Objective: Existing cognitive and clinical predictors of treatment response to date are not of sufficient strength to meaningfully impact treatment decision making and are not readily employed in clinical settings. This study investigated whether clinical and cognitive markers used in a tertiary care clinic could predict response to usual treatment over a period of 4 to 6 months in a sample of 75 depressed adults.
Methods: Patients (N = 384) were sequentially tested in 2 half-day clinics as part of a quality improvement project at an outpatient tertiary care center between August 2003 and September 2007; additional subjects evaluated in the clinic between 2007 and 2009 were also included. Diagnosis was according to DSM-IV-TR criteria and completed by residents and attending faculty. Test scores obtained at intake visits on a computerized neuropsychological screening battery were the Parametric Go/No-Go task and Facial Emotion Perception Task. Treatment outcome was assessed using 9-item Patient Health Questionnaire (PHQ-9) self-ratings at follow-up (n = 75). Usual treatment included psychotropic medication and psychotherapy. Decline in PHQ-9 scores was predicted on the basis of baseline PHQ-9 score and education, with neuropsychological variables entered in the second step.
Results: PHQ-9 scores declined by 46% at follow-up (56% responders). Using 2-step multiple regression, baseline PHQ-9 score (P ≤ .05) and education (P ≤ .01) were significant step 1 predictors of percent change in PHQ-9 follow-up scores. In step 2 of the model, faster processing speed with interference resolution (go reaction time) independently explained a significant amount of variance over and above variables in step 1 (12% of variance, P < .01), while other cognitive and affective skills did not. This 2-step model accounted for 28% of the variance in treatment change in PHQ-9 scores. Processingspeed with interference resolution also accounted for 12% variance in treatment and follow-up attrition.
Conclusions: Use of executive functioning assessments in clinics may help identify individuals with cognitive weaknesses at risk for not responding to standard treatments.
ABSTRACT
Objective: Existing cognitive and clinical predictors of treatment response to date are not of sufficient strength to meaningfully impact treatment decision making and are not readily employed in clinical settings. This study investigated whether clinical and cognitive markers used in a tertiary care clinic could predict response to usual treatment over a period of 4 to 6 months in a sample of 75 depressed adults.
Methods: Patients (N = 384) were sequentially tested in 2 half-day clinics as part of a quality improvement project at an outpatient tertiary care center between August 2003 and September 2007; additional subjects evaluated in the clinic between 2007 and 2009 were also included. Diagnosis was according to DSM-IV-TR criteria and completed by residents and attending faculty. Test scores obtained at intake visits on a computerized neuropsychological screening battery were the Parametric Go/No-Go task and Facial Emotion Perception Task. Treatment outcome was assessed using 9-item Patient Health Questionnaire (PHQ-9) self-ratings at follow-up (n = 75). Usual treatment included psychotropic medication and psychotherapy. Decline in PHQ-9 scores was predicted on the basis of baseline PHQ-9 score and education, with neuropsychological variables entered in the second step.
Results: PHQ-9 scores declined by 46% at follow-up (56% responders). Using 2-step multiple regression, baseline PHQ-9 score (P ≤ .05) and education (P ≤ .01) were significant step 1 predictors of percent change in PHQ-9 follow-up scores. In step 2 of the model, faster processing speed with interference resolution (go reaction time) independently explained a significant amount of variance over and above variables in step 1 (12% of variance, P < .01), while other cognitive and affective skills did not. This 2-step model accounted for 28% of the variance in treatment change in PHQ-9 scores. Processing speed with interference resolution also accounted for 12% variance in treatment and follow-up attrition.
Conclusions: Use of executive functioning assessments in clinics may help identify individuals with cognitive weaknesses at risk for not responding to standard treatments.
Prim Care Companion CNS Disord 2017;19(1):16m01949
https://doi.org/10.4088/PCC.16m01949
© Copyright 2017 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, University of Michigan Medical Center, Ann Arbor
†Deceased.
*Corresponding author: Scott A. Langenecker, PhD, Department of Psychiatry, The University of Illinois at Chicago, 1601 W Taylor Ave, Chicago, 60612 ([email protected]).
Despite significant advances in the field of psychiatry, there is currently no empirically derived method for the order or combination in which medications and therapies should be employed to treat depressive symptoms.1,2 Indeed, half of the adults hospitalized for a major depressive episode take at least a year to achieve symptom remission with standard treatments.3 Additionally, approximately 30% of individuals who respond well to cognitive-behavioral therapy relapse within a 12-month period.4 Therefore, the identification of prognostic characteristics for treatment responsiveness and sustained wellness may help maximize the efficacy of health care delivery and improve the quality of life among individuals with depression. If patients can be identified earlier as having a low probability of response to usual treatment, alternative or augmented treatment plans might be developed in an effort to reduce short- and longer-term morbidity in this population.
Previous studies3,5,6 have identified a number of clinical characteristics that predict treatment outcomes in depressed adults (eg, age at depression onset, number of prior hospitalizations, psychiatric comorbidity, baseline depression severity). However, predictors in these models often account for only a small portion of the variance in treatment response (eg, 5%-20% cumulative variance accounted for), and these studies typically do not examine longer-term outcomes. Other studies6-9 support modest links between cognitive strengths and better treatment responsiveness, and the combination of clinical characteristics and cognition often yields a stronger predictive model. In 1 study10 that assessed cognition as a predictor of treatment responsiveness, poorer performance on measures of novel problem-solving and verbal inhibition at baseline significantly predicted a lack of response to fluoxetine in a sample of 14 women experiencing a major depressive episode. Poorer divided attention has also been associated with less responsiveness to antidepressants 6 months after psychiatric hospitalization in a sample of 73 adults with major depressive disorder or bipolar disorder.11 Additionally, for 32 adults experiencing a major depressive episode, poorer performance at baseline on measures of attentional shifting, working memory, and verbal fluency predicted an unfavorable response to selective serotonin reuptake inhibitors (SSRIs) within 3 months.8 Other studies,9 in contrast, have failed to support cognitive strengths as a predictor of improved treatment responsiveness among depressed adults. Of note, the sample sizes, measures used, and degrees of freedom available in each statistical model varied significantly across these studies, limiting the degree to which they can be compared. More recent studies and reviews12,13 completed after the trial reported here have solidified the importance of executive functioning measures in prediction of treatment response.
