Lessons Learned at the Interface of Medicine and Psychiatry
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord 2024;26(4):23f03702
Author affiliations are listed at the end of this article.
Have you ever wondered when your patients should receive or avoid taking clozapine? Have you been uncertain about what laboratory tests are required before and during clozapine administration? Have you puzzled over drug interactions associated with clozapine use? If you have, the following case vignette and discussion should prove useful.
CASE VIGNETTE
Ms K, a 67-year-old woman, was brought to the emergency department (ED) by her family due to worsening depression, thoughts of suicide, and bizarre behavior. Her psychiatric history was notable for schizoaffective disorder, bipolar type, for which she had been hospitalized multiple times (most recently 2 years earlier, at which time she received electroconvulsive therapy [ECT]); she also had a history of catatonia and several suicide attempts. Her family reported that while she was traveling in Europe several weeks earlier, her mood was “elated.” However, since she returned home, she had become paranoid. She appeared depressed and had delusions (believing that she had committed crimes for which she would be incarcerated). She was eating poorly, although she had been drinking a lot of water. Her outpatient psychiatrist increased her risperidone and lorazepam, but Ms K’s symptoms failed to improve. In the ED, Ms K appeared disheveled and had a long speech latency as well as ambitendency (ie, saying “yes” to a question and then quickly saying “no”). Her thoughts were negativistic, and she perseverated on being a “bad” and “stupid” mother. She reported having a plan to overdose on her medications but denied intent. Laboratory testing (ie, complete blood count [CBC] and differential, electrolytes, liver function tests, urine toxicology, ethanol level, and urinalysis) revealed the following abnormalities: white blood cells: 14.39 k/uL, sodium: 124 mg/dL, potassium: 3.2 mg/dL, chloride: 86 mg/dL, creatinine: 1.53 mg/dL, and alkaline phosphatase: 118 mg/dL.
She was admitted to the inpatient psychiatric unit. On hospital day 1, she scored a 12 on the Bush-Francis Catatonia Rating Scale.1 Over the first week, risperidone was increased to 6 mg/d, while lorazepam was increased to 4 mg/d, without a significant decrease in her symptoms, although this was complicated by her tendency to refuse medications. Given that her symptoms had previously responded to ECT, she restarted ECT 3 times/wk. After 10 sessions, her catatonia resolved, but her psychotic symptoms persisted (with ongoing delusions and disorganized thinking). Additional treatment was pursued.
DISCUSSION
What Is Clozapine and How Does It Work?
Clozapine is an atypical (second-generation) antipsychotic that remains the only treatment approved by the US Food and Drug Administration (FDA) for either treatment-resistant schizophrenia (TRS) or thoughts of suicide in those with schizophrenia-spectrum disorders. Clozapine is broadly considered to be the most effective antipsychotic for a variety of psychiatric conditions, including psychosis with sensitivity to extrapyramidal symptoms (EPS) or tardive dyskinesia (TD), catatonic schizophrenia, Parkinson disease with psychosis, aggression secondary to psychosis, refractory mood disorders, and primary polydipsia.2
The precise mechanism for clozapine’s superior efficacy remains unknown. Its mechanism of action includes atypical dopaminergic activity (ie, with weak D2 antagonism and strong D4 antagonism), strong serotonergic antagonism at 5-HT2A, and N-methyl-D aspartate glutamatergic agonism.3 Its broad blockade at α1-adrenergic, M1-muscarinic, and H1-histaminergic receptors accounts for many of its adverse effects. There is also evidence that clozapine increases neuroplasticity and levels of brain-derived neurotrophic factor, decreases inflammation through glial modulation, and normalizes excitatory to inhibitory tone across neural circuits.4
Shortly after clozapine’s introduction in the early 1970s, reports of agranulocytosis and death among clozapine-treated patients in Finland led to its withdrawal from the market5 until a seminal 1988 study confirmed its superior efficacy in TRS.6 The FDA responded by granting approval to clozapine alongside the establishment of a mandated registry-based monitoring system for neutrophil counts, the Clozapine Risk Evaluation and Mitigation Strategy (Clozapine REMS). Clozapine carries a unique set of adverse effects that necessitates clinician expertise and close patient monitoring. However, fears surrounding the prescribing of clozapine and a lack of familiarity with it led to its underutilization and delayed initiation.7 It has become increasingly well established that clozapine’s unique efficacy, tolerability among certain groups, and ability to reduce thoughts of suicide make it a life-saving medication.
How Is Clozapine Metabolized/Excreted and How Long Does It Last?
Clozapine’s pharmacokinetics play a crucial role in determining its duration of action; its metabolism and elimination involve hepatic biotransformation and renal excretion. Clozapine is primarily metabolized by the cytochrome P450 (CYP) isoenzyme system, with CYP1A2 being the main enzyme responsible for its metabolism. N-desmethylclozapine (norclozapine) and clozapine N-oxide are clozapine’s major metabolites. Norclozapine is a pharmacologically active metabolite that contributes to clozapine’s overall therapeutic actions. On average, the half-life of clozapine is approximately 12 hours. In contrast, norclozapine’s half life is around 23 hours. The extended duration of norclozapine contributes to clozapine’s sustained therapeutic effects, even after it has been discontinued.
Following its hepatic metabolism, clozapine and its metabolites are excreted in both urine and feces. However, clozapine’s primary route of elimination is via the kidneys, where both unchanged clozapine and its metabolites are excreted. Renal excretion plays a significant role in the body’s elimination of clozapine, and this process is facilitated by glomerular filtration and active tubular secretion.
Factors that can influence the metabolism of clozapine include patient-level factors (eg, older age, female sex, cigarette smoking, and genetics [such as the rs762551 polymorphism in CYP1A2 enzymes]), hepatic dysfunction, and drug interactions (eg, with coadministered medications that induce [eg, omeprazole, barbiturates, and carbamazepine] or inhibit [eg, cimetidine and ciprofloxacin] CYP1A2 enzymes).8,9 In addition, renal insufficiency can delay the excretion of clozapine, leading to the potential accumulation of clozapine and its metabolites. This, in turn, necessitates clozapine dosage adjustments.
Who Should Receive Clozapine (and When)?
Nearly one-third of patients with schizophrenia do not respond to first-line antipsychotic treatment.10 Although clozapine has consistently demonstrated clinical superiority in comparison with other antipsychotics, it remains woefully underutilized by clinicians. Clozapine is indicated for individuals with TRS and schizoaffective disorder, with treatment resistance being defined as persistent symptoms of at least moderate severity with at least moderate functional impairment despite 2 adequate antipsychotic trials.11–13
However, other patient populations can also benefit from earlier consideration of clozapine, eg, patients who have had EPS in association with use of other antipsychotics. Because of its weak D2 receptor antagonism, clozapine is less likely than other atypical and typical antipsychotics to cause akathisia, parkinsonism, and TD.14,15 The weak D2 receptor binding also makes clozapine an excellent choice for patients with Parkinson disease–related psychosis who have not yet achieved symptom remission with other antipsychotics.16 Finally, studies have demonstrated that clozapine can decrease aggression and thoughts of suicide.17,18
Which Laboratory Tests Should Be Obtained Before Prescribing Clozapine (and Why)?
