Lessons Learned at the Interface of Medicine and Psychiatry
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord 2024;26(3):23f03662
Author affiliations are listed at the end of this article.
Have you ever wondered what the acronym PANDAS stands for? Have you ever considered how an infectious disease might lead to neuropsychiatric symptoms? Have you been unsure about how best to evaluate and treat someone with sudden-onset obsessions and compulsions? If so, the following case vignette and discussion will review these concepts and provide an overview of the diagnosis and treatment of pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections (PANDAS).
CASE VIGNETTE
Miss K, a 6-year-old girl, was in her normal state of good health until she developed an upper respiratory infection during the summer before starting second grade. She received a diagnosis of group A streptococcal (GAS) infection from her pediatrician after reviewing the results of a rapid throat culture. She was placed on a 14-day course of amoxicillin. Her sore throat remitted approximately 3 days after beginning the antibiotic, and she completed the antibiotic course as prescribed. Several days after her last antibiotic dose, Miss K became excessively worried about germs; she would wash her hands until they became raw and bled. At night, she would refuse to go to bed until her mother repeatedly promised her that everyone in her family was safe. Although Miss K had been potty trained at the age of 2.5 years, she began to experience nocturnal enuresis. Her mother reported that Miss K developed new worries about leaving the house for fear that she would need to be close to a bathroom. Miss K refused to attend summer camp because she would not have regular access to a sink or a bathroom. Her parents became concerned that her symptoms would adversely impact Miss K’s transition to second grade, and they sought guidance from her pediatrician. A subsequent rapid strep test was negative, and she was referred for psychiatric evaluation. Miss K began seeing a psychologist for cognitive-behavioral therapy (CBT) and received a diagnosis of obsessive compulsive disorder (OCD).
Once Miss K began second grade, her teacher noticed that she was frequently distracted in class. Her psychologist and parents worked with her school to provide Miss K with school-based therapy and accommodations that allowed her to use the restrooms as often as needed, take movement breaks, and sit in the front of the class. Miss K’s symptoms steadily improved until she contracted another GAS infection at the beginning of winter. She experienced a sudden reemergence of OCD symptoms. She feared that whatever her brother touched was contaminated, and thus, Miss K refused to enter rooms where her brother had been. She also exhibited a strong aversion to certain foods and required excessive reassurance from her family that her food was safe to eat. Miss K’s psychologist recommended that she be evaluated by a treatment team that included a pediatric immunologist and a child psychiatrist, who diagnosed Miss K with PANDAS. Another course of antibiotics and a nonsteroidal anti-inflammatory drug (NSAID) was prescribed. Miss K’s symptoms subsided within the next 2 months.
Two years later, her parents reported that Miss K’s symptoms of anxiety returned intermittently but were manageable with ongoing therapy and a low dose of a selective serotonin reuptake inhibitor (SSRI). Despite concerns that Miss K had ongoing problems with attention, she was doing well in school and developed fulfilling social relationships with peers.
DISCUSSION
What Is PANDAS?
PANDAS is a disorder of acute onset with OCD and/or tics in children following a GAS infection.1 Symptom onset is often dramatic and may include other behavioral or cognitive symptoms, including irritability and separation anxiety (Table 1). A related diagnosis (pediatric acute neuropsychiatric syndrome [PANS]) was introduced later to describe the acute onset of either OCD or avoidant restrictive food intake disorder (ARFID) following any infectious trigger (other than GAS) or no infectious trigger at all.2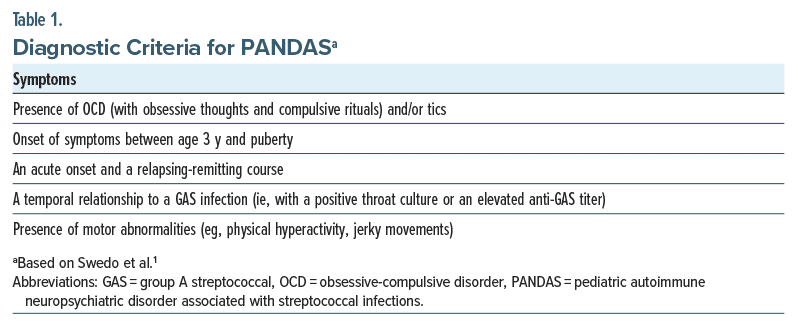
The onset of the OCD or tics symptoms is often accompaprice
nied by additional behavioral symptoms, some of which may differentiate children with PANDAS and children with OCD.3 These include (1) increased anxiety (eg, separation anxiety), (2) emotional lability or depression, (3) irritability or aggressive behavior, (4) new-onset concentration difficulties or worsening of school performance, (5) sleep difficulties (eg, insomnia, night terrors, and a refusal to sleep alone), (6) significant worsening of handwriting or new manifestations of motoric 2 dysfunction (including new-onset hyperactivity, the presence of choreiform movements, pronator drift, or truncal instability), and (7) urinary frequency or an increased urge to urinate or daytime or nighttime enuresis.
