Recovery—the absence of all abnormal mood symptoms—is the goal of treatment for bipolar disorder. Unfortunately, a minority of people suffering from bipolar disorder achieve sustained recovery. Improving recovery rates for this population will require clinicians in the primary care setting to be familiar with appropriate treatments for acute bipolar mania and depression and for the maintenance phase. Efficacy and tolerability of pharmacotherapeutic and psychotherapeutic options for all phases of treatment and each type of mood episode are discussed. Primary care physicians are encouraged to avoid prescribing antidepressant monotherapy for any patient with depression and a history of mania or hypomania.
A Critical Appraisal of Treatments for Bipolar Disorder
From the Department of Psychiatry, Harvard Medical School and Massachusetts General Hospital, Boston.
This article is derived from the planning teleconference series “Improving the Recognition and Treatment of Bipolar Disorder in Primary Care,” which was held in September 2009 and supported by an educational grant from AstraZeneca.
As of September 2, 2009, Dr Nierenberg is a consultant for Appliance Computing (Mindsite), BrainCells, Brandeis University, PGx Health, Shire, Schering-Plough, Targacept, and Takeda; has received grant/research support from National Institute of Mental Health, Pamlab, Pfizer, and Shire; has received honoraria from Belvior Publishing, University of Texas Southwestern Dallas, Hillside Hospital, American Drug Utilization Review, American Society for Clinical Psychopharmacology, Baystate Medical Center, Columbia University, IMEDEX, MJ Consulting, New York State, MBL Publishing, Physicians Postgraduate Press, SUNY Buffalo, University of Wisconsin, and the University of Pisa; is on the advisory boards of Appliance Computing, BrainCells, Eli Lilly, and Takeda; is a stock shareholder of Appliance Computing and BrainCells; and, through Massachusetts General Hospital, owns copyrights to the Clinical Positive Affect Scale and the MGH Structured Clinical Interview for the Montgomery-Asberg Depression Rating Scale exclusively licensed to the MGH Clinical Trials Network and Institute.
Corresponding author: Andrew A. Nierenberg, MD, Massachusetts General Hospital, 15 Parkman St, WACC-812, Boston, MA 02114 ([email protected]).
doi:10.4088/PCC.9064su1c.04
© Copyright 2010 Physicians Postgraduate Press, Inc.
Recovery—the absence of all abnormal mood symptoms—is the goal of treatment for bipolar disorder. Unfortunately, a minority of people suffering from bipolar disorder achieve sustained recovery. Improving recovery rates for this population will require clinicians in the primary care setting to be familiar with appropriate treatments for acute bipolar mania and depression and for the maintenance phase. Efficacy and tolerability of pharmacotherapeutic and psychotherapeutic options for all phases of treatment and each type of mood episode are discussed. Primary care physicians are encouraged to avoid prescribing antidepressant monotherapy for any patient with depression and a history of mania or hypomania.
(Prim Care Companion J Clin Psychiatry 2010;12[suppl 1]:23–29)
When people who are depressed visit a primary care physician, the physician should question the patient about a history of mania or hypomania so that a possible bipolar disorder diagnosis can be discerned. The reason for this is that, while antidepressant monotherapy is appropriate for patients with major depressive disorder, this treatment should be avoided in patients with bipolar disorder, even when they are experiencing a depressive episode. Remembering this single point will be extraordinarily helpful to primary care physicians treating patients with bipolar disorder.
Patients with bipolar disorder are more likely to visit physicians when they are depressed than when manic. Besides seeing patients who are experiencing an acute episode of depression, primary care physicians may also see patients who already have a diagnosis of bipolar disorder and are taking medications for the long-term prevention of mood episodes; these patients may visit primary care offices for other psychiatric or physical health problems. Primary care physicians should be familiar with bipolar medications in order to help patients in both acute and maintenance phases of the illness.
Mood stabilization is the primary focus in the treatment of bipolar disorder, and therefore effective treatments have been called mood stabilizers. Ketter and Calabrese1 clarified the term mood stabilizer when they characterized bipolar disorder as mood aberration from baseline (ie, one’s mood is either above or below a euthymic midpoint) and explained that there are 2 types of mood stabilizers: those that stabilize mood from above baseline (ie, have an antimanic effect) and those that stabilize mood from below baseline (ie, have an antidepressant effect). Drugs that stabilize mood from above the baseline are used to treat mania, mixed states, hypomania, and subsyndromal mood elevation and include lithium, divalproex, carbamazepine, and the atypical or second-generation antipsychotic agents. Medications that stabilize mood from below the baseline are used to treat depression and subsyndromal depression, such as lithium, lamotrigine, the olanzapine/fluoxetine combination, and quetiapine.