Because clozapine can cause agranulocytosis, several laboratory tests (eg, CBC with a differential, including an absolute neutrophil count [ANC]) should be obtained before starting clozapine (Table 1 and Table 2). For those in the general population, the ANC must be at least 1,500/μL before clozapine can be initiated.19 For patients with benign ethnic neutropenia (BEN), the ANC must be greater than 1,000/μL before starting clozapine. BEN is diagnosed when neutropenia (an ANC ≤ 1,500/μL) has been detected on repeated blood draws, and there have been no pathologic consequences, such as repeated infections (which are not typical of BEN).20 BEN is most frequently observed in individuals of African descent (with an approximate prevalence of 25%–50%), in some Middle Eastern ethnic groups, and in other ethnic groups who have darker complexions.19 Most patients with BEN have a fluctuating level of the ANC (above 1,000/μL), and they can be managed safely while taking clozapine.21 If the ANC drops below 1,000/μL, it may be helpful to obtain a hematology consultation when deciding whether to use clozapine.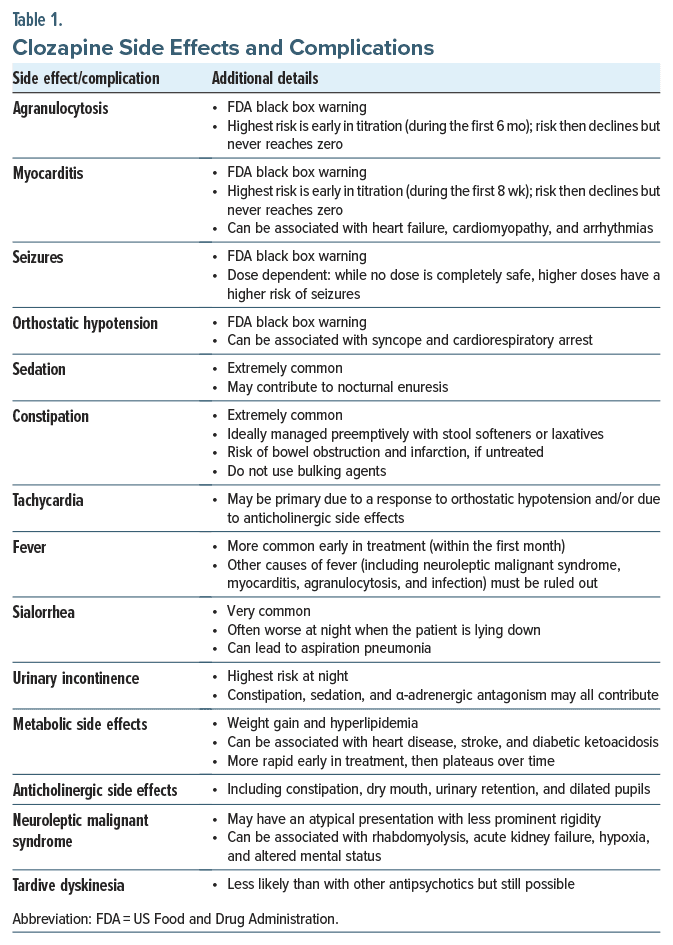
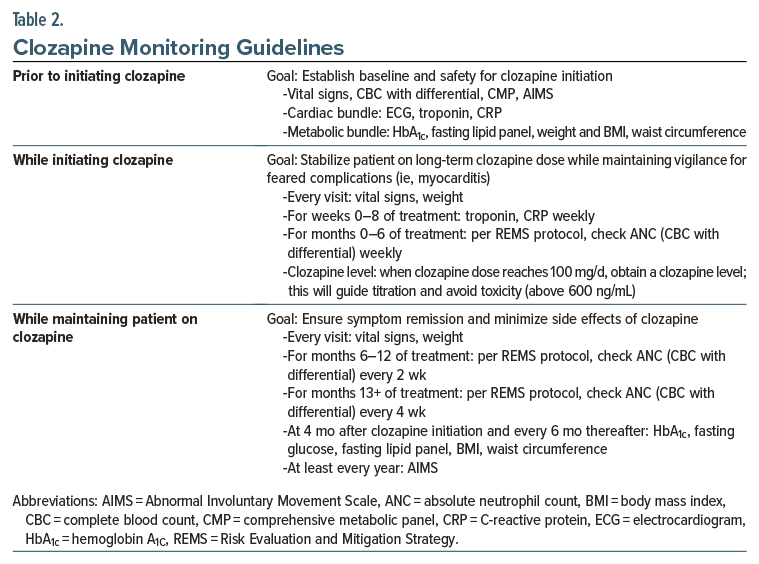
In addition to hematological complications, clozapine can also cause myocarditis and orthostatic hypotension. Therefore, before beginning clozapine, all patients should have their vital signs assessed. In addition, for all patients in whom clozapine treatment is considered, a baseline electrocardiogram (ECG) and levels of troponin and C-reactive protein (CRP) should be obtained.