What Looks Like PANDAS but Is Not?
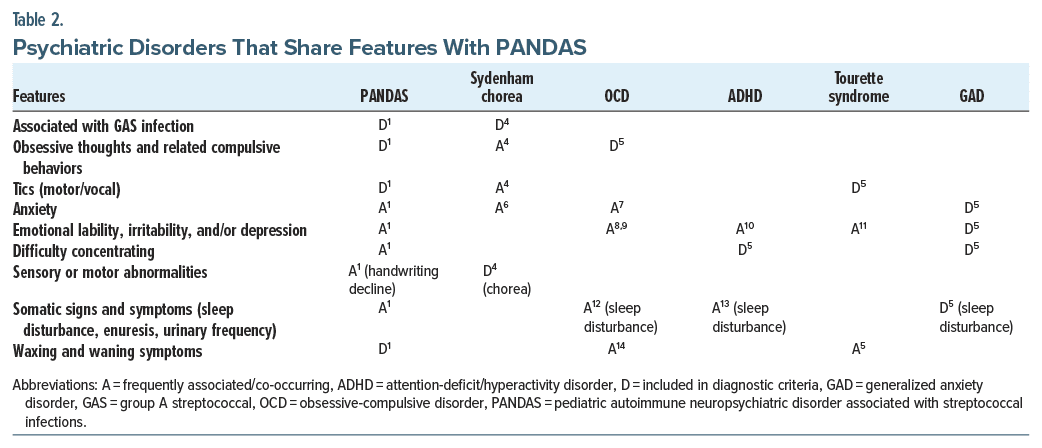
PANDAS represents a hypothesis regarding a child’s etiology of OCD and/or tic, symptoms, but the individual symptoms of PANDAS are shared among multiple psychiatric disorders, including OCD, Tourette syndrome, attention-deficit/hyperactivity disorder (ADHD), and generalized anxiety disorder (Table 24–14). Most children with PANDAS show a progression from symptom onset to severe symptoms in a matter of days to weeks.1 Medical conditions that should be considered during the workup of PANDAS include Sydenham chorea, autoimmune encephalitis, neuropsychiatric lupus, central nervous system vasculitis, systemic autoimmune disease, and Wilson disease.15,16 As PANDAS is both a clinical diagnosis and a diagnosis of exclusion, a thorough psychiatric and medical evaluation is necessary to determine the appropriate treatment.
What Is the Usual Course of PANDAS?
Most children with PANDAS display a relapsing and remitting course, experiencing cycles of symptom exacerbation or improvement with or without treatment. A variety of treatments have been utilized for the treatment of PANDAS symptoms, although few have been investigated using current clinical trial methodology. While the rates of symptom remission are unknown, many children have persistent difficulties that last through childhood and into their adolescence.17–20
What Should the Evaluation of Obsessions, Compulsions, and Tics Include?
If a patient presents with obsessions, compulsions, and tics, a complete medical and psychiatric history should be obtained. A thorough review of the onset and course of symptoms is crucial, including current and previous OCD and tic symptoms and recent and preceding infections. Medical data should be reviewed for autoimmune or immunodeficiency status. An understanding of one’s family history of obsessions, compulsions, and tics is helpful in making the diagnosis and planning treatment, as research suggests that genetic factors may play a role in one’s vulnerability to develop OCD and Tourette syndrome.21 Inquiry into the family history should include questions about neurological, psychiatric, autoimmune, inflammatory, and immunodeficiency syndromes.4
If symptoms of a streptococcal infection are present, a rapid throat swab to assess for GAS is warranted, even in the absence of obsessions, compulsions, or tics.4 If the patient’s symptoms began weeks or months after a strep infection, it is reasonable to obtain an infectious disease panel looking at anti-streptolysin O and anti-DNase B titers. Laboratory analyses are warranted to rule out related and potentially serious inflammatory conditions, such as Sydenham chorea, neurological Lyme disease, and autoimmune encephalitis. If the suspicion for autoimmune encephalitis is high, cerebrospinal fluid analyses may be warranted. Currently, there are no laboratory tests that confirm the diagnosis of PANS/PANDAS, although a workup that includes a complete blood count, comprehensive metabolic panel, erythrocyte sedimentation rate, C-reactive protein, and urinalysis is recommended based on the guidelines of a panel of PANS experts.4 If there are clinical signs of inflammation or autoimmunity, an antinuclear antibody test may be warranted. Finally, brain magnetic resonance imaging and an electroencephalogram are indicated if signs of encephalitis, encephalopathy, or focal neurological symptoms are present, though these tests are often within normal limits for most children presenting with PANS/PANDAS.
