovic A, et al. Catatonia: an approach to diagnosis and treatment. Prim Care Companion CNS Disord. 2018;20(1):17f02202. PubMed CrossRef
- Bartolommei N, Lattanzi L, Callari A, et al. Catatonia: a critical review and therapeutic recommendations. J Psychopathology. 2012;18:234–246.
Please sign in or purchase this PDF for $40.
Save
Cite