Since clozapine can also induce considerable weight gain, all patients should have a baseline weight, body mass index, and waist circumference checked before clozapine is started. Patients should also have a baseline hemoglobin A1c level and a cholesterol panel checked.
Like all antipsychotic medications, clozapine can cause abnormal movements (although it does so uncommonly). Nevertheless, all patients should be screened with the Abnormal Involuntary Movement Scale,22 and the results of the screening should be documented in their medical record. Due to other physical and metabolic effects of clozapine and its unknown effects on the fetus when it is taken during pregnancy, patients should have a complete metabolic panel, including tests of renal and liver function, and a urine pregnancy test (for people of child-bearing potential).
Which Drug Interactions With Clozapine Can Be Problematic?
Clozapine is largely metabolized by the hepatic CYP450 isoenzyme system, specifically the isoform CYP1A2.23 As such, prescribers should be aware of CYP1A2 inducers and inhibitors, as these will affect rates of clozapine metabolism and therefore serum clozapine drug levels, which will necessitate dose adjustments. Prescribers should also be aware that a patient’s baseline CYP1A2 activity may vary due to genetic polymorphisms, such as a trend toward lower CYP1A2 activity in East and South Asian individuals compared to white populations.24
Drugs that inhibit CYP1A2 will impede the breakdown of clozapine, thereby increasing serum clozapine levels. Commonly encountered strong inhibitors of CYP1A2 include the selective serotonin reuptake inhibitors (SSRIs), such as fluvoxamine, and several antibiotics (ie, ciprofloxacin, enoxacin, and erythromycin). Moderate CYP1A2 inhibitors include cimetidine and oral contraceptives that contain ethinylestradiol. While not technically a CYP1A2 inhibitor, caffeine competes with clozapine as a substrate, which can also increase serum clozapine levels.23 Lastly, systemic inflammatory responses (eg, sepsis) downregulate CYP1A2 activity.25,26 By contrast, CYP1A2 inducers facilitate clozapine metabolism and thus decrease its serum levels.23 Multiple psychotropics are categorized as strong CYP1A2 inducers (including carbamazepine, modafinil, phenobarbital, and phenytoin), as is the proton pump inhibitor, omeprazole. Importantly, cigarette smoking and smoking cannabis lead to the generation of polycyclic aromatic hydrocarbons (PAHs), which induce CYP1A2 metabolism.27 PAH formation occurs through the combustion process; as such, smoking induces CYP1A2, whereas alternatives such as nicotine replacement therapy do not.
What Side Effects and Complications Are Associated With Clozapine Use?
Clozapine use has been associated with a wide range of side effects and complications, spanning those that are common and manageable to those that are rare and potentially life-threatening. Table 1 summarizes clozapine’s side effects and complications.28
In addition to the rare and potentially life-threatening side effects (eg, agranulocytosis and myocarditis), clozapine has also been linked with the onset of tachycardia, orthostatic hypotension, urinary retention, sedation, drowsiness, sexual dysfunction, and pulmonary emboli.28,29 Therefore, prescribing clozapine has consistently posed a challenge for physicians, as it requires adept navigation around potential side effects and complications.
What Are Contraindications to Clozapine Use?
Failure to adhere to a clozapine treatment regimen (eg, when clozapine is abruptly stopped or restarted) can lead to serious complications, such as cholinergic rebound and syncope, respectively. In addition, nonadherence with FDA-mandated regular blood draws to monitor ANCs renders some patients unable to remain on clozapine.
Although an allergic reaction to clozapine is the only absolute contraindication to use, risk factors for agranulocytosis, myocarditis, cardiomyopathy, and seizures should be considered carefully, including prior occurrences of these side effects while taking clozapine. The benefits of rechallenge with clozapine (after it had been discontinued following development of a serious side effect) may outweigh the risks.
Consensus is lacking regarding rechallenge following severe agranulocytosis, despite a high risk of recurrence.30 Although myocarditis also has a high risk of recurrence, recent literature suggests that rechallenge is better tolerated with a slow clozapine titration and a lower target dose.31 Seizures are more likely when clozapine levels are high, but they can be managed with use of antiepileptic drugs and frequent monitoring of clozapine blood levels. In general, those with medical comorbidities and the elderly tend to be more sensitive to side effects, especially agranulocytosis. Other rare side effects—including but not limited to EPS and neuroleptic malignant syndrome—may also occur, but these conditions are not absolute contraindications to use of clozapine.