Accurate facial affect recognition is also frequently impacted by depression, but its relationship with treatment responsiveness is currently unknown. Depressed individuals typically exhibit difficulty recognizing subtle positive facial expressions14 and can have difficulty disengaging from the negative affect of others.15 These findings are even more robust with greater depression severity and lower educational attainment.14 Given that affective signals of others represent important social cues, impaired facial affect recognition may have negative psychosocial implications. There appears to be no treatment prediction studies in adults with major depressive disorder using facial affect recognition measures, although there is a recent neuroimaging study16 related to response and other studies17,18 related to outcome. In addition, at least 1 study19 supports an association between higher Global Assessment of Functioning scores20 and more accurate recognition of happiness.
The present study is an extension of our previous cross-sectional work,21 which evaluated executive functioning and affective processing in a naturalistic sample of adults presenting to outpatient mental health clinics for initiation of treatment for mood and anxiety disorders. The present study used a longitudinal design to evaluate whether psychomotor speed, attention/executive functioning, and emotion perception at baseline would predict treatment responsiveness in a sample of depressed adults receiving usual, “open-label” treatment. Our hypothesis was that participants who demonstrated better executive functioning and emotion perception at baseline would respond more robustly to usual psychiatric and psychological treatments at follow-up. The naturalistic approach to participant recruitment was intended to promote generalizability to real-world applications, and the duration of 4 to 6 months was intended to evaluate more sustained remission. A secondary goal of this study was to evaluate the potential utility of administering a computerized cognitive screening battery in outpatient mental health clinics as part of the intake process by identifying cognitive limitations early in the treatment process, which might inform subsequent treatment plans. The ultimate goal of this study, while not directly assessed, was to identify whether the process of cognitive screening at intake could inform empirically based treatment choices. We are unaware of any existing study that has analyzed the potential value of such clinical screening as a standard procedure.

- Limited knowledge exists in predicting which individuals might benefit most from the existing treatments for depression.
- Executive functioning is a useful, prospective predictor of likelihood of treatment response to usual care.
METHODS
Participants
Participants represented a portion of the larger sample of adults (N = 384) with various psychiatric conditions seen between August 2003 and September 2007 that is described in an earlier article,21 including additional subjects evaluated in the clinic between 2007 and 2009. Inclusion criteria for the current sample were a current or lifetime diagnosis of major depressive disorder or mood disorder not otherwise specified, an estimated verbal IQ of at least 75, mild to severe depression (defined by baseline scores ≥ 5 on the 9-item Patient Health Questionnaire [PHQ-9]22), and completion of a follow-up PHQ-9 within 4 to 6 months after intake. The sample was restricted to adults aged ≤ 65 years to reduce the potential effects of age-related cognitive changes. Exclusion criteria were a current alcohol or substance abuse disorder or a history of a medical condition likely to negatively impact cognition or the ability to complete computerized testing (eg, developmental disability, cerebrovascular accident). Because this study aimed to capture a representative sample of individuals initiating care for depression, participants were not excluded for other comorbid conditions.
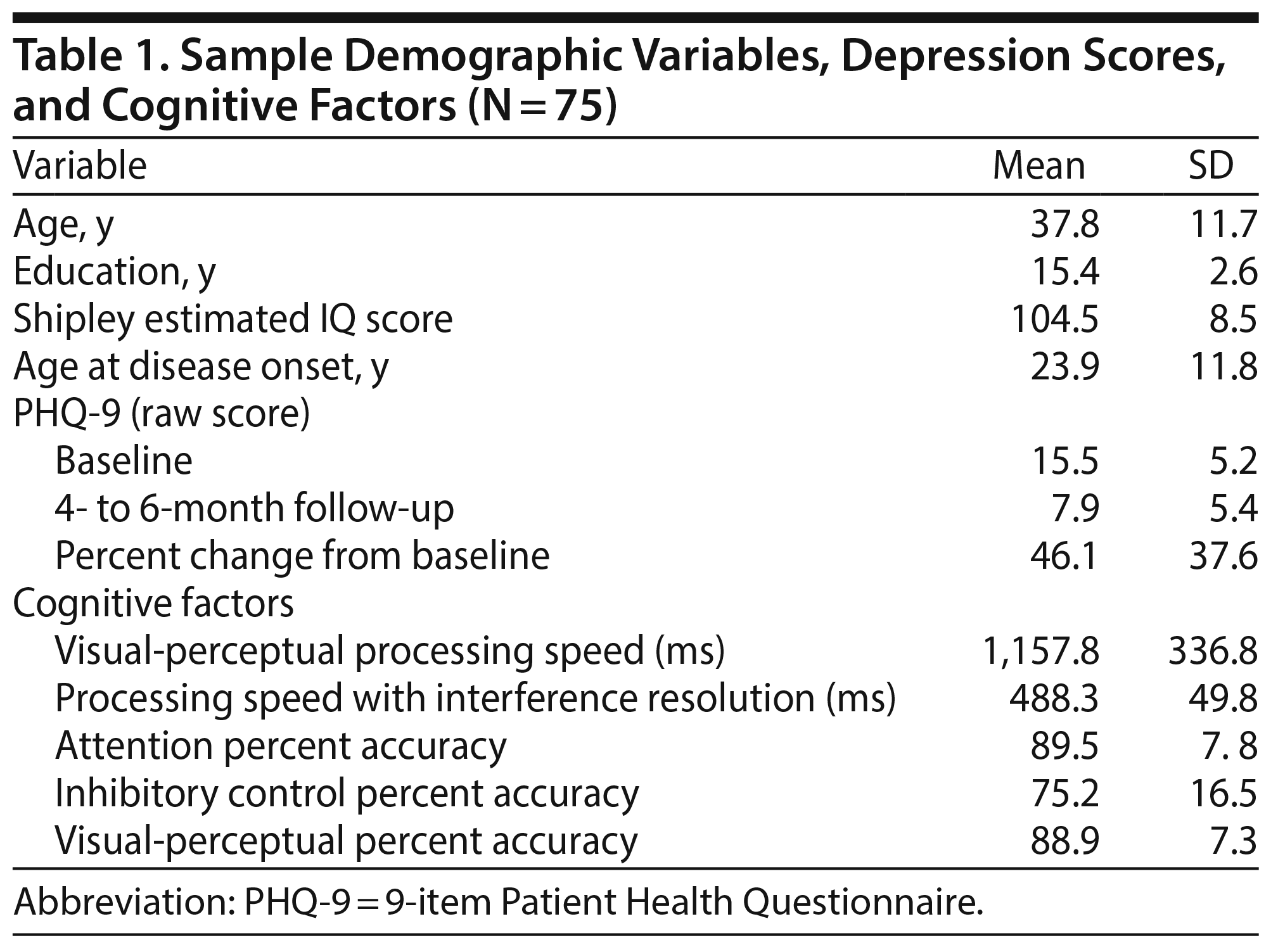
The final sample included 75 adults diagnosed (per DSM-IV-TR20) with major depressive disorder (n = 67) or mood disorder not otherwise specified (n = 8). The sample was largely female (n = 60) and white (n = 56). Most participants had completed some college (n = 69; range, 9-22 years), typical of the setting from which they were recruited. There was wide variability in age at disease onset (ranging from 5-54 years). IQs were estimated using the Shipley Institute of Living Scale,23 with 67% (n = 50) falling within the average range (score range, 82-121). Most participants endorsed experiencing moderately severe depression at the point of treatment initiation at the clinic (PHQ-9 score range, 5-24).
Design and Procedure
Institutional review board approval was obtained from the University of Michigan, as was permission to waive written informed consent and acquire verbal assent for use of clinical data for research purposes. Testing occurred prior to intake evaluations at University of Michigan, Department of Psychiatry tertiary care treatment facilities in 2 afternoon (1 women’s mental health) and 1 morning clinic. Diagnoses were made using DSM-IV-TR criteria by board-certified psychiatrists or psychologists.24 Research assistants administered the cognitive screening battery described here. A brief, automated report describing the patient’s strengths and weaknesses in comparison to normative data was provided to the treating physician, psychologist, or social worker, typically prior to or during the visit. Additional measures not described here were administered as part of a broader clinical care improvement initiative. The PHQ-9 was completed at baseline and at variable follow-up intervals. We analyzed changes from baseline to follow-up at 4 to 6 months to assess sustained improvement.
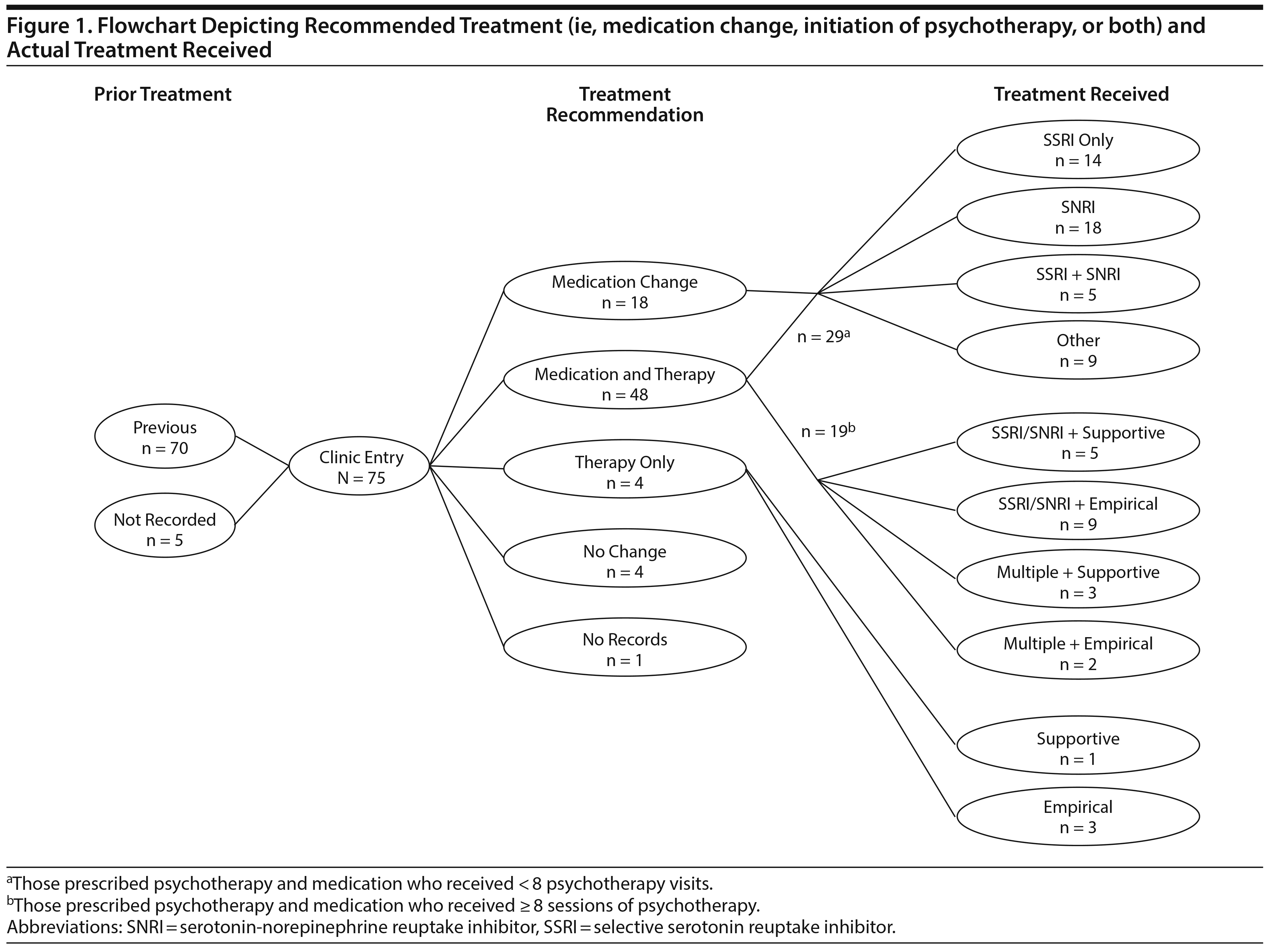
Due to the naturalistic, observational nature of this study, treatments included psychotropic medication only, psychotherapy only, or a combination of medication and therapy (Figure 1). Due to the range of treatments employed, we monitored treatments after intake primarily for descriptive purposes. Of note, several participants were transferring care from different organizations and were therefore already receiving treatment. Since most participants’ depressive symptoms were in the moderate to severe range despite the presence or absence of a prior treatment regimen, we identified participants who completed a new trial of psychotherapy or a change in psychotropic medication (eg, increased dosage, typically of SSRI, substitution of new SSRI, often followed later by augmentation) after intake as a means of assessing response to the intervention. A completed treatment trial was defined as (1) participation in 8 or more individual psychotherapy sessions after intake, (2) at least 8 weeks of treatment with an increased dosage of at least 1 psychotropic medication after intake, and (3) a minimum 8-week trial of 1 or more new psychotropic medications within the 4- to 6-month treatment window.
Sixty-six participants completed 8-week trials of at least 1 psychotropic medication. Of the 71 patients with psychotherapy data, 52 were referred to individual psychotherapy, but only 23 of those patients (44%) completed at least 8 sessions within 6 months, with 14 (60%) receiving an evidence-based treatment (ie, cognitive-behavioral, interpersonal, or dialectical behavioral therapies25) and 9 receiving supportive therapy. Of note, the final sample included 2 patients who failed to meet criteria for a completed treatment trial due to medication noncompliance, 4 patients who had imprecise medical records due to ongoing treatment at outside facilities, and 1 patient whose treatment information was missing. We chose to retain participants with missing or incomplete data in the current sample for better representativeness given the naturalistic recruitment method. The exclusion of these subjects did not change the treatment prediction estimates.
Measures
The following cognitive screening battery used in this study was selected to rapidly evaluate frequently impacted cognitive abilities among depressed adults, including psychomotor speed, attention and executive functioning, and affect perception. This specific combination of tests was chosen in 2002 at the start of this project because both tests are easily administered via computer in less than 25 minutes, enhancing accessibility to outpatient treatment providers.
The Parametric Go/No-Go (PGNG) task26,27 was used to assess inhibitory control, attention, and set-shifting abilities. The task has been well described in previous literature,26,27 and an overview is presented here. The PGNG consists of a rapid (interstimulus interval = 500 ms) serial presentation of letters, with target letters of “x,” “y,” and “z.” Subjects follow a “respond to all targets” rule in the easiest level of the test (level 1) and a “do not repeat” rule in the 2 no-go levels (levels 2 and 3) of the test. The task is a specialized measure of contextual inhibition, as go and no-go response sets change with each response. Contextual inhibition is thought to reflect the same neural regions that support emotion regulation.21 Two aspects of inhibitory control are measured as part of contextual inhibition28: behavioral response inhibition (eg, impulsivity) and removal inhibition (eg, updating working memory rules or set-shifting). Psychomotor speed is also assessed during each level of the task. Outcome measures include reaction time across levels 1 to 3, percentage of correct hits across levels 1 to 3, and percentage of correct inhibited responses during levels 2 to 3.
The Facial Emotion Perception Task (FEPT)21,27,29,30 assesses emotion perception and processing, both of which can be impaired in depressed adults.31-33 Participants are asked to rapidly categorize a series of faces exhibiting happiness, sadness, anger, and fearfulness.34 Categorization of animals (dogs, cats, primates, and birds) is included as a control task. The visual stimulus is presented for 300 ms, followed by a mask (ie, a gray checkerboard mask) for 100 ms, and then 2,600 ms are provided as a response window. Trials are separated by the presentation of a fixation cross for 500 ms. Outcome variables include reaction time across all trials and the percentage of correctly identified emotional expressions and animal categories.
A modified version of the vocabulary subtest of the Shipley Institute of Living Scale23 was used to estimate verbal intelligence. Participants are presented with a word and then asked to choose a synonym among 4 options. There is no penalty for guessing, and we did not impose a time limit for responding. Raw scores were prorated and converted to IQ estimates on the basis of age norms.
The PHQ-9 is a self-report measure of depression symptoms. This measure categorizes 4 degrees of depression severity on the basis of the individual’s total score (ie, 5-9 = mild, 10-14 = moderate, 15-19 = moderately severe, and 20-27 = severe). This measure does not require specialized training to administer or score and is therefore easily incorporated into clinic intake and follow-up procedures.
Analyses
As described in our previous cross-sectional study,21 a principal axis factor analysis with oblique rotation was computed using the FEPT and PGNG raw scores in the sample from the larger study (n = 73 healthy adults and n = 367 adult psychiatric outpatients) to consolidate the number of variables to be used in subsequent analyses. The resulting factors included (1) visual-perceptual processing speed (reaction time during all trials of the FEPT), (2) inhibitory processing speed (reaction time during all levels of the PGNG, hereafter described as processing speed with interference resolution [PSIR] to reflect changes in the way the variable has been conceptualized and described following publication of the initial study),21 (3) attention accuracy (the percentage of hits during levels 1-3 of the PGNG), (4) inhibitory control accuracy (the percentage of correctly inhibited responses to lures during levels 2 to 3 of the PGNG), and (5) visual-perceptual accuracy (the percentage of correct identifications of different human expressions and animal categories). These factor scores were used in all subsequent analyses as key predictor variables (Supplementary Table 1). Correlations between cognitive/affective variables and initial PHQ-9 scores were not significant (all r < 0.17, all P > .13).
Cognitive factor scores were assessed for invalid or out-of-range data. Outliers underwent a Winsor truncation procedure.34 To address the primary aim of determining whether executive functioning and affect perception predict treatment responsiveness, a 2-step, full-model multiple regression analysis was computed. Given the significant association between baseline depression severity and the percent change in follow-up depression scores (2-tailed Pearson r73 = 0.34, P < .05), baseline PHQ-9 scores were included in the first step of the model as a covariate. Age and education were also included as covariates, as these variables are known to be related to these cognitive and affective measures. In addition, as there was substantial variability in baseline and follow-up PHQ-9 scores, we used percent change in PHQ-9 scores as an outcome measure. The executive functioning and affect perception variables were entered in a second step, with the goal of determining whether cognition contributed significantly in predicting treatment outcome after consideration for the effects of clinical/demographic characteristics. Percent change in PHQ-9 scores (from baseline to follow-up) was used as the primary outcome variable of the multiple regression analysis. A second post hoc regression model was computed using backward elimination to further clarify the association between baseline cognitive functioning and follow-up depression scores. Attrition from treatment was also predicted using cognitive and affective measures. Finally, a planned exploratory 1-way analysis of variance was conducted to determine whether treatment type represented an independent predictor of depression 4 to 6 months after baseline.
RESULTS
With regard to treatment response, PHQ-9 scores improved an average of 46% within 4 to 6 months after intake, with most participants improving from moderately severe depression at baseline to mild depression at follow-up (Table 1). Over half the sample experienced a 50% or larger reduction in depression symptoms (eg, full responders, 56% of those treated). Thirty-two percent of the sample scored within the minimal range of depression on the PHQ-9 at follow-up.