Treatment of Acute Bipolar Mania
In primary care, it is unlikely that clinicians will have to treat acute mania, but knowledge of therapies for acute mania and hypomania is helpful. Medications currently approved by the US Food and Drug Administration (FDA) for the treatment of acute mania include lithium, the anticonvulsant divalproex, and several atypical antipsychotics.
Lithium and Divalproex
Although lithium has long been used to treat manic episodes, the drug has a narrow therapeutic window and must be dosed carefully to avoid adverse events and toxicity. The anticonvulsant agent divalproex was the first alternative for patients with mania who could not tolerate lithium or for whom it was ineffective. In a study2 comparing lithium (n = 36), divalproex (n = 69), and placebo (n = 74) for the reduction of manic symptomatology during the acute phase, about half of the sample had been previously unresponsive to lithium. Results showed that response to divalproex was independent of prior response to lithium, and both active agents were significantly better than placebo in the reduction of manic symptoms (P < .05) according to the Mania Rating Scale, Schedule for Affective Disorders and Schizophrenia–Change version. Improvement of 50% or more was found in 49% of lithium-treated patients, 48% of divalproex-treated patients, and 25% of placebo-treated patients. But, even after 3 weeks, patients taking either lithium or divalproex still had residual manic symptoms, indicating that full remission with either agent might require an extended period of treatment.
Lithium and divalproex were both well tolerated.2 Lithium was associated with significantly more fever than divalproex and more twitching and vomiting than placebo (all P ≤ .05). Divalproex was associated with significantly more general pain than lithium and more vomiting than placebo (both P ≤ .05). The authors concluded that, despite the efficacy and tolerability of these 2 agents in much of the sample, more effective therapies were needed for the many patients like those in the rest of the sample who had inadequate response or could not tolerate the medications.
Carbamazepine
Besides divalproex, another anticonvulsant agent, carbamazepine, is used to treat manic or mixed bipolar episodes, although it has no indication to treat bipolar disorder. A study3 comparing extended-release (XR) carbamazepine (n = 101) with placebo (n = 103) assessed efficacy weekly for 3 weeks. The study found a benefit for the active agent in the reduction of manic symptomatology. The Young Mania Rating Scale (YMRS) showed significantly greater improvement for carbamazepine starting at week 2 (P = .032), and, at endpoint, the rates of response (ie, decrease of 50% or more in YMRS scores) were 42% for carbamazepine and 22% for placebo (P = .007).
Carbamazepine was associated with significantly more subjects with any adverse event (P = .007) and study discontinuation due to adverse events (P = .095) compared with placebo.3 An identical number of serious adverse events were reported for the treatment group and the placebo group. Carbamazepine was associated with more dizziness, nausea, vomiting, and somnolence than placebo.
Atypical Antipsychotics
Risperidone, olanzapine, quetiapine, ziprasidone, and
aripiprazole carry an indication to treat acute manic or mixed episodes of bipolar I disorder as either monotherapy or an adjunct to lithium or divalproex. In a meta-analysis of 12 monotherapy trials (N = 3,114), Perlis et al4 compared the efficacy of lithium and the atypical antipsychotics risperidone, olanzapine, quetiapine, ziprasidone, and aripiprazole versus placebo in the treatment of acute mania as measured by difference in YMRS change (Figure 1). Response (ie, decrease of 50% or more in YMRS scores) occurred in 53% of patients taking antipsychotics and 30% of those taking placebo. Furthermore, no significant differences in efficacy were found among the different antipsychotics.
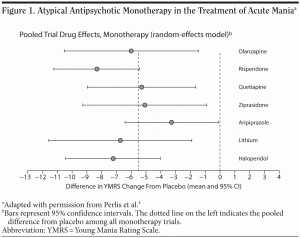
A randomized, double-blind, placebo-controlled 3-week trial5 by Sachs et al found that the combination of an antipsychotic (risperidone) and a mood stabilizer (lithium or divalproex) was more efficacious than a mood stabilizer plus placebo for the treatment of acute mania according to outcomes measured with the YMRS. Subjects receiving the atypical antipsychotic/mood stabilizer combination (n = 52) reported adverse events with similar occurrence rates and severity as subjects receiving a mood stabilizer and placebo (n = 51). Compared with those receiving mood stabilizer monotherapy, those receiving the combination therapy experienced more somnolence, extrapyramidal symptoms, and dizziness. Perlis et al4 pooled the results of 6 studies (including the Sachs et al5 study) of antipsychotic/mood stabilizer combination treatment versus mood stabilizer plus placebo for mania and found a 4.1-point greater improvement on the YMRS score with combination therapy. Primary care clinicians may notice that their patients with bipolar disorder are often taking multiple medications, which, as demonstrated in these studies, is because combinations of certain drugs may be more efficacious than either drug alone, although the side effect burden may be greater.