What Type of Monitoring Is Advised or Required During Clozapine Administration?
Before beginning clozapine, baseline ANC levels should be obtained; this value should be ≥ 1,500/mm3 in the general patient population and ≥ 1,000/mm3 for patients with BEN.32 The ANC must be monitored weekly for the first 6 months of clozapine treatment, and then every other week until the patient has completed 1 year of clozapine treatment. After the first year of treatment, the ANC should be monitored monthly on an indefinite basis for as long as the patient remains on clozapine.
In addition, all patients who are receiving clozapine in the United States must be registered in the Clozapine REMS database before initiating this medication. Furthermore, prescribers must be certified in the Clozapine REMS before prescribing clozapine; certification entails a process that includes a review of agranulocytosis risk during clozapine treatment, completion of an enrollment form, and a knowledge assessment. Similarly, pharmacists must also be certified to receive and dispense clozapine prescriptions. Before clozapine can be dispensed, a clinician will need to enter their patient’s ANC level into the REMS database on a regular basis—weekly during the first 6 months of treatment, every 2 weeks for months 7–12 of treatment, and monthly thereafter. Any disruptions in clozapine treatment and monitoring may require dose adjustments; see the Clozapine REMS website for more details. Notably, the FDA has acknowledged recent disruptions in clozapine access for patients who have been recently discharged from inpatient settings. The FDA has signaled to inpatient pharmacies that they have discretion to dispense “a days’ supply of clozapine that aligns with the patient’s monitoring frequency (eg, weekly monitoring=7-day supply, twice monthly monitoring=14- day supply, and monthly monitoring=30-day supply) upon discharge from an inpatient facility.”33 Prescribers are explicitly encouraged to use their clinical judgment in prescribing clozapine to patients with acceptable ANC values, as disruption or discontinuation of clozapine can lead to negative patient outcomes.
CASE VIGNETTE: WHAT HAPPENED TO THE PATIENT?
Clozapine was considered, given her refractory psychosis and catatonia and history of treatment failure with multiple antipsychotics (including risperidone, haloperidol, olanzapine, quetiapine, lurasidone, and perphenazine). Clozapine was initiated after her CRP, ECG, and troponins were within normal limits. ECT was continued (3 times/wk). Within 1 week, Ms K’s psychotic symptoms remitted; she was no longer paranoid, and her thought process became linear. She began to care for her hygiene and eagerly participated in therapy groups. She was discharged on clozapine and continued with weekly maintenance ECT.
CONCLUSION
Clozapine is a second-generation antipsychotic that remains the only drug approved by the FDA for either TRS or thoughts of suicide in those with schizophrenia spectrum disorders. Clozapine’s unique efficacy, its tolerability among those who are prone to EPS, and its ability to reduce thoughts of suicide make it a life-saving medication. Unfortunately, clozapine has a unique set of adverse side effects (eg, agranulocytosis, myocarditis, cardiomyopathy, and seizures) that necessitates close patient monitoring. However, fears surrounding prescribing clozapine and a lack of familiarity with its use have led to its underutilization and delayed initiation.
Because of clozapine’s weak D2 receptor antagonism, it is less likely than other atypical and typical antipsychotics to cause akathisia, parkinsonism, and TD. Since clozapine is largely metabolized by CYP1A2, clinicians should be mindful of coadministered drugs that inhibit this enzyme (eg, SSRIs, such as fluvoxamine, and several antibiotics, such as ciprofloxacin, enoxacin, and erythromycin) and impede the breakdown of clozapine, thereby increasing serum clozapine levels and contributing to toxicity, and of CYP1A2 inducers (eg, carbamazepine, modafinil, phenobarbital, phenytoin, and omeprazole) that facilitate its metabolism and thus decrease its serum levels. Although an allergic reaction to clozapine is the only absolute contraindication to clozapine use, risk factors for its other serious complications should be considered carefully.