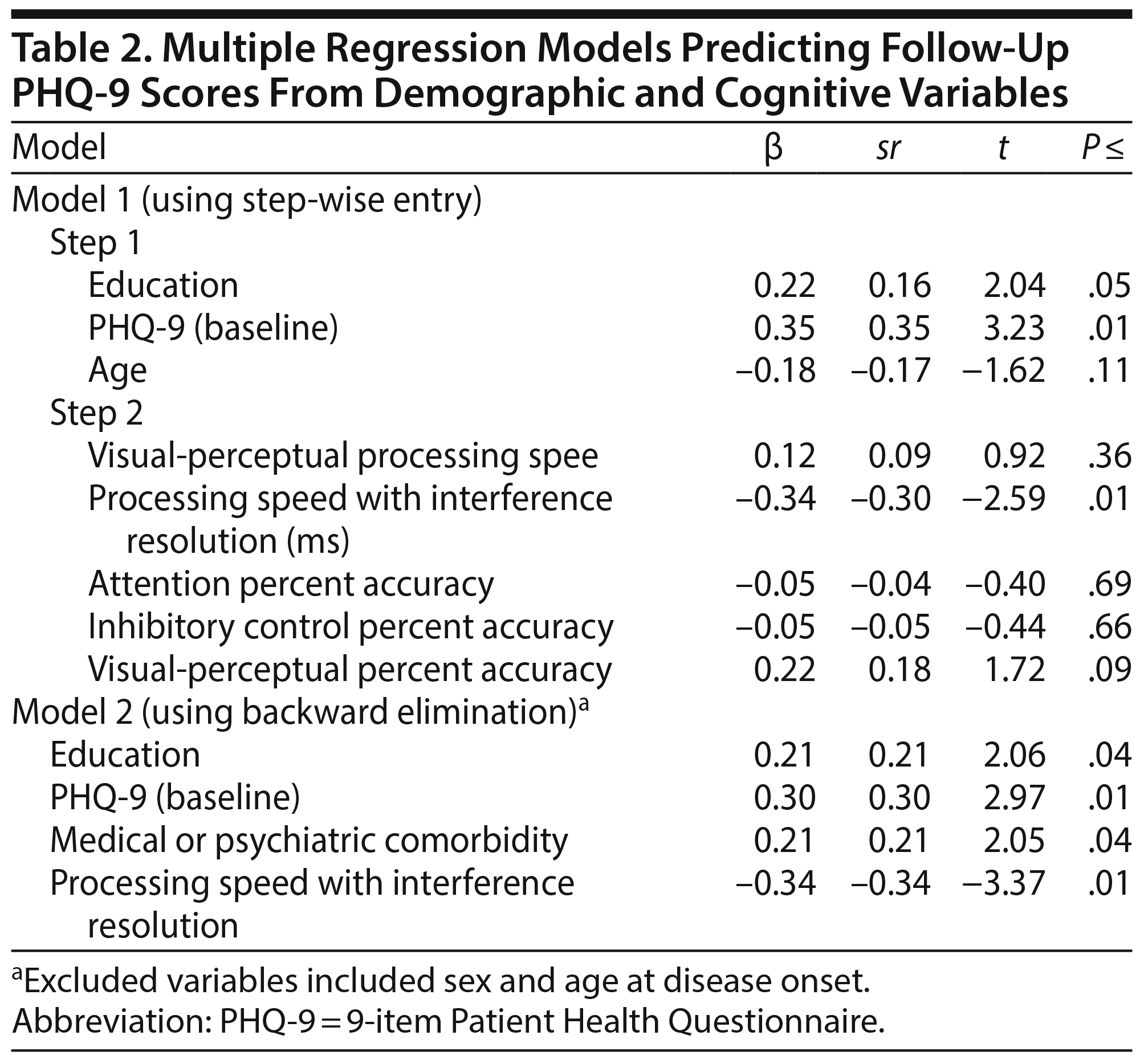
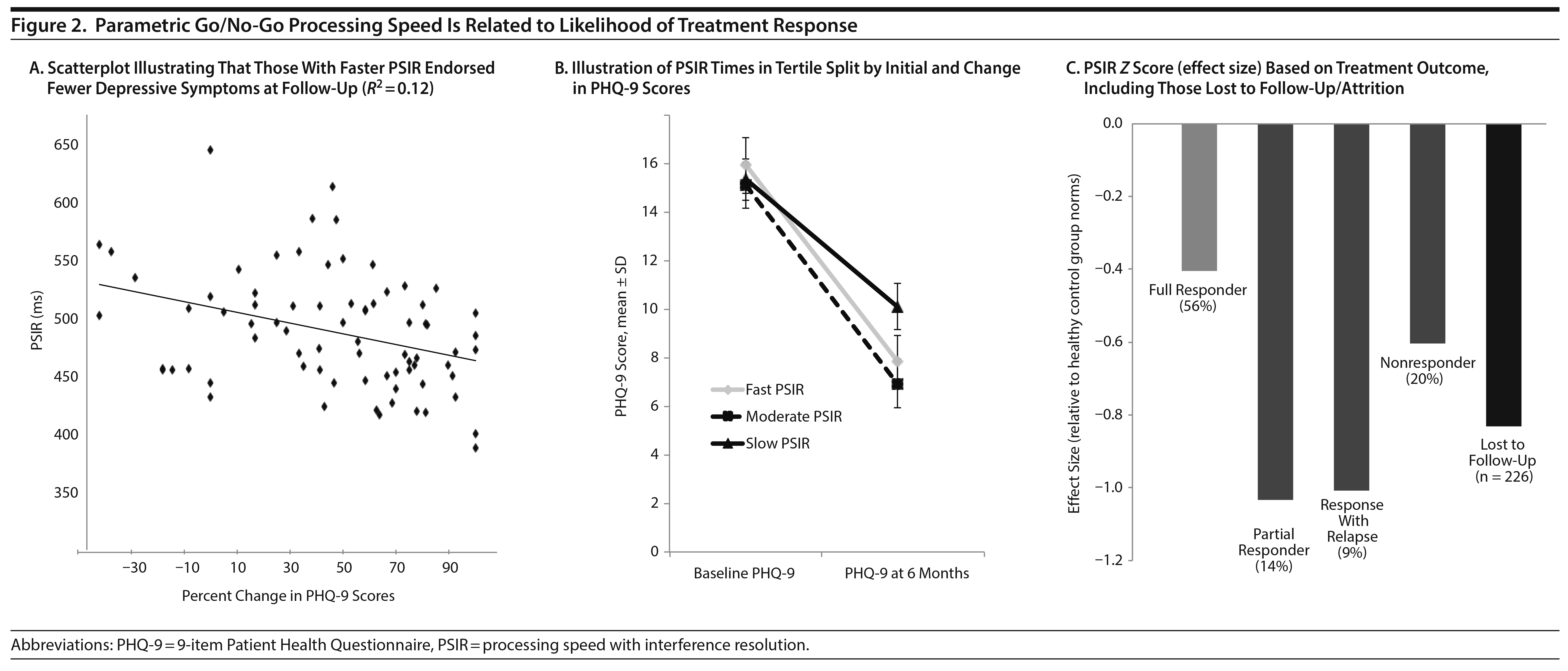
To address our hypothesis that cognitive test performance would significantly predict response to usual treatment, a 2-step multiple regression was computed using the percent change in follow-up PHQ-9 scores as the outcome variable. The first step of the model using education, age, and baseline PHQ-9 scores was significant (F3,73 = 4.79, P < .01, R2 = 0.17, Table 2). When the executive functioning and affect perception variables were added as predictors in the second step, R2 notably increased (F8,68 = 3.35, P < .01, R2 = 0.28), enhancing the prediction of treatment outcomes from 14% to 28%. Among the cognitive variables entered in the model, only PSIR added significant, unique variance to the model, with faster PSIR predicting a greater percent change in PHQ-9 follow-up scores (illustrated in Figure 2, B = -0.34, P = .01). The other cognitive factor scores were not significant unique predictors in the model. Better emotion/visual processing accuracy was at the trend level for prediction of treatment response (B = 0.22, P = .09). In a larger model with all participants, we used the same predictors to evaluate ability to predict attrition to treatment or PHQ-9 follow-up. Step 1 was the same as above, now predicting binary continue versus attrition (χ2 = 4.79, P = .19, R2 = 0.03). We also compared those who were included to those who were lost to attrition. The 226 individuals who were lost to attrition were significantly slower in PSIR relative to those who remained in treatment and completed multiple PHQ-9 measurements (F1,310 = 37.61, P < .0001, B = 0.02, P < .0001, sR2 = 0.16). Those who did not complete treatment or for which multiple PHQ-9 measurements were not different in age, education, initial PHQ-9 score, or age at onset relative to those who continued on in treatment and completed multiple PHQ-9 measurements (P > .25). More males tended to be lost to attrition in measurement compared to females, but this was not significant (χ2 = 1.27, P = .26). Models had similar predictive value if those with mood disorder not otherwise specified were excluded or if those with PHQ-9 scores in the mild range were excluded, consistent with current formulations for how treatment evaluation and prediction might be transdiagnostic.36
In an effort to further evaluate the meaningfulness of PSIR as a predictor of treatment outcome, a post hoc regression model was computed using backward elimination to determine whether PSIR would remain a significant contributor to the model when additional demographic characteristics were considered (ie, age at disease onset, presence of medical or psychiatric comorbidity, and sex). The combination of age, education, percent change in PHQ-9 scores, presence of a comorbid medical or psychiatric condition, and PSIR explained approximately 30% of the variance in the percent change in PHQ-9 scores (F4,70 = 7.43, P < .01, R2 = 0.30). PSIR remained a significant unique predictor (semipartial correlation = 0.11).
Finally, a planned, exploratory post hoc t test was computed to evaluate whether the type of treatment the patients received was associated with the percent change in depression scores at follow-up. Possible treatment outcomes were limited to (1) medication change only (n = 47) versus (2) combined medication change and completed psychotherapy (n = 19) because very few participants completed psychotherapy only (n = 4). There were no significant differences in the change in PHQ-9 scores between treatment groups (t25 = 1.50, P = .14, Supplementary Figure 1). Likewise, there was no difference between groups on the basis of subsequent treatment allocation in baseline PHQ-9 scores (F3,64 = 0.26, P = .85, see Supplementary Figure 1).
DISCUSSION
This study evaluated whether performance on a brief computerized cognitive screening battery would prospectively predict responsiveness to usual treatment among adults with depressive disorders in a naturalistic clinical setting. We hypothesized that better executive functioning and affect perception at baseline would be associated with a greater reduction in depressive symptoms 4 to 6 months after treatment initiation. As expected, faster processing speed with interference resolution was a significant predictor of greater improvement in depressive symptoms at follow-up, contributing variance beyond that explained by baseline depression symptoms and educational attainment. Therefore, inefficient executive functioning at baseline, exemplified by slowed interference resolution, appears to represent a risk factor for poorer response to standard treatments. Visual processing speed and accuracy and other attention/inhibition variables did not contribute significant unique variance in the change in depression severity.
Response inhibition has been assessed as a prospective predictor of treatment outcome using paper-and-pencil tests with variable results.10,11 Some of this variability may be related to the relatively small number of participants in most of these studies in proportion to the relatively large number of cognitive variables, which increases the risk for type II errors. Paper-and-pencil tests are also unable to measure inhibitory processing speed, which is a unique capability of computerized go/no go tasks such as the PGNG. Executive functioning, more broadly, appears well supported as a meaningful predictor of treatment responsiveness.6,8,10,37,38 These findings are consistent with the theory that the executive system regulates behavior and also support the potential role of the executive system in emotion regulation and perseveration of negative mood states in depression.21,27,39 Additionally, this is the first study of which we are aware to demonstrate that cognitive measures can be incorporated into the intake process at outpatient psychiatry clinics, thereby supporting the direct translation of research findings to clinical practice.
Our primary finding that efficient behavioral responses on a contextual inhibition task predicts treatment responsiveness among depressed adults fits well with available literature on the structural and functional impact of depression on neural circuits. Specifically, the dorsolateral prefrontal cortex, orbital frontal cortex, and anterior cingulate cortex, regions associated with cognitive and inhibitory control, are often reduced in volume and underactive at rest in depressed adults compared to healthy adults39-41 and have been implicated in responsiveness to treatment for depression. Several studies9,39,42-44 support the utility of neuroimaging techniques for identifying individuals at risk for treatment resistance or individuals who may respond better to 1 treatment versus another, yet neuroimaging is typically costly, time-consuming, and limited in availability and is sometimes medically contraindicated. As a more feasible alternative, the current results support the administration of a brief computerized screening battery for this purpose. Computerized neuropsychological screening batteries can easily be administered by supervised trainees, technicians, and research assistants; require minimal resources; and, as this study illustrates, can be easily adapted as part of the intake process in any outpatient setting. Additional measures might be added to the PGNG task, such as auditory memory, to increase the predictive power. Furthermore, this added intake procedure could be useful for identifying individuals who might benefit from a more thorough neuropsychological evaluation—to rule out significant cognitive impairment that could impede the patient’s ability to effectively participate in psychotherapy, manage his or her medications, or successfully function in social, familial, and occupational domains.
The sample treated herein is relatively large and had a similar level of treatment response compared to that observed in the Sequenced Treatment Alternatives to Relieve Depression study (STAR*D)45 (32% remission rate compared to about 33% in STAR*D, not accounting for relapse). In fact, as the University of Michigan was a site in STAR*D, drawing patients from some of the same clinics, some of the patients in the present study could have been previously enrolled in STAR*D.
The present study has limitations to acknowledge. First, a naturalistic sample is less well-controlled and restricts the ability to compare efficacy of different treatments. We were, therefore, unable to reliably assess the potential relationships among cognitive functioning and specific treatment methods. The sample is also restricted to those who continued to receive care in our clinics for at least 4 months. The strength, however, is the representativeness of this sample to other outpatient settings and the substantial implications in real-world settings, particularly because medical and psychiatric comorbidities among depressed individuals are common and relevant to treatment considerations, yet are often exclusionary in treatment trials. Second, there was substantial heterogeneity in initial depression severity, which was also related to our sample selection method. There is substantial attrition in the sample, including about 44% of those who were prescribed psychotherapy following through with the advised number of sessions. It is unclear if the high attrition and fewer than 8 sessions of prescribed psychotherapy might be related to patient burden (more frequent visits are needed) or more rapid return to wellness, and we did not have data available to answer these questions. Another limitation is that, with the current sample, we were unable to model specific cognitive variables by depression severity, and the current test battery was selected on the basis of its relative ease of application and its relevance to the cognitive domains frequently implicated in depression but was therefore limited in scope. Also, unfortunately, broader measures of functioning were not evaluated. Finally, we were unable to reliably control for type of intervention on depression severity at follow-up due to range restriction.
As a proof-of-concept study, this is the first of which we are aware that uses a brief, computer-administered cognitive screening battery as a clinical tool in tertiary care mental health clinics. We found that patients demonstrating slower reaction times during a task involving response inhibition (ie, PGNG) at intake responded less robustly to outpatient treatment for depression. Indeed, response inhibition efficiency in this study represented a primary predictor of treatment responsiveness beyond other demographic characteristics previously associated with treatment outcomes. These findings suggest that patients with impaired executive functioning may benefit from augmented or alternative treatment if such impairments can be identified early, thereby supporting the utility of streamlining the inclusion of a brief cognitive screen as part of the intake process in outpatient mental health clinics. Selection accuracy with cognitive and affective measures could decrease time to wellness, a key concept in the precision medicine initiative of the National Institute of Mental Health.16-18 Use of executive functioning weakness in treatment decision-making to enhance selection accuracy may be particularly valuable if some newer medications and treatments are available to potentially improve executive functioning.13,46 Future studies might iteratively develop a battery of sensitive measures that is somewhat broader-based, with the ultimate goal of reducing time to recovery and incidence of relapse through more personalized care.
Submitted: March 6, 2016; accepted October 19, 2016.
Published online: February 9, 2017.
Drug names: fluoxetine (Prozac and others).
Potential conflicts of interest: None.
Funding/support: This work was supported by National Institutes of Health grant MH074459, Brain and Behavior Research NARSAD Young Investigator Award to Dr Langenecker, and Rachel Upjohn Clinical Scholars Award and internal support from the Depression and Neuropsychology Sections of the Department of Psychiatry at the University of Michigan Medical Center, Ann Arbor.
Role of the sponsor: The sponsors played no role in design or conduct of the study, analysis of the data, or interpretation and reporting of the results.
Supplementary material: See accompanying pages.
REFERENCES
1. Gaynes BN, Rush AJ, Trivedi MH, et al. The STAR*D study: treating depression in the real world. Cleve Clin J Med. 2008;75(1):57-66. PubMed doi:10.3949/ccjm.75.1.57
2. Siegle GJ. Beyond depression commentary: wherefore art thou, depression clinic of tomorrow? Clin Psychol (New York). 2011;18(4):305-310. PubMed
3. Keitner GI, Ryan CE, Miller IW, et al. Recovery and major depression: factors associated with twelve-month outcome. Am J Psychiatry. 1992;149(1):93-99. PubMed doi:10.1176/ajp.149.1.93
4. Hollon SD, Stewart MO, Strunk D. Enduring effects for cognitive behavior therapy in the treatment of depression and anxiety. Annu Rev Psychol. 2006;57:285-315. PubMed doi:10.1146/annurev.psych.57.102904.190044
5. Roca M, Garc×a-Toro M, Garc×a-Campayo J, et al. Clinical differences between early and late remission in depressive patients. J Affect Disord. 2011;134(1-3):235-241. PubMed doi:10.1016/j.jad.2011.05.051
6. Withall A, Harris LM, Cumming SR. The relationship between cognitive function and clinical and functional outcomes in major depressive disorder. Psychol Med. 2009;39(3):393-402. PubMed doi:10.1017/S0033291708003620
7. Kampf-Sherf O, Zlotogorski Z, Gilboa A, et al. Neuropsychological functioning in major depression and responsiveness to selective serotonin reuptake inhibitors antidepressants. J Affect Disord. 2004;82(3):453-459. PubMed
8. Gorlyn M, Keilp JG, Grunebaum MF, et al. Neuropsychological characteristics as predictors of SSRI treatment response in depressed subjects. J Neural Transm (Vienna). 2008;115(8):1213-1219. PubMed doi:10.1007/s00702-008-0084-x
9. Taylor BP, Bruder GE, Stewart JW, et al. Psychomotor slowing as a predictor of fluoxetine nonresponse in depressed outpatients. Am J Psychiatry. 2006;163(1):73-78. PubMed doi:10.1176/appi.ajp.163.1.73
10. Dunkin JJ, Leuchter AF, Cook IA, et al. Executive dysfunction predicts nonresponse to fluoxetine in major depression. J Affect Disord. 2000;60(1):13-23. PubMed doi:10.1016/S0165-0327(99)00157-3
11. Majer M, Ising M, Künzel H, et al. Impaired divided attention predicts delayed response and risk to relapse in subjects with depressive disorders. Psychol Med. 2004;34(8):1453-1463. PubMed doi:10.1017/S0033291704002697
12. Langenecker SA, Lee J, Bieliauskas LA. Neuropsychology of depression and related mood disorders. In: Grant I, Adams KM, eds. Neuropsychological Assessment of Neuropsychiatric and Neuromedical Disorders. New York, NY: Oxford University Press; 2009.