What Types of Interventions or Treatments Can Result in Clinical Improvement?
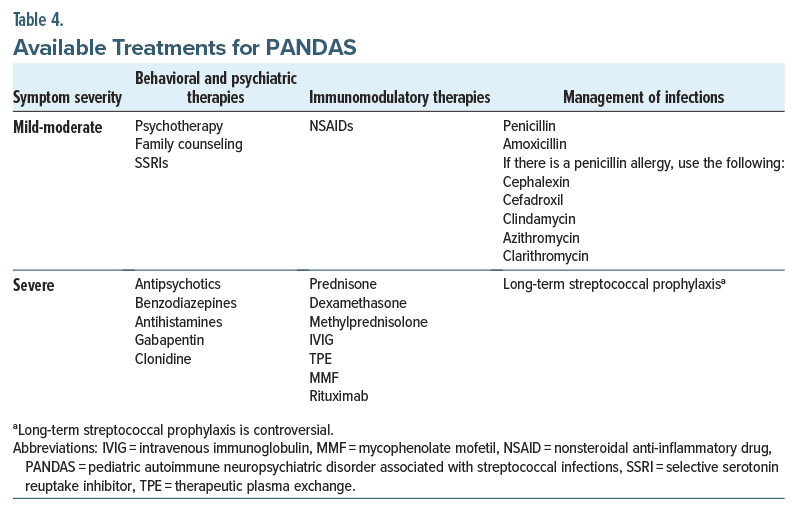
Since constellations of symptoms can differ among patients with PANDAS, therapeutic interventions will vary between patients to meet the patient’s specific needs.22 A 3-pronged approach recommended by a consortium of experts in PANS/PANDAS provides a general framework. The 3 tenets—psychiatric/behavioral treatment, immunomodulatory therapies, and antimicrobial treatment—are briefly summarized (Table 3).5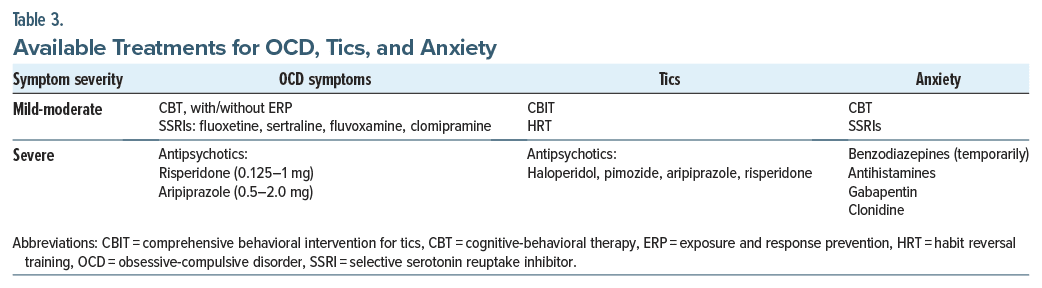
Psychiatric/behavioral treatment. Education, supportive treatment, behavioral therapies, and psychoactive medications can all be beneficial for PANDAS. While physicians should treat a child’s psychiatric symptoms, they must also avoid reacting to temporary psychiatric symptom exacerbations with frequent psychopharmacologic adjustments.22 Neuropsychiatric symptoms frequently change during PANDAS. For example, a child may alternate between severe anxiety and aggression or between emotional lability and depression.
Symptoms that pose a risk to the child or to the child’s family should be addressed urgently with appropriate environmental, educational, and pharmacologic measures. Examples of potentially high-risk symptoms in PANDAS include impulsivity, aggression, refusal to eat or drink, and thoughts of suicide.22 Crisis management in PANDAS is similar to that of other disorders that present with the risk of self harm or the risk of harm to others.
To address symptoms of OCD in PANDAS, CBT is an effective, empirically validated treatment for OCD symptoms in children.23 CBT should utilize exposure and response prevention (ERP) techniques, which aim to desensitize patients to fearful stimuli while promoting the resistance of ritualistic or compulsive behaviors. Two pilot studies24,25 have evaluated the effect of CBT on OCD symptoms in those with PANS/PANDAS. Both studies were limited by a lack of an active control group. One study24 found that 6 of 7 participants were classified as treatment responders (much or very much improved) posttreatment based on a reduction in OCD symptoms. In the second study,25 all 8 participants who completed treatment were considered improved after treatment.