Article Information
Published Online: July 30, 2024. https://doi.org/10.4088/PCC.23f03702
© 2024 Physicians Postgraduate Press, Inc.
Submitted: January 5, 2024; accepted March 27, 2024.
To Cite: Lissanu DS, Vyas CM, Lim CS, et al. Clozapine: its use and monitoring.
Prim Care Companion CNS Disord. 2024;26(4):23f03702.
Author Affiliation: Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, Boston, Massachusetts (all authors).
Corresponding Author: Desta S. Lissanu, MD, Department of Psychiatry, Massachusetts General Hospital and Harvard Medical School, 55 Fruit St, Warren 12-20, Boston, MA 02114 ([email protected]).
Drs Lissanu, Vyas, Lim, Daneshvari, and Donovan are co-first authors.
Relevant Financial Relationships: Dr Vyas has received research support from Nestlé-Purina Petcare Company, Mars Edge, and American Foundation for Suicide Prevention. Drs Lissanu, Lim, Daneshvari, Donovan, and Stern report no conflicts of interest related to the subject of this article.
Funding/Support: None.
Clinical Points
- Clozapine is a second-generation antipsychotic primarily metabolized by cytochrome P450 1A2; renal excretion plays a significant role in its elimination from the body.
- A complete blood count (with a differential, including an absolute neutrophil count [ANC]) should be obtained before starting clozapine, as it can cause agranulocytosis; for those in the general population, the ANC must be at least 1,500/μL before clozapine can be initiated.
- Since clozapine can cause myocarditis, all patients in whom clozapine is being considered should have a baseline electrocardiogram, and levels of troponin and C-reactive protein should be obtained; given clozapine’s propensity to induce weight gain, all patients should have a baseline weight, body mass index, and waist circumference before clozapine is started.
- All patients receiving clozapine in the United States must be registered in the Clozapine Risk Evaluation and Mitigation Strategy database before initiating this medication.
References (33)

- Bush G, Fink M, Petrides G, et al. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129–136. PubMed CrossRef
- Young CR, Longhurst JG, Bowers MB Jr., et al. The expanding indications for clozapine. Exp Clin Psychopharmacol. 1997;5(3):216–234. PubMed CrossRef
- Meltzer HY. An overview of the mechanism of action of clozapine. J Clin Psychiatry. 1994;55(suppl B):47–52. PubMed
- Gammon D, Cheng C, Volkovinskaia A, et al. Clozapine: why is it so uniquely effective in the treatment of a range of neuropsychiatric disorders? Biomolecules. 2021;11(7):1030. PubMed CrossRef
- Idänpään-Heikkilä J, Alhava E, Olkinuora M, et al. Agranulocytosis during treatment with chlozapine. Eur J Clin Pharmacol. 1977;11(3):193–198. PubMed
- Kane J, Honigfeld G, Singer J, et al. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789–796. PubMed CrossRef
- Shah P, Iwata Y, Plitman E, et al. The impact of delay in clozapine initiation on treatment outcomes in patients with treatment-resistant schizophrenia: a systematic review. Psychiatry Res. 2018;268:114–122. PubMed CrossRef
- Haring C, Fleischhacker WW, Schett P, et al. Influence of patient-related variables on clozapine plasma levels. Am J Psychiatry. 1990;147(11):1471–1475. PubMed CrossRef
- Bersani FS, Capra E, Minichino A, et al. Factors affecting interindividual differences in clozapine response: a review and case report. Hum Psychopharmacol. 2011;26(3):177–187. PubMed CrossRef
- Kane JM, Agid O, Baldwin ML, et al. Clinical guidance on the identification and management of treatment-resistant schizophrenia. J Clin Psychiatry. 2019;80(2):18com12123. PubMed CrossRef
- Kelly DL, Freudenreich O, Sayer MA, et al. Addressing barriers to clozapine underutilization: a national effort. Psychiatr Serv. 2018;69(2):224–227. PubMed CrossRef
- McEvoy JP, Lieberman JA, Stroup TS, et al. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600–610. PubMed CrossRef
- Howes OD, McCutcheon R, Agid O, et al. Treatment-resistant schizophrenia: treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174(3):216–229. PubMed CrossRef
- Niemann N, Jankovic J. Treatment of tardive dyskinesia: a general overview with focus on the vesicular monoamine transporter 2 inhibitors. Drugs. 2018;78(5):525–541. PubMed CrossRef
- Lieberman JA, Safferman AZ, Pollack S, et al. Clinical effects of clozapine in chronic schizophrenia: response to treatment and predictors of outcome. Am J Psychiatry. 1994;151(12):1744–1752. PubMed CrossRef
- Pollak P, Tison F, Rascol O, et al. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75(5):689–695. PubMed CrossRef
- Meltzer HY, Alphs L, Green AI, et al. Clozapine treatment for suicidality in schizophrenia: international suicide prevention trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82–91. PubMed CrossRef
- Volavka J, Czobor P, Nolan K, et al. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. J Clin Psychopharmacol. 2004;24(2):225–228. PubMed CrossRef
- Clozapine REMS. Clozapine REMS Program. Accessed on May 12, 2023. https://www.newclozapinerems.com/
- Freudenreich O. Clozapine. In: Freudenreich O, ed. Psychotic Disorders: A Practical Guide. 2nd ed. Humana Press; 2020:231–248.
- Manu P, Sarvaiya N, Rogozea LM, et al. Benign ethnic neutropenia and clozapine use: a systematic review of the evidence and treatment recommendations. J Clin Psychiatry. 2016;77(7):e909–e916. PubMed CrossRef
- Guy W. ECDEU Assessment Manual for Psychopharmacology, Revised. US Department of Health, Education, and Welfare Publication (ADM). National Institute of Mental Health; 1976:76–338.
- Taylor D. Pharmacokinetic interactions involving clozapine. Br J Psychiatry. 1997;171:109–112. PubMed CrossRef
- Gunes A, Dahl ML. Variation in CYP1A2 activity and its clinical implications: influence of environmental factors and genetic polymorphisms. Pharmacogenomics. 2008;9(5):625–637. PubMed CrossRef
- Hägg S, Spigset O, Mjörndal T, et al. Effect of caffeine on clozapine pharmacokinetics in healthy volunteers. Br J Clin Pharmacol. 2000;49(1):59–63. PubMed
- Leung JG, Nelson S, Takala CR, et al. Infection and inflammation leading to clozapine toxicity and intensive care: a case series. Ann Pharmacother. 2014;48(6):801–805. PubMed CrossRef
- Wagner E, McMahon L, Falkai P, et al. Impact of smoking behavior on clozapine blood levels - a systematic review and meta-analysis. Acta Psychiatr Scand. 2020;142(6):456–466. PubMed CrossRef
- De Berardis D, Rapini G, Olivieri L, et al. Safety of antipsychotics for the treatment of schizophrenia: a focus on the adverse effects of clozapine. Ther Adv Drug Saf. 2018;9(5):237–256. PubMed CrossRef
- Sarvaiya N, Lapitskaya Y, Dima L, et al. Clozapine-associated pulmonary embolism: a high-mortality, dose-independent and early-onset adverse effect. Am J Ther. 2018;25(4):e434–e438. PubMed CrossRef
- Manu P, Lapitskaya Y, Shaikh A, et al. Clozapine rechallenge after major adverse effects: clinical guidelines based on 259 cases. Am J Ther. 2018;25(2):e218–e223. PubMed CrossRef
- Wagner E, Siskind D, Falkai P, et al. Clozapine optimization: a delphi consensus guideline from the treatment response and resistance in psychosis working group. Schizophr Bull. 2023;49(4):962–972. PubMed CrossRef
- Correll CU, Agid O, Crespo-Facorro B, et al. A guideline and checklist for initiating and managing clozapine treatment in patients with treatment-resistant schizophrenia. CNS Drugs. 2022;36(7):659–679. PubMed CrossRef
- Food and Drug Administration. Clozapine REMS announcement; 2022. Accessed December 3, 2024. www.newclozapinerems.com
Please sign in or purchase this PDF for $40.
Save
Cite