Asenapine is a new atypical antipsychotic that has
received FDA approval for the treatment of acute mania or mixed episodes in adults with bipolar I disorder. A randomized, double-blind, placebo-controlled trial6 found that asenapine was superior to placebo in the reduction of manic symptoms. A study7 comparing asenapine with olanzapine found that asenapine was similarly efficacious to olanzapine in reducing manic symptomatology. Asenapine has been found to be well-tolerated6,7 and is not associated with significant weight gain; however, asenapine is associated with a higher incidence of extrapyramidal symptoms than olanzapine.8
Some atypical antipsychotics may increase the risk of weight gain and diabetes. Clozapine and olanzapine are most associated with weight gain and decreased insulin sensitivity, followed by risperidone and quetiapine. Aripiprazole and ziprasidone are thought to have the lowest risk for weight gain or diabetes.9
Treatment of Acute Bipolar Depression
Antidepressants
Patients suffering from bipolar major depressive episodes are often prescribed antidepressant monotherapy, a treatment strategy that should be avoided, especially for patients with bipolar I disorder (ie, a history of mania). Controversy exists concerning what is best for patients with a history of hypomania (ie, bipolar II disorder), but, for now, clinicians should not employ antidepressant monotherapies in the treatment of bipolar depression of any type.
A meta-analysis of 12 trials (N = 1,088) by Gijsman and colleagues10 evaluating the efficacy of antidepressants in the amelioration of a bipolar depressive episode found a 1.86 risk ratio for response to antidepressants versus placebo. However, many of the studies included were very small and had high dropout rates. Also, about 75% of the patients were taking a concurrent mood stabilizer or antipsychotic agent. Thus, the meta-analysis did not completely clarify whether antidepressants are efficacious for bipolar depression.
The Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD)11 was a large, seminaturalistic study embedded with several smaller randomized clinical trials. One STEP-BD randomized trial12 compared the combination of a mood stabilizer plus an antidepressant (n = 179) versus a mood stabilizer plus placebo (n = 187) in the treatment of acute bipolar depression. On every outcome, no difference was found between groups. Durable recovery (≤ 2 mood symptoms sustained for at least 8 weeks) occurred in 24% of patients receiving adjunctive antidepressants and 27% of those taking mood stabilizer monotherapy (P = .40). Also of interest, the frequency of treatment-emergent affective switch—that is, the emergence of mania or hypomania after initiation of an
antidepressant—was no different whether subjects received an antidepressant (10%) or placebo (11%), suggesting that there is neither advantage nor harm in adding an antidepressant to a mood stabilizer. Further analysis showed that patients with a history of treatment-emergent affective switch were more likely to experience a treatment-emergent affective switch again if exposed to an antidepressant.
Lithium
In a review, Keck13 found that 8 of 9 studies demonstrated efficacy for lithium versus placebo in the treatment of bipolar depression, but the total sample size for all the studies combined was only about 160. The average onset of action occurred between 6 and 8 weeks, and 80% of subjects had a “positive response” to treatment with lithium while 36% had an “unequivocal response.” Unfortunately, “positive” and “unequivocal” were not well defined. A more definitive study14 of the antidepressant effects of lithium used lithium as a control condition to assess the antidepressant effects of quetiapine for bipolar depression. This placebo-controlled study14 was larger than any other study of lithium for bipolar depression, with 136 patients receiving lithium and 129 receiving placebo for 8 weeks. No differences were found between lithium and placebo on measures of depression (Montgomery-Asberg Depression Rating Scale [MADRS] and the Hamilton Depression Rating Scale), clinical global impression, anxiety, disability, or cognition.
Lamotrigine
Lamotrigine has not been approved by the FDA for the treatment of acute bipolar depression, and its efficacy has been debated.15 Lamotrigine must be uptitrated slowly at a rate of 25 mg/wk until the target dose of 200 mg/d is reached. Otherwise, patients are at risk of developing Stevens-Johnson syndrome or a toxic epidermal necrolysis.
