When clinically indicated, some antipsychotic medications can be switched from oral administration to a long-acting injectable formulation. Guidelines are available for converting the oral dosage to an equivalent intramuscular (IM) dosage,1 but to our knowledge, little has been published on converting from IM to oral.2,3 We report a case in which steady-state concentrations of IM risperidone (half-life of 3–6 days) were used to determine an equivalent oral dosage of risperidone (half-life of 20 hours).4
Case Report
A middle-aged resident who lives in a long-term care facility has a diagnosis of DSM-5 schizophrenia and had been in partial remission for the preceding 5 years. Current psychotropic medications included maintenance Risperdal Consta 50 mg IM every 2 weeks (IM risperidone started 15 years ago), clonazepam 1.0 mg in the evening for anxiety, and fluoxetine 40 mg in the morning for depression. The resident had no physical comorbidities and was taking no medications known to alter the metabolism of risperidone.5 The patient gave consent to switch from IM risperidone to the oral form and remained in partial yet stable remission during the transition period.
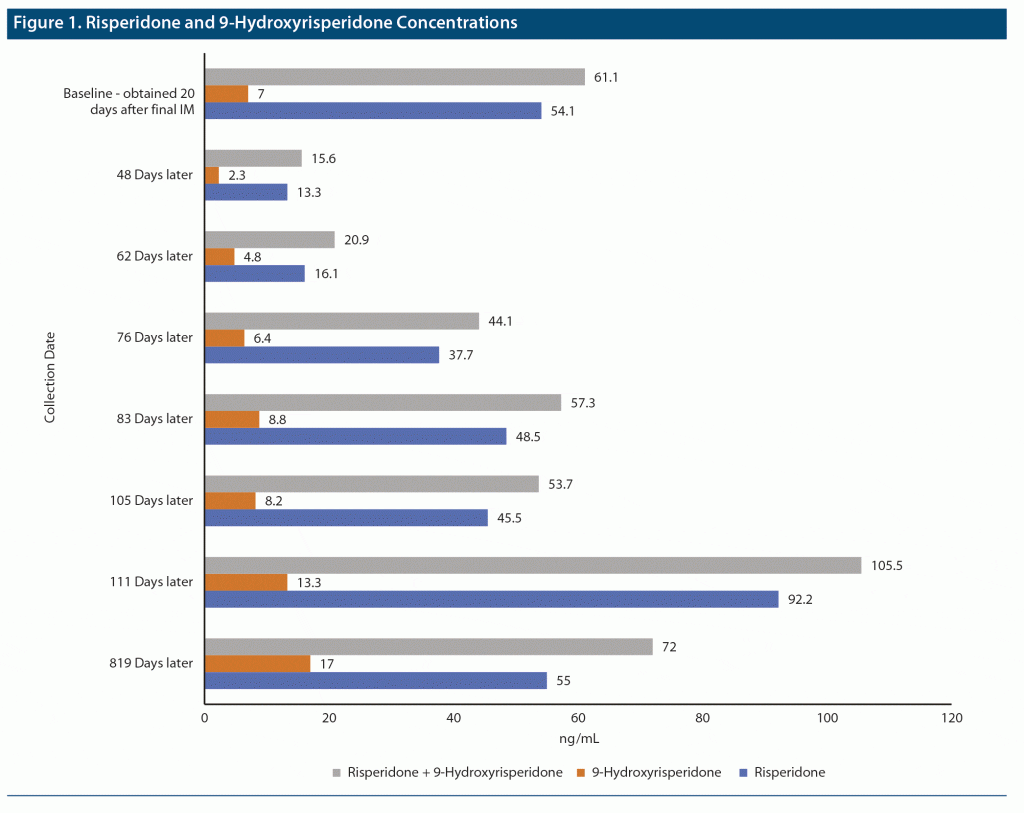
Steady-state concentrations of risperidone and its only active metabolite, 9-hydroxyrisperidone, were determined from a blood sample drawn 20 days after the previous administration of Risperdal Consta 50 mg IM (risperidone = 54.1 ng/mL, 9-hydroxyrisperidone = 7.0 ng/mL). Because the patient’s symptoms had been under adequate control on this dosage, these steady-state concentrations were considered as target endpoints for gradually increasing dosages of oral risperidone and were consistent with the therapeutic reference range of 20–60 ng/mL (risperidone plus 9-hydroxyrisperidone).6 One month after discontinuation of IM risperidone, oral risperidone was started at 1.0 mg/d and increased by 1.0 mg/d every week. Steady-state concentrations of risperidone/9-hydroxyrisperdone were obtained following dosages of 1 mg/d, 3 mg/d, 5 mg/d, and 6 mg/d. After approximately 6 weeks of upward titration of oral risperidone, steady-state concentrations of risperidone/9-hydroxyrisperidone closely approximated the steady-state baseline IM levels, and the resulting oral dosage of 6 mg/d was considered equivalent to the baseline IM dosage of 50 mg every 2 weeks (Figure 1).