SSRIs can be used to treat moderate-to-severe OCD symptoms and are most effective when used in combination with CBT.23 The following medications have an indication for the treatment of pediatric OCD from the US Food and Drug Administration (FDA): fluoxetine, sertraline, and fluvoxamine, as well as the tricyclic antidepressant clomipramine. SSRIs are the preferred medication for the treatment of OCD based on multiple placebo-controlled clinical trials26,27 and thus can be considered as a treatment in PANS and PANDAS in which OCD symptoms are clinically significant.
The use of antipsychotics should be reserved for those whose OCD and tics are debilitating, as these medications may have significant side effects. Antipsychotics for debilitating OCD include risperidone (with a dose between 0.125 and 1 mg/d) and aripiprazole (with a dose between 0.5 and 2.0 mg/d).22 For treatment-refractory OCD, open-label trials and case studies have shown that augmentation of SSRIs with risperidone or aripiprazole can reduce OCD symptoms in children.28–30
For the treatment of tics, comprehensive behavioral intervention for tics (CBIT) and habit reversal training (HRT) have been efficacious for children31,32 and are recommended for the treatment of tics in children with PANDAS.22 Pharmacologic interventions for tics include a short course of an antipsychotic medication for debilitating tics.32 FDA-approved antipsychotics for Tourette syndrome include haloperidol, pimozide, and aripiprazole. Risperidone has evidence supporting its use for the treatment of tics but is not yet FDA approved for this indication.33
Behavioral and pharmacologic treatments can also be used to treat co-occurring symptoms of PANDAS. Anxiety, including separation anxiety, can be addressed with psychotherapy (such as CBT), pharmacotherapy (such as SSRIs and/or a short course of a benzodiazepine, an antihistamine, gabapentin, or clonidine),22 or both. Symptoms of ADHD may represent either an underlying ADHD or behaviors related to anxiety or OCD. Treatment teams should conduct an evaluation for ADHD. Treatment for ADHD’s symptoms may include stimulants22 and/or behavioral treatments, such as parent management training and social skills training.34
Anti-inflammatory and immunomodulatory therapies. Antibiotics and immunotherapies may dramatically reduce or eliminate psychiatric symptoms,22 as there is strong anecdotal evidence to support the use of anti-inflammatory and immunomodulatory therapies in PANDAS.35 Similar to treatment with behavioral and psychiatric approaches, immunomodulatory treatments for PANDAS should be tailored to the severity of the patient’s symptoms and disease trajectory. For example, a child with mild-to-moderate symptoms may only require treatment with an NSAID, whereas children with a more severe presentation may require treatment with prednisone, dexamethasone, or methylprednisolone.35 One observational study reported a decrease in the duration of symptom exacerbations (termed “flares”) with NSAID therapy in children with PANS (by a mean of 4 weeks compared to those not treated with NSAIDs), lasting a mean of 12.2 weeks. If NSAIDs were administered within 30 weeks of flare onset, the flares were approximately 2.6 weeks shorter than those not treated with NSAIDs.36
In cases of severe and life-threatening disease, providers can also choose to use a corticosteroid-sparing agent (eg, a therapy used in conjunction with a steroid or to replace corticosteroids) in treating PANDAS. Examples include intravenous immunoglobulin (IVIG), therapeutic plasma exchange (TPE), mycophenolate mofetil, or rituximab.35 Placebo-controlled and open-label studies investigating the therapeutic response to IVIG and TPE in PANS/PANDAS have been mixed. One trial compared IVIG (Gammagard 1 g/kg daily on 2 consecutive days), TPE (5 single-volume exchanges over 2 weeks), and placebo, although only the IVIG and placebo conditions were double-blinded. At the 1-month follow up, children who had received either IVIG or TPE showed a significant improvement in OCD symptoms, anxiety, depression, emotional lability, and global functioning compared to baseline.37 In another randomized controlled trial of IVIG versus placebo,38 the IVIG group did not demonstrate a statistically significant improvement in OCD severity compared to the control group. During the following open-label phase of the trial, those treated with IVIG showed a significant improvement in OCD severity compared to baseline.38 In a recent open-label trial of IVIG (OPHELIA 2 g/kg monthly) in children with PANS/ PANDAS, there was a significant reduction in PANS symptoms and OCD severity after 3 months.39 Currently, there is no empirical evidence to support the use of dietary changes to reduce inflammation or the addition of supplements (such as cannabidiol [CBD] oil).