Monotherapy. A 7-week study16 evaluating lamotrigine monotherapy versus placebo in a group of outpatients with bipolar I disorder experiencing an acute depressive episode (N = 195) demonstrated significant efficacy for lamotrigine (200 mg/d) versus placebo according to multiple standardized measurement tools. Significant improvements versus placebo (P < .05) were seen beginning at week 3, when the dose got above 50 mg/d. The occurrence of adverse events was similar for both treatment groups, with only headache occurring significantly more often with lamotrigine than placebo (P < .05). Negative studies17 of lamotrigine for acute bipolar depression have since emerged. As such, the usefulness of lamotrigine for this population is unclear.
A 7-week head-to-head comparison18 of lamotrigine monotherapy versus the olanzapine/fluoxetine combination in 410 patients with acute bipolar I depression demonstrated similar response rates for both comparators, ie, 60% with lamotrigine and 69% with olanzapine/fluoxetine (P = .073), as measured by ≥ 50% reduction in score on the MADRS. The olanzapine/fluoxetine combination was associated with a faster response (P = .01) and lower rates of suicidality (P = .037) but caused more side effects (including somnolence, weight gain, dry mouth, tremor [all P < .05], and hyperlipidemia [P ≤ .001]) than lamotrigine.
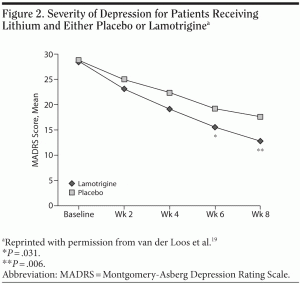
Adjunctive therapy. Van der Loos and colleagues19 compared lamotrigine plus lithium with lithium plus placebo in 124 outpatients with a bipolar depressive episode. Patients receiving the lithium/lamotrigine combination for 8 weeks had significantly reduced symptom scores as measured by the MADRS (Figure 2). No statistically significant differences in occurrence of adverse events were reported between the lamotrigine and the placebo groups, including the incidence of skin rash.
Olanzapine/Fluoxetine Combination
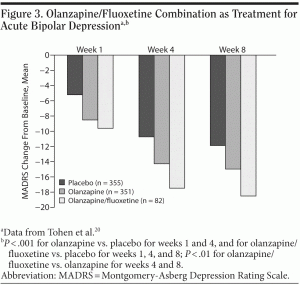
The olanzapine/fluoxetine combination and quetiapine are the only treatments approved by the FDA for acute bipolar depression. A key multisite study20 compared olanzapine, olanzapine/fluoxetine, and placebo (N = 833). Of the 3 options, the combination treatment was associated with the largest change in MADRS total scores (Figure 3). Patients taking the olanzapine/fluoxetine combination treatment reported an occurrence of adverse events similar to those taking only olanzapine, including somnolence, weight gain, increased appetite, headache, nausea, and diarrhea. Nausea and diarrhea were the only side effects occurring significantly more often for the olanzapine/fluoxetine group than for the olanzapine group (P = .02 and P = .001, respectively).11 Unfortunately, olanzapine puts patients at risk for the development of metabolic syndrome.9 Clinicians may attempt to mitigate the risk for metabolic syndrome with antidiabetic drugs or statins,21 but no definitive data support making a recommendation for this strategy. Clinicians using the olanzapine/fluoxetine combination to treat bipolar depression should regularly screen patients for the development of metabolic syndrome and take steps to manage its emergence.
Quetiapine
Quetiapine, administered at 300 mg/d (n = 172) or 600 mg/d (n = 170), was compared with placebo (n = 169) for efficacy in the treatment of acute bipolar depression.22 Quetiapine was significantly superior to placebo for both doses (P < .001) beginning at week 1, according to change from baseline in MADRS scores. No significant difference in efficacy was found between dosage levels, indicating that the lower dose is sufficient for most patients. Response rates for both 300 mg/d and 600 mg/d of quetiapine were 58% versus 36% for placebo, and remission rates for both 300 mg/d and 600 mg/d of quetiapine were 53% versus 28% for placebo. Quetiapine was also shown to be beneficial for subjects with anxiety, which is an often-overlooked component of bipolar disorder.
Dry mouth, sedation, somnolence, dizziness, and constipation were reported significantly more often (P < .05) for both quetiapine groups than for placebo.22 Quetiapine is FDA-approved for the treatment of acute bipolar depression and, like olanzapine, increases patients’ risk for the development of metabolic syndrome.9
The efficacy of quetiapine in acute bipolar depression was found to be superior not only to that of placebo but also that of lithium in an 8-week trial.14
Aripiprazole
Aripiprazole, unlike other atypical antipsychotics, has not been associated with an increased risk for metabolic syndrome.23 However, aripiprazole has not demonstrated efficacy in the treatment of bipolar depression. Two 8-week studies by Thase et al.24 (N = 749) showed that, at endpoint, aripiprazole (up to 30 mg/d) was no more effective than placebo in reducing bipolar I depressive symptoms according to MADRS total scores, although significant differences were seen in weeks 1 to 6.