Since conversion from IM to oral risperidone, the resident has remained clinically unchanged for the past 2 years and has indicated a preference for the oral administration.
Discussion
For this resident, switching from IM to oral administration had the advantage of eliminating the discomfort of receiving an injection every 2 weeks and avoiding potential complications including muscle fibrosis and contracture, infection, and nerve injury. Also, medication errors can possibly be reduced by changing from a more complicated route of administration to a simpler one. There have also been times at our facility when shortages of IM medications have forced providers to find alternative medications (A. Weir, PharmD, RPh, e-mail communication, March 31, 2022). For the facility staff, medication monitoring was a routine procedure by the unit nurse dispensing medications, so IM administration was not needed to assure compliance. For the facility, a significant cost savings was realized: compared to the annual cost of giving Risperdal Consta 50 mg IM every 2 weeks ($24,157), oral risperidone cost $37, which was a cost savings to the facility of $24,120 per year (A. Weir, PharmD, RPh, e-mail communication, March 31, 2022). Given that these are cost savings for 1 individual, a broader cost savings could be achieved should clinicians consider changing from IM to oral antipsychotic medications for others who live under 24-hour supervision where medication administration is closely monitored.
A limitation of this report is that baseline blood levels were determined at 3 weeks instead of the intended 2 weeks after the final dose of IM risperidone. However, because of the long half-lives of IM risperidone/9-hydroxyrisperidone, the difference in blood concentrations were probably negligible. Another limitation is that blood samples were taken during the morning when oral risperidone was scheduled for administration (4-hour window), so it was not always clear if the blood draw occurred before the administration of oral risperidone. Future protocols should ensure that blood draws occur prior to the administration of oral risperidone.
Article Information
Published Online: July 27, 2023. https://doi.org/10.4088/PCC.22cr03430
© 2023 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2023;25(4):22cr03430
Submitted: October 7, 2022; accepted January 4, 2023.
To Cite: MacNeill BR, Opatrny PM, Burke MS, et al. Converting from intramuscular to oral risperidone in a nursing home resident. Prim Care Companion CNS Disord. 2023;25(4):22cr03430.
Author Affiliations: Minnesota Department of Veterans Affairs (MacNeill, Opatrny, Burke, Heinen, Dysken); Minneapolis VA Health Care System, Minneapolis, Minnesota (Dysken).
Corresponding Author: Brian R. MacNeill, MA, BCBA, 20 W 12th St, Room 206, St Paul, Minnesota 55155 ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Patient Consent: Consent was obtained from the patient to publish the case report, and patient information was de-identified to protect anonymity.
References (6)

- Eerdekens M, Van Hove I, Remmerie B, et al. Pharmacokinetics and tolerability of long-acting risperidone in schizophrenia. Schizophr Res. 2004;70(1):91–100. PubMed CrossRef
- Walling DP, Hassman HA, Anta L, et al. The steady-state comparative bioavailability of intramuscular risperidone ISM and oral risperidone: an open-label, one-sequence study. Drug Des Devel Ther. 2021;15:4371–4382. PubMed CrossRef
- Schoretsanitis G, Kane JM, Correll CU, et al; American Society of Clinical Psychopharmacology. Blood levels to optimize antipsychotic treatment in clinical practice: a joint consensus statement of the American Society of Clinical Psychopharmacology and the Therapeutic Drug Monitoring Task Force of the Arbeitsgemeinschaft für Neuropsychopharmakologie und Pharmakopsychiatrie. J Clin Psychiatry. 2020;81(3):19cs13169. PubMed CrossRef
- Physicians’ Desk Reference: PDR. Oradell, NJ: Medical Economics Co.
- Schoretsanitis G, de Leon J, Haen E, et al. Pharmacokinetics of risperidone in different application forms: comparing long-acting injectable and oral formulations. Eur Neuropsychopharmacol. 2018;28(1):130–137. PubMed CrossRef
- Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(01–02):9–62. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite