Prim Care Companion CNS Disord 2021;23(1):20br02621
To cite: Gandotra K, Jaskiw GE, Williams SG, et al. Development of insomnia associated with different formulations of bupropion. Prim Care Companion CNS Disord. 2021;23(1):20br02621.
To share: https://doi.org/10.4088/PCC.20br02621
aLouis Stokes VA Medical Center, Cleveland, Ohio
bPsychiatry Service, Louis Stokes VA Medical Center, Cleveland, Ohio
cDepartment of Psychiatry, Case Western Reserve University, Cleveland, Ohio
dDivision of Pulmonary, Critical Care, and Sleep Medicine, Louis Stokes VA Medical Center, Cleveland, Ohio
eSleep Disorders Center, Department of Medicine, Walter Reed National Military Medical Center, Bethesda, Maryland
fDepartment of Medicine, Uniformed Services University of the Health Sciences, Bethesda
gNational PBM Clinical Pharmacy Program Manager VHA Pharmacy Benefits Management Services, Hines, Illinois
hDepartments of Psychiatry and Psychology, Case Western Reserve University, Cleveland, Ohio
iGeriatric Research Educational and Clinical Center (GRECC), Louis Stokes VA Medical Center, Cleveland, Ohio
jDivision of Pulmonary, Critical Care and Sleep Medicine, University Hospitals, Case Western Reserve University, Cleveland, Ohio
*Corresponding author: Kamal Gandotra, MD, Louis Stokes VAMC, 10701 East Blvd, Cleveland, OH 44106 ([email protected]).
Bupropion is a widely prescribed antidepressant with efficacy for major depressive disorder, seasonal affective disorder, smoking tobacco cessation, and attention-deficit/hyperactivity disorder.1 While bupropion’s advantages include a relatively low risk of inducing restless leg syndrome2 or rapid eye movement sleep behavioral disorder,3 its alerting effects may disrupt sleep, particularly when a short-acting formulation is administered late in the day.4 Awareness of the kinetics and pharmacology of bupropion can mitigate such sleep-disturbing effects while exploiting antidepressant effects. Bupropion is a weak norepinephrine-dopamine reuptake inhibitor that does not show significant antagonism at histaminic or muscarinic receptors and has a low prevalence of sexual side effects.5 While bupropion is not a classic stimulant,6 its ability to increase dopamine and norepinephrine can result in nonspecific stimulation.5 Sleep medicine practitioners and others can now optimize selection of bupropion formulations to minimize the risk of insomnia.
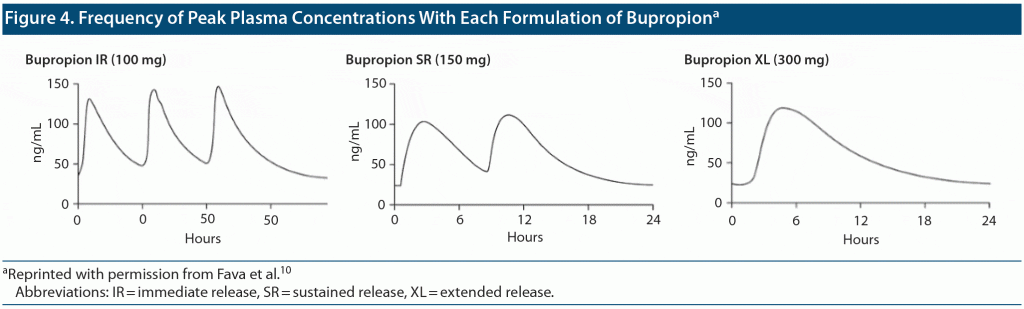
The stimulating effects in wakefulness of bupropion include restlessness, activation, tremors, and nausea and typically coincide with peak (Cmax) plasma concentrations. The original immediate-release (IR) preparation has a half-life requiring 3 dosing times/day. The sustained-release (SR) (twice/day dosing) and extended-release (XL) (once/day dosing) formulations were developed to improve tolerability and adherence. Given the dosing regimen, patients with IR, SR, and XL formulations are exposed to 3, 2, and 1 instances of Cmax daily, respectively.3 Furthermore, the time to achieve Cmax (Tmax) is shorter for the IR dosage form (2 hours) relative to the SR (3 hours) and XL (5 hours) dosage forms.3 In combination, these characteristics may affect tolerability. A morning dosing of the XL formulation, in particular, results in lower drug levels during evening hours3 and may result in a lower risk of insomnia. We evaluated the effects of a possible association between the different bupropion formulations and treatment-emergent insomnia in veterans with major depressive disorder prescribed bupropion based on provider discretion.
Methods
A retrospective chart review was conducted of outpatients treated at the Veterans Affairs Northeast Ohio Healthcare System, Cleveland, Ohio. Charts were selected of patients who presented over a 3-month period and met the following criteria: (1) referred to mental health clinic for symptoms of major depressive disorder (DSM-5 criteria), (2) did not have comorbid insomnia (initial Insomnia Severity Index [ISI, see Supplementary Appendix 1]7 score <7 with no subjective sleep complaints), (3) Apnea-Hypopnea Index score <5/h, (4) were started on any formulation of bupropion (IR, SR, or XL), (5) were not on medications that influence sleep, and (6) did not have a hypersomnolence condition. ISI, Clinically Useful Depression Outcome Scale (CUDOS, see Supplementary Appendix 2),8 and Epworth Sleepiness scale (ESS)9 scores were determined at baseline and at 4–6 weeks and 7–12 weeks after initiation of bupropion. Self-reporting timing of doses was extracted from the chart. For ISI and CUDOS scores, linear mixed-effects models were used to assess the interaction of treatment formulation (IR, SR, XL) and time. Analyses were performed in R 3.5.1 using the lme4 and emmeans packages (https://www.r-project.org/). A random intercept for each subject was estimated given unbalanced groups and missing observations due to discontinuations. For each of 3 time points, differences across fixed-effect estimates of treatments were determined and Tukey P value adjustments imposed (P<.05).
Results
A total of 30 veterans (aged 25 to 58 years) met entry criteria. In 2 patients, bupropion was discontinued secondary to tinnitus or agitation, yielding a final sample of 28 (27 men, 1 woman).
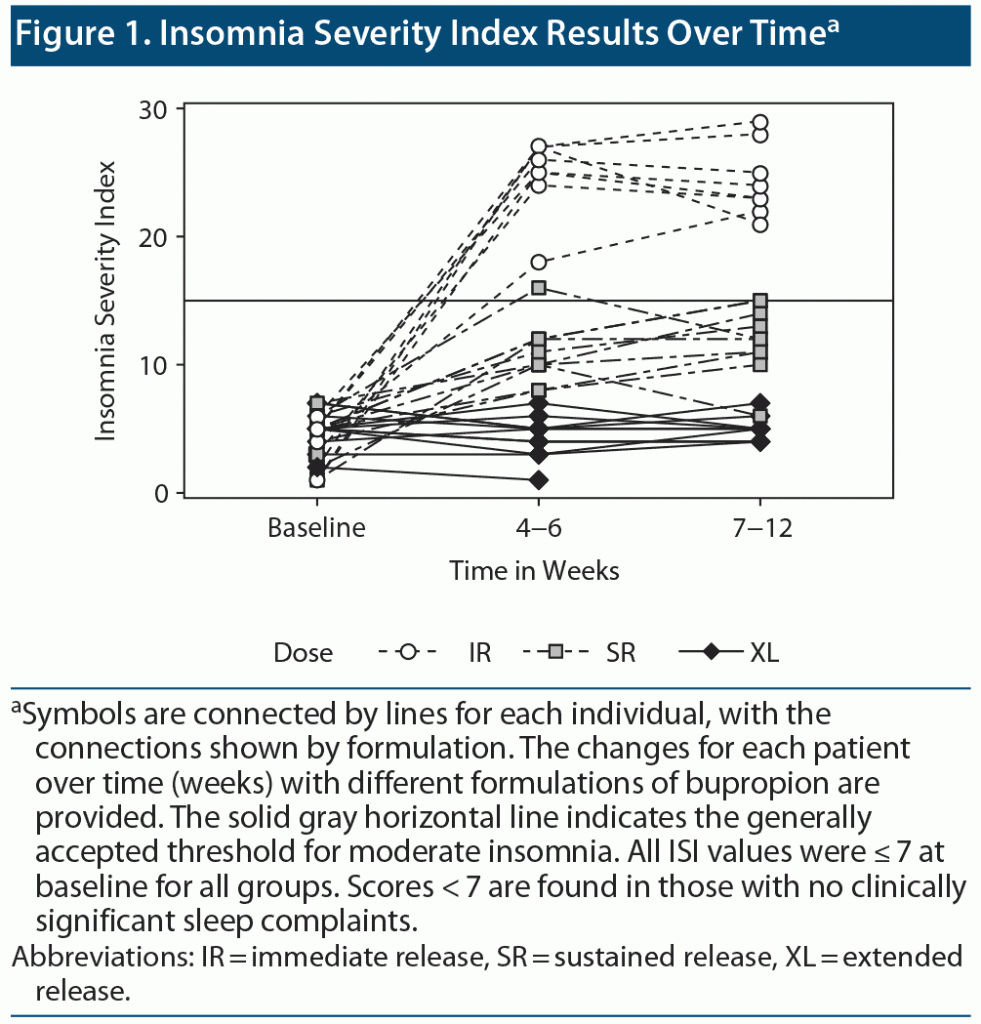
Veterans treated with both SR and IR formulations presented more often with complaints of insomnia based on the ISI compared to patients treated with the XL dosage form. We observed a statistically significant interaction of dose and time (F=99.7, df=4, P<.01). The mean post baseline ISI score was significantly lower in patients treated with bupropion XL than in patients treated with bupropion IR or bupropion SR (Figure 1).
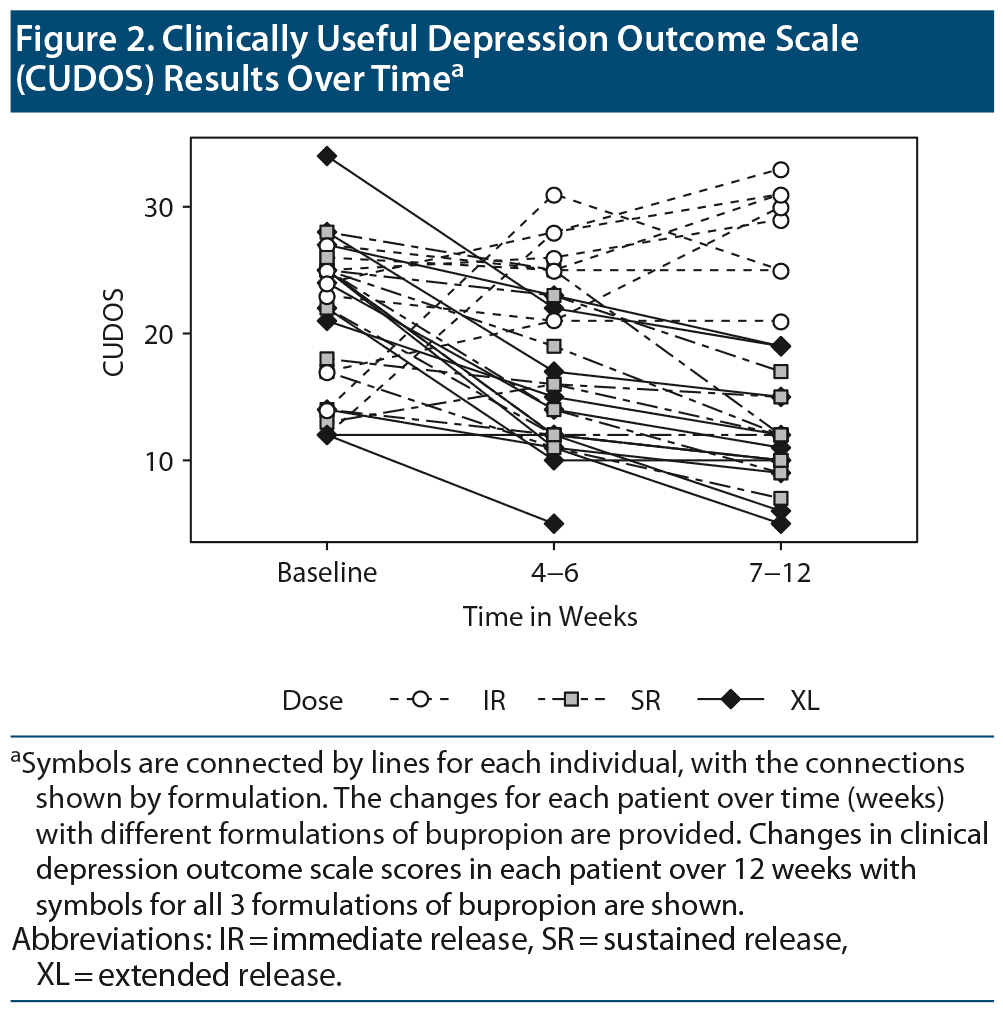
A similar model predicting CUDOS scores included a significant interaction of dose and time (F=18.9, df=4, P<.01), indicating the IR formulation of bupropion was least effective in lowering the depression score (Figure 2).
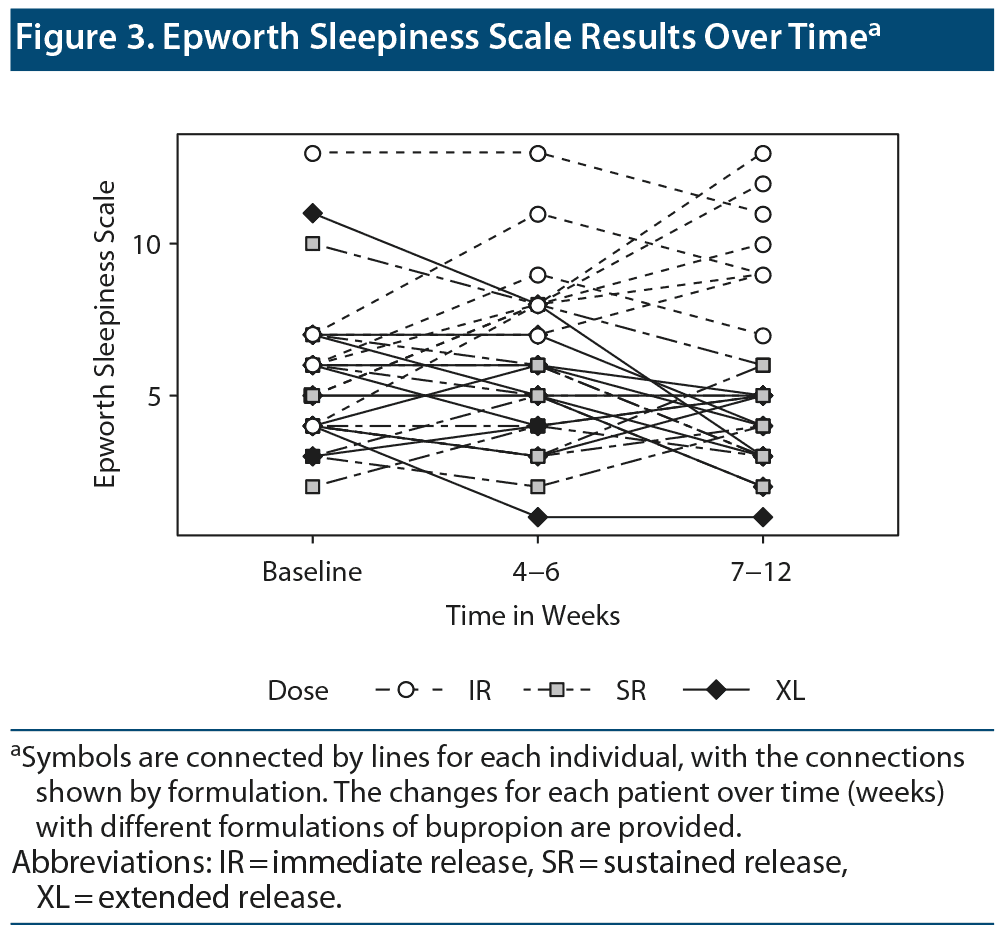
For the ESS, there was no difference among groups at baseline. While the ESS scores for the IR formulation were significantly higher than other 2 formulations at other time points, no difference was observed between SR and XL at any time point on the ESS (Figure 3). The ESS values did not change significantly over time during administration of any of the formulations. This finding may be attributed to the pathophysiology of insomnia, as it is considered to be a disorder of arousal. Alternatively, the IR formulation causes medication-induced insomnia, which leads to next-day tiredness as a result of sleeping poorly.
Discussion
Psychotropic drugs can contribute to the pathogenesis and maintenance of insomnia. Activating antidepressants such as bupropion may be especially culpable.5 Our chart review found that in outpatients presenting with depression but without comorbid insomnia, high rates of insomnia developed after initiation of treatment with IR formulations of bupropion but not after a single-dose morning administration of a slow-release, long-acting formulation (see Figure 1).
These findings underscore the importance of considering potential stimulating effects of bupropion expected to correlate with peak (Cmax) plasma concentrations. Given the dosing regimens, patients with IR, SR, and XL formulations are exposed to 3, 2, and 1 instances of Cmax daily. Furthermore, the time to achieve Cmax (Tmax) is shorter for IR (2h) relative to SR (3h) and XL (5h) formulations3 (see Figure 3). In combination, these characteristics affect tolerability. The morning dosing of the XL formulation, in particular, limits drug peak levels during evening hours10 and may thus explain lower risk of insomnia in our patient sample. Assuming a bedtime approximately 14 hours after the first dose, the median time from last dose to bedtime was 4.5 hours for bupropion IR and 8 hours for bupropion SR. While all formulations of bupropion showed a good antidepressant effect, our experience suggests a possible antidepressant advantage for an early morning administration of the slow-release, long-acting XL preparation in patients experiencing insomnia. If the IR formulation is chosen, it is recommended that the final dose not be administered after 2 pm or 3 pm to avoid peak levels in the evening. The last dose of the IR and SR formulations should be taken as early as possible to prevent peak plasma levels in the evening (Figure 4).
Limitations of this study include the retrospective approach, the male predominance of the referral population, an inability to ensure proper medication adherence, and lack of a control group. It is becoming increasingly apparent that the relationship between insomnia and other psychiatric disorders such as depression is complex, at times bidirectional, and incompletely characterized. Insomnia can constitute an independent risk factor for depression, precede the onset of depression, develop in conjunction with other symptoms of depression, and complicate the treatment response. The recognition of such associations underscores the need for randomized, controlled prospective studies.
Conclusion
In this retrospective study, the introduction of bupropion IR or SR for antidepressant purposes was associated with significantly more insomnia compared to the XL dosage form. This finding is most likely attributable to differences in the pharmacokinetics of the formulations. Total exposure (area under the curve) at steady-state of all 3 formulations is equivalent, and all 3 formulations have similar peak concentrations (Cmax). However, by utilizing an XL dosage form, the patient is exposed to 1 daily peak exposure at a later time (Tmax) compared to 2 and 3 daily peak exposures for the SR and IR dosage forms, respectively. Further, morning administration of bupropion XL is associated with a lower evening plasma concentration compared to the SR dosage form; as adverse effects have been associated with plasma levels, a lower evening concentration may result in lower rates of insomnia. Physicians should be aware of such differences when assessing insomnia in patients treated with shorter-acting bupropion formulations and should consider the XL formulation used once daily in the morning to lower disordered sleep while maintaining antidepressant efficacy.
Published online: February 18, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Supplementary material: See accompanying pages.
References (10)

- Huecker MR, Smiley A, Saadabadi A. Bupropion. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2020. https://www.ncbi.nlm.nih.gov/books/NBK470212/.
- Bayard M, Bailey B, Acharya D, et al. Bupropion and restless legs syndrome: a randomized controlled trial. J Am Board Fam Med. 2011;24(4):422–428. PubMed CrossRef NLM
- McCarter SJ, Howell MJ. Importance of rapid eye movement sleep behavior disorder to the primary care physician. Mayo Clin Proc. 2016;91(10):1460–1466. PubMed CrossRef NLM
- Thase ME, Haight BR, Richard N, et al. Remission rates following antidepressant therapy with bupropion or selective serotonin reuptake inhibitors: a meta-analysis of original data from 7 randomized controlled trials. J Clin Psychiatry. 2005;66(8):974–981. PubMed CrossRef NLM
- Stahl SM, Pradko JF, Haight BR, et al. A review of the neuropharmacology of bupropion, a dual norepinephrine and dopamine reuptake inhibitor. Prim Care Companion J Clin Psychiatry. 2004;6(4):159–166. PubMed CrossRef NLM
- Hamilton MJ, Smith PR, Peck AW. Effects of bupropion, nomifensine and dexamphetamine on performance, subjective feelings, autonomic variables and electroencephalogram in healthy volunteers. Br J Clin Pharmacol. 1983;15(3):367–374. PubMed CrossRef NLM
- Morin CM. Insomnia Severity Index (ISI) [Database record]. APA PsychNet website. https://doi.org/10.1037/t07115-000. 1993.
- Zimmerman M, Chelminski I, McGlinchey JB, et al. A clinically useful depression outcome scale. Compr Psychiatry. 2008;49(2):131–140. PubMed CrossRef NLM
- Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. PubMed CrossRef NLM
- Fava M, Rush AJ, Thase ME, et al. 15 years of clinical experience with bupropion HCl: from bupropion to bupropion SR to bupropion XL. Prim Care Companion J Clin Psychiatry. 2005;7(3):106–113. PubMed CrossRef NLM
Please sign in or purchase this PDF for $40.
Save
Cite