Modafinil
Modafinil, a wakefulness-promoting agent, is not approved by the FDA for the treatment of bipolar depression, and evidence supporting the agent’s efficacy is limited. A preliminary 6-week study25 found that adjunctive modafinil therapy administered at a mean dose of 177 mg/d significantly reduced bipolar depressive symptoms compared with placebo (P < .01), but more research is needed before specific recommendations can be made regarding the use of modafinil in bipolar depression.
Psychotherapy
A 1-year STEP-BD study26 evaluated the efficacy of medication plus 1 of 3 intensive psychotherapies—cognitive-behavioral therapy, family-focused therapy, and interpersonal and social rhythm therapy—in comparison with medication plus collaborative care, which comprised a brief psychoeducational program in which patients were given a workbook and met with a therapist up to 3 times to be educated about bipolar disorder. Patients with acute bipolar depression who received intensive psychotherapy (n = 163) recovered sooner than those who received psychoeducation (n = 130; P = .01) and had a higher recovery rate (64% vs 52%, P = .01). Primary care physicians should be aware of the potential benefits of adjunctive psychotherapy for their patients with bipolar disorder.
Maintenance Treatment
Individuals with bipolar disorder should be treated indefinitely to avoid an increased risk of experiencing recurrent mood episodes. The agents with an indication for maintenance treatment of bipolar disorder are lithium; lamotrigine; aripiprazole; and quetiapine and ziprasidone as adjuncts to lithium or divalproex. When interpreting studies of maintenance pharmacotherapy for bipolar disorder, considering whether subjects’ most recent episodes were manic or
depressive is helpful.
Lithium and Lamotrigine
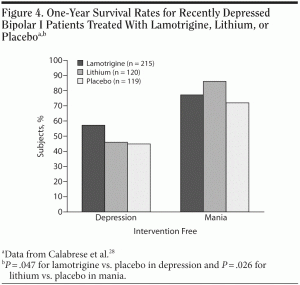
A meta-analysis by Muzina and Calabrese27 showed that lithium is the gold standard for the prevention of bipolar relapse, particularly for episodes of mania or hypomania. One head-to-head 18-month study28 showed that, among 463 patients who had recovered from a depressive episode with lamotrigine therapy, lithium was significantly superior to placebo (P = .026) in the prevention of a manic or hypomanic episode (Figure 4). Lithium, however, was not as effective as lamotrigine in preventing bipolar depressive relapse, which is consistent with clinical experience. Lamotrigine was significantly better at the long-term prevention of a depressive episode than placebo (P = .047).
Headache was the most common adverse event reported, but no statistically relevant difference across active treatment groups was found.28 The only adverse event occurring significantly more often for lamotrigine than placebo was the development of nonserious rash (P < .05). Tremor and somnolence occurred significantly more often with lithium than with placebo (P < .05). Lithium is known to inhibit thyroid hormone release,29 and, after long-term treatment, patients are at risk of having kidney problems such as polyuria and even nephrotoxicity.29,30 Clinicians should regularly screen patients for the development of lithium-related health problems.
Lithium may have specific antisuicidal properties. A meta-analysis by Cipriani et al31 found that patients taking lithium were less likely to die by suicide or to experience deliberate self-harm than patients taking other compounds for the treatment of mood disorders.
Atypical Antipsychotics
In a 12-month study,32 patients with a history of manic or mixed bipolar episodes who achieved remission while being treated with a combination of lithium plus olanzapine were randomly assigned to receive lithium (n = 217) or olanzapine (n = 214) as maintenance therapy. Patients in the olanzapine group had a significantly longer time to relapse (P = .04) to any mood episode compared with patients in the lithium group. Symptomatic recurrence was experienced by 30% of patients in the olanzapine group and 39% of patients receiving lithium. Depressive recurrence occurred more
often in the olanzapine group than in the lithium group (16% and 11%, respectively); olanzapine was more effective in preventing manic recurrence than lithium, with respective recurrence rates of 14% and 23%.
In maintenance treatment, as in the acute phase, clinicians prescribing olanzapine must monitor patients for weight gain and the development of metabolic syndrome. In the above study,32 patients taking olanzapine gained a mean 1.8 kg compared with a 1.4 kg weight loss for lithium subjects (P < .001). Adverse events occurring more often in the lithium group than in the olanzapine group were insomnia, worsening of mania, headache not otherwise specified, and nausea.
Aripiprazole is an approved maintenance monotherapy for bipolar disorder. Its efficacy versus placebo was demonstrated in a 100-week study.33 Patients stabilized with aripiprazole after manic or mixed episodes were randomly assigned to aripiprazole (n = 78) or placebo (n = 83) and monitored for relapse. Prevention of mania with aripiprazole was superior to that of placebo (P = .005) but not prevention of depression.
As adjunctive therapy to lithium or divalproex, quetiapine and ziprasidone have maintenance efficacy, and combination treatment may be cost-effective over the long-term.34 The efficacy of the combination of quetiapine and lithium or divalproex was compared with that of either mood stabilizer plus placebo in 2 studies35,36 lasting up to 104 weeks, and quetiapine was associated with equal efficacy in preventing both manic and depressive recurrences. A 6-month maintenance trial37 for bipolar mania comparing ziprasidone adjunctive to a mood stabilizer with placebo plus mood stabilizer found that ziprasidone was significantly more effective in preventing relapse than placebo (P = .010).
Psychotherapy and Psychoeducation
Even with appropriate pharmacotherapy, patients with bipolar disorder are at risk of relapse while euthymic. Psychotherapy and psychoeducation can help prevent relapse of bipolar mood episodes. One study38 demonstrated that family-focused therapy plus pharmacotherapy was significantly better at preventing relapse (P = .003) than a combination of a less intensive crisis management intervention and pharmacotherapy. Medication adherence was also greater with family-focused therapy. In another study,39 standard group psychoeducation plus pharmacotherapy also demonstrated significant efficacy in prolonging time to recurrence of any bipolar mood episode compared with pharmacotherapy alone (P < .001).
Summary
During the acute phase of bipolar treatment, selection of pharmacotherapy should be made according to the type of episode a patient is experiencing, ie, manic or depressive. Many patients may require a combination of treatments to keep them well during maintenance treatment, even though the combinations carry a greater side effect burden. Antidepressant monotherapy should be avoided whenever possible, and psychotherapy or psychoeducation should be employed when those options are available. If a psychiatrist is the primary clinician handling a patient’s bipolar treatment, then a collaborative effort between the primary care physician and the psychiatrist is vital, particularly when dealing with health issues such as metabolic syndrome associated with the use of atypical antipsychotics.
Drug Names: aripiprazole (Abilify), asenapine (Saphris), carbamazepine (Tegretol, Equetrol, and others), clozapine (Clozaril, FazaClo, and others), divalproex (Depakote and others), haloperidol (Haldol and others), lamotrigine (Lamictal and others), lithium (Lithobid and others), modafinil (Provigil), olanzapine (Zyprexa), olanzapine/fluoxetine (Symbyax), quetiapine (Seroquel), risperidone (Risperdal and others), ziprasidone (Geodon).
Disclosure of off-label usage: The author has determined that, to the best of his knowledge, modafinil is not approved by the US Food and Drug Administration for the treatment of bipolar disorder.
References
1. Ketter TA, Calabrese JR. Stabilization of mood from below versus above baseline in bipolar disorder: a new nomenclature. J Clin Psychiatry. 2002;
63(2):146–151. PubMed
2. Bowden CL, Brugger AM, Swann AC, et al, for the Depakote Mania Study Group. Efficacy of divalproex vs lithium and placebo in the treatment of mania. JAMA. 1994;271(12):918–924. doi:10.1001/jama.271.12.918 PubMed
3. Weisler RH, Kalali AH, Ketter TA, et al. A multicenter, randomized, double-blind, placebo-controlled trial of extended-release carbamazepine capsules as monotherapy for bipolar disorder patients with manic or mixed episodes. J Clin Psychiatry. 2004;65(4):478–484. doi:10.4088/JCP.v65n0405 PubMed
4. Perlis RH, Welge JA, Vornik LA, et al. Atypical antipsychotics in the treatment of mania: a meta-analysis of randomized, placebo-controlled trials. J Clin Psychiatry. 2006;67(4):509–516. doi:10.4088/JCP.v67n0401 PubMed
5. Sachs GS, Grossman F, Ghaemi SN, et al. Combination of a mood
stabilizer with risperidone or haloperidol for treatment of acute mania:
a double-blind, placebo-controlled comparison of efficacy and safety.