13. McIntyre RS, Cha DS, Soczynska JK, et al. Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety. 2013;30(6):515-527. PubMed doi:10.1002/da.22063
14. Kohler CG, Hoffman LJ, Eastman LB, et al. Facial emotion perception in depression and bipolar disorder: a quantitative review. Psychiatry Res. 2011;188(3):303-309. PubMed doi:10.1016/j.psychres.2011.04.019
15. Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol. 2010;6:285-312. PubMed doi:10.1146/annurev.clinpsy.121208.131305
16. Fu CH, Mourao-Miranda J, Costafreda SG, et al. Pattern classification of sad facial processing: toward the development of neurobiological markers in depression. Biol Psychiatry. 2008;63(7):656-662. PubMed doi:10.1016/j.biopsych.2007.08.020
17. Bouhuys AL, Geerts E, Gordijn MCM. Depressed patients’ perceptions of facial emotions in depressed and remitted states are associated with relapse: a longitudinal study. J Nerv Ment Dis. 1999;187(10):595-602. PubMed doi:10.1097/00005053-199910000-00002
18. Bouhuys AL, Geerts E, Mersch PPA, et al. Nonverbal interpersonal sensitivity and persistence of depression: perception of emotions in schematic faces. Psychiatry Res. 1996;64(3):193-203. PubMed doi:10.1016/S0165-1781(96)02930-7
19. Hoertnagl CM, Muehlbacher M, Biedermann F, et al. Facial emotion recognition and its relationship to subjective and functional outcomes in remitted patients with bipolar I disorder. Bipolar Disord. 2011;13(5-6):537-544. PubMed doi:10.1111/j.1399-5618.2011.00947.x
20. Amercan Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000.
21. Langenecker SA, Caveney AF, Giordani B, et al. The sensitivity and psychometric properties of a brief computer-based cognitive screening battery in a depression clinic. Psychiatry Res. 2007;152(2-3):143-154. PubMed doi:10.1016/j.psychres.2006.03.019
22. Kroenke K, Spitzer RL, Williams JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. PubMed doi:10.1046/j.1525-1497.2001.016009606.x
23. Shipley WC. Institute of Living Scale. Los Angeles, CA: Western Psychological Services; 1946.
24. First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV Axis I Disorders. New York, NY: New York State Psychiatric Institute, Biometrics Research; 1996.
25. Society of Clinical Psychology. Research-Supported Clinical Treatments. https://www.div12.org/psychological-treatments/. Accessed December 2, 2016.
26. Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96(14):8301-8306. PubMed doi:10.1073/pnas.96.14.8301
27. Langenecker SA, Bieliauskas LA, Rapport LJ, et al. Face emotion perception and executive functioning deficits in depression. J Clin Exp Neuropsychol. 2005;27(3):320-333. PubMed doi:10.1080/13803390490490515720
28. Hasher L, Zacks RT. Working memory, comprehension, and aging: a review and a new view. In: Bower GH, ed. The Psychology of Learning and Motivation. Vol. 22. New York, NY: Academic Press; 1988:193-225. doi:10.1016/S0079-7421(08)60041-9
29. Rapport LJ, Friedman SR, Tzelepis A, et al. Experienced emotion and affect recognition in adult attention-deficit hyperactivity disorder. Neuropsychology. 2002;16(1):102-110. PubMed doi:10.1037/0894-4105.16.1.102
30. Vederman AC, Weisenbach SL, Rapport LJ, et al. Modality-specific alterations in the perception of emotional stimuli in bipolar disorder compared to healthy controls and major depressive disorder. Cortex. 2012;48(8):1027-1034. PubMed doi:10.1016/j.cortex.2011.03.017
31. Gur RC, Erwin RJ, Gur RE, et al. Facial emotion discrimination, II: behavioral findings in depression. Psychiatry Res. 1992;42(3):241-251. PubMed doi:10.1016/0165-1781(92)90116-K
32. Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156(5):675-682. PubMed
33. LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155-184. PubMed doi:10.1146/annurev.neuro.23.1.155
34. Ekman P, Friesen WV. Measuring facial movement. J Environ Psychol. 1976;(1):56-75.
35. Tabachnick BG, Fidell LS. Using Multivariate Statistics. Needham Heights, MA: Allyn and Bacon; 2006.
36. Simpson HB. The RDoC project: a new paradigm for investigating the pathophysiology of anxiety. Depress Anxiety. 2012;29(4):251-252. PubMed doi:10.1002/da.21935
37. Kalayam B, Alexopoulos GS. Prefrontal dysfunction and treatment response in geriatric depression. Arch Gen Psychiatry. 1999;56(8):713-718. PubMed doi:10.1001/archpsyc.56.8.713
38. Mattis S. Dementia Rating Scale. Odessa, FL: Psychological Assessment Resources; 1989.
39. Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology. 2011;36(1):183-206. PubMed doi:10.1038/npp.2010.166
40. Murrough JW, Iacoviello B, Neumeister A, et al. Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiol Learn Mem. 2011;96(4):553-563. PubMed doi:10.1016/j.nlm.2011.06.006
41. Peterson BS, Warner V, Bansal R, et al. Cortical thinning in persons at increased familial risk for major depression. Proc Natl Acad Sci U S A. 2009;106(15):6273-6278. PubMed doi:10.1073/pnas.0805311106
42. Kennedy SH, Konarski JZ, Segal ZV, et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. Am J Psychiatry. 2007;164(5):778-788. PubMed doi:10.1176/ajp.2007.164.5.778
43. Joormann J, Nee DE, Berman MG, et al. Interference resolution in major depression. Cogn Affect Behav Neurosci. 2010;10(1):21-33. PubMed doi:10.3758/CABN.10.1.21
44. Siegle GJ, Thompson WK, Collier A, et al. Toward clinically useful neuroimaging in depression treatment: prognostic utility of subgenual cingulate activity for determining depression outcome in cognitive therapy across studies, scanners, and patient characteristics. Arch Gen Psychiatry. 2012;69(9):913-924. PubMed doi:10.1001/archgenpsychiatry.2012.65
45. Rush AJ, Trivedi MH, Wisniewski SR, et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry. 2006;163(11):1905-1917. PubMed doi:10.1176/ajp.2006.163.11.1905
46. Anaya C, Martinez Aran A, Ayuso-Mateos JL, et al. A systematic review of cognitive remediation for schizo-affective and affective disorders. J Affect Disord. 2012;142(1-3):13-21. PubMed doi:10.1016/j.jad.2012.04.020
Please sign in or purchase this PDF for $40.00.
Save
Cite