Am J Psychiatry. 2002;159(7):1146–1154. doi:10.1176/appi.ajp.159.7.1146 PubMed
6. McIntyre RS, Cohen M, Zhao J, et al. Asenapine in the treatment of acute mania in bipolar I disorder: a randomized, double-blind, placebo-controlled trial. J Affect Disord. 2010;122(1–2):27–38. doi:10.1016/j.jad.2009.12.028 PubMed
7. McIntyre RS, Cohen M, Zhao J, et al. Asenapine versus olanzapine
in acute mania: a double-blind extension study. Bipolar Disord. 2009;
11(8):815–826. doi:10.1111/j.1399-5618.2009.00749.x PubMed
8. Bishara D, Taylor D. Asenapine monotherapy in the acute treatment of both schizophrenia and bipolar I disorder. Neuropsychiatr Dis Treat. 2009;5:483–490. PubMed
9. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, et al. Consensus
development conference on antipsychotic drugs and obesity and
diabetes. J Clin Psychiatry. 2004;65(2):267–272. doi:10.4088/JCP.v65n0219 PubMed
10. Gijsman HJ, Geddes JR, Rendell JM, et al. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials.
Am J Psychiatry. 2004;161(9):1537–1547. doi:10.1176/appi.ajp.161.9.1537 PubMed
11. Sachs GS, Thase ME, Otto MW, et al. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry. 2003;53(11):1028–1042. doi:10.1016/S0006-3223(03)00165-3 PubMed
12. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med. 2007;
356(17):1711–1722. doi:10.1056/NEJMoa064135 PubMed
13. Keck PE Jr. Pharmacologic treatment of depression. In: Kupfer DJ, ed. Bipolar Depression: The Clinicians Reference Guide. Montvale, NJ: Current Psychiatry; 2004:27–43.
14. Young AH, McElroy SL, Bauer M, et al, for the EMBOLDEN I (Trial 001) Investigators. A double-blind, placebo-controlled study of quetiapine and lithium monotherapy in adults in the acute phase of bipolar depression (EMBOLDEN I). J Clin Psychiatry. 2010;71(2):150–162. doi:10.4088/JCP.08m04995gre PubMed
15. Nassir Ghaemi S, Shirzadi AA, Filkowski M. Publication bias and the pharmaceutical industry: the case of lamotrigine in bipolar disorder. Medscape J Med. 2008;10(9):211. PubMed
16. Calabrese JR, Bowden CL, Sachs GS, et al, for the Lamictal 602 Study Group. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry. 1999;
60(2):79–88. PubMed
17. Calabrese JR, Huffman RF, White RL, et al. Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disord. 2008;10(2):323–333. doi:10.1111/j.1399-5618.2007.00500.x PubMed
18. Brown EB, McElroy SL, Keck PE Jr, et al. A 7-week, randomized, double-blind trial of olanzapine/fluoxetine combination versus lamotrigine in the treatment of bipolar I depression. J Clin Psychiatry. 2006;67(7):
1025–1033. doi:10.4088/JCP.v67n0703 PubMed
19. van der Loos ML, Mulder PG, Hartong EG, et al, for the LamLit Study Group. Efficacy and safety of lamotrigine as add-on treatment to lithium in bipolar depression: a multicenter, double-blind, placebo-controlled trial. J Clin Psychiatry. 2009;70(2):223–231. doi:10.4088/JCP.08m04152 PubMed
20. Tohen M, Vieta E, Calabrese J, et al. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I
depression. Arch Gen Psychiatry. 2003;60(11):1079–1088. doi:10.1001/archpsyc.60.11.1079 PubMed
21. Moisan J, Grégoire JP, Gaudet M, et al. Exploring the risk of diabetes mellitus and dyslipidemia among ambulatory users of atypical antipsychotics: a population-based comparison of risperidone and olanzapine. Pharmacoepidemiol Drug Saf. 2005;14(6):427–436. doi:10.1002/pds.1093 PubMed
22. Calabrese JR, Keck PE Jr, Macfadden W, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. Am J Psychiatry. 2005;162(7):1351–1360. doi:10.1176/appi.ajp.162.7.1351 PubMed
23. Newcomer JW, Campos JA, Marcus RN, et al. A multicenter, randomized, double-blind study of the effects of aripiprazole in overweight subjects with schizophrenia or schizoaffective disorder switched from olanzapine. J Clin Psychiatry. 2008;69(7):1046–1056. doi:10.4088/JCP.v69n0702 PubMed
24. Thase ME, Jonas A, Khan A, et al. Aripiprazole monotherapy in nonpsychotic bipolar I depression: results of 2 randomized, placebo-controlled studies. J Clin Psychopharmacol. 2008;28(1):13–20. doi:10.1097/jcp.0b013e3181618eb4 PubMed
25. Frye MA, Grunze H, Suppes T, et al. A placebo-controlled evaluation
of adjunctive modafinil in the treatment of bipolar depression.
Am J Psychiatry. 2007;164(8):1242–1249. doi:10.1176/appi.ajp.2007.06060981 PubMed
26. Miklowitz DJ, Otto MW, Frank E, et al. Psychosocial treatments for bipolar depression: a 1-year randomized trial from the Systematic Treatment Enhancement Program. Arch Gen Psychiatry. 2007;64(4):419–426. doi:10.1001/archpsyc.64.4.419 PubMed
27. Muzina DJ, Calabrese JR. Maintenance therapies in bipolar disorder: focus on randomized controlled trials. Aust N Z J Psychiatry. 2005;
39(8):652–661. PubMed
28. Calabrese JR, Bowden CL, Sachs G, et al, for the Lamictal 605 Study Group. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. J Clin Psychiatry. 2003;64(9):1013–1024. PubMed
29. Grandjean EM, Aubry JM. Lithium: updated human knowledge
using an evidence-based approach, part III: clinical safety. CNS Drugs. 2009;23(5):397–418. doi:10.2165/00023210-200923050-00004 PubMed
30. Grünfeld JP, Rossier BC. Lithium nephrotoxicity revisited. Nat Rev Nephrol. 2009;5(5):270–276. doi:10.1038/nrneph.2009.43 PubMed
31. Cipriani A, Pretty H, Hawton K, et al. Lithium in the prevention of suicidal behavior and all-cause mortality in patients with mood disorders:
a systematic review of randomized trials. Am J Psychiatry. 2005;162(10):
1805–1819. doi:10.1176/appi.ajp.162.10.1805 PubMed
32. Tohen M, Greil W, Calabrese JR, et al. Olanzapine versus lithium in the maintenance treatment of bipolar disorder: a 12-month, randomized, double-blind, controlled clinical trial. Am J Psychiatry. 2005;162(7):
1281–1290. doi:10.1176/appi.ajp.162.7.1281 PubMed
33. Keck PE Jr, Calabrese JR, McIntyre RS, et al, for the Aripiprazole Study Group. Aripiprazole monotherapy for maintenance therapy in bipolar I disorder: a 100-week, double-blind study versus placebo. J Clin Psychiatry. 2007;68(10):1480–1491. doi:10.4088/JCP.v68n1003 PubMed
34. Woodward TC, Tafesse E, Quon P, et al. Cost-effectiveness of quetiapine with lithium or divalproex for maintenance treatment of bipolar I disorder. J Med Econ. 2009;12(4):259–268. doi:10.3111/13696990903266612
35. Suppes T, Vieta E, Liu S, et al, for the Trial 127 Investigators. Maintenance treatment for patients with bipolar I disorder: results from a North American study of quetiapine in combination with lithium or divalproex (trial 127). Am J Psychiatry. 2009;166(4):476–488. doi:10.1176/appi.ajp.2008.08020189 PubMed
36. Vieta E, Suppes T, Eggens I, et al. Efficacy and safety of quetiapine in combination with lithium or divalproex for maintenance of patients with bipolar I disorder (international trial 126). J Affect Disord. 2008;109(3):
251–263. doi:10.1016/j.jad.2008.06.001 PubMed
37. Bowden CL, Vieta E, Ice KS, et al. Ziprasidone plus a mood stabilizer in subjects with bipolar I disorder: a 6-month, randomized, placebo-controlled, double-blind trial. J Clin Psychiatry. 2010;71(2):130–137. doi:10.4088/JCP.09m05482yel PubMed
38. Miklowitz DJ, George EL, Richards JA, et al. A randomized study of family-focused psychoeducation and pharmacotherapy in the outpatient management of bipolar disorder. Arch Gen Psychiatry. 2003;60(9):
904–912. doi:10.1001/archpsyc.60.9.904 PubMed
39. Colom F, Vieta E, Martinez-Aran A, et al. A randomized trial on the efficacy of group psychoeducation in the prophylaxis of recurrences in bipolar patients whose disease is in remission. Arch Gen Psychiatry. 2003;60(4):402–407. doi:10.1001/archpsyc.60.4.402 PubMed
Please sign in or purchase this PDF for $40.00.
Save
Cite