Management of infections. Children with a rapid onset of obsessions, compulsions, or tics (as well as their family members and close contacts) should be observed closely for symptoms of pharyngitis or another streptococcal infection with prompt clinical evaluation and diagnostic testing when appropriate.4 Antimicrobial treatment is indicated for all individuals with pharyngeal GAS. The first-line treatment is oral or intramuscular penicillin.40 Amoxicillin is often prescribed to younger children due to its palatability. In the case of a child having a penicillin allergy, cephalexin, cefadroxil, clindamycin, azithromycin, or clarithromycin can be prescribed. While data from controlled clinical trials are lacking, clinicians have noticed that many children with recent-onset PANDAS experience a reduction in neuropsychiatric symptoms shortly after starting antimicrobial treatment for an acute GAS infection.41 Secondary antimicrobial prophylaxis may be beneficial for preventing neural injury from future GAS-associated exacerbations of PANDAS. At present, there is insufficient evidence to support the use of long-term GAS prophylaxis for children with PANDAS. However, at times, practitioners initiate long-term GAS prophylaxis in the most severely affected children or for those with frequent, multiple GAS associated neuropsychiatric exacerbations.41 If a clinician recommends embarking on a course of GAS prophylaxis, the antimicrobial regimen should be based on guidelines developed for the prevention of rheumatic fever.40 Systematic reviews of treatment options for PANDAS (Table 4) have failed to show that adenotonsillectomies have a statistically significant impact on symptom improvement.42,43
Who Is at Risk for Developing PANDAS?
Diagnostic criteria specify that PANDAS arises in children during the years that they are most susceptible to developing a streptococcal infection (typically between the ages of 5 and 15 years).44 However, case reports document that some people develop symptoms of PANDAS in young adulthood.45–48 Besides age, little is known about the demographic risk factors for PANDAS. While the research on children with PANDAS is skewed toward white, non-Latino children,49–51 it is not known whether this is consistent with GAS infection rates of different demographic groups within the US population, as the Centers for Disease Control does not publish these data. As it is the infectious agent that puts the individual at risk for developing PANDAS, it is likely that all racial, ethnic, and demographic groups are at risk for developing PANDAS. Little is also known about genetic risk factors that influence one’s susceptibility to PANDAS; however, both clinical and population-based studies suggest that children with PANS/PANDAS have a strong family history of autoimmune diseases.52,53
How Common Is PANDAS?
To date, no epidemiological studies of PANDAS or PANS have been conducted. While the prevalence of PANDAS is unknown, it is likely that a subset of the 2%–3% of children worldwide who are diagnosed with OCD 54 and the 0.3%–0.8% of children who are diagnosed with Tourette syndrome also meet criteria for PANDAS.55,56
What Evidence Suggests That PANDAS Has an Immunologic Etiology?
The specific etiology of PANDAS remains unknown, and it is unclear whether PANDAS has a separate etiology from pediatric OCD and tic disorders or whether it is a subgroup of these disorders. The hypothesis that a subset of children have symptoms of OCD triggered by a GAS is supported by 2 population-based studies utilizing the Danish Health Registry, which reports a significantly increased risk of developing OCD following either a GAS infection or another bacterial infection.57,58 In addition, antibodies from children with PANDAS specifically bind to a population of neurons present in the basal ganglia (cholinergic interneurons), a brain structure implicated in OCD symptoms.59 Following treatment with IVIG, OCD symptom improvement was observed to correlate with reduced antibody binding to these neurons,59 suggesting that the etiology of PANDAS may be similar to other types of autoimmune encephalitis. While in need of expansion and replication, the discovery of these autoantibodies may result in future therapies for these conditions.
Which Brain Territories Are Associated With PANDAS?
The basal ganglia have been implicated in meta analyses of OCD and tic disorders.60,61 Therefore, neuropsychiatric symptoms seen in PANDAS may be related to basal ganglia dysfunction. Evidence of this hypothesis has come from both molecular and cognitive neuroscience. Antibodies from children with PANDAS specifically bind to cholinergic interneurons in the basal ganglia and cause disruption in the corticostriatal pathway.59 Neuroimaging studies comparing healthy children and children with PANDAS showed increased volumes of basal ganglia nuclei (ie, caudate, putamen, and globus pallidus) in children with PANDAS.62,63 Diffuse neurological changes may also be present, as one study64 showed differences in voxel-based morphometry between children with PANDAS and healthy controls in the cortex, subcortex, and cerebellum.
What Type of Help in School and in Social Situations Do Those Afflicted With PANDAS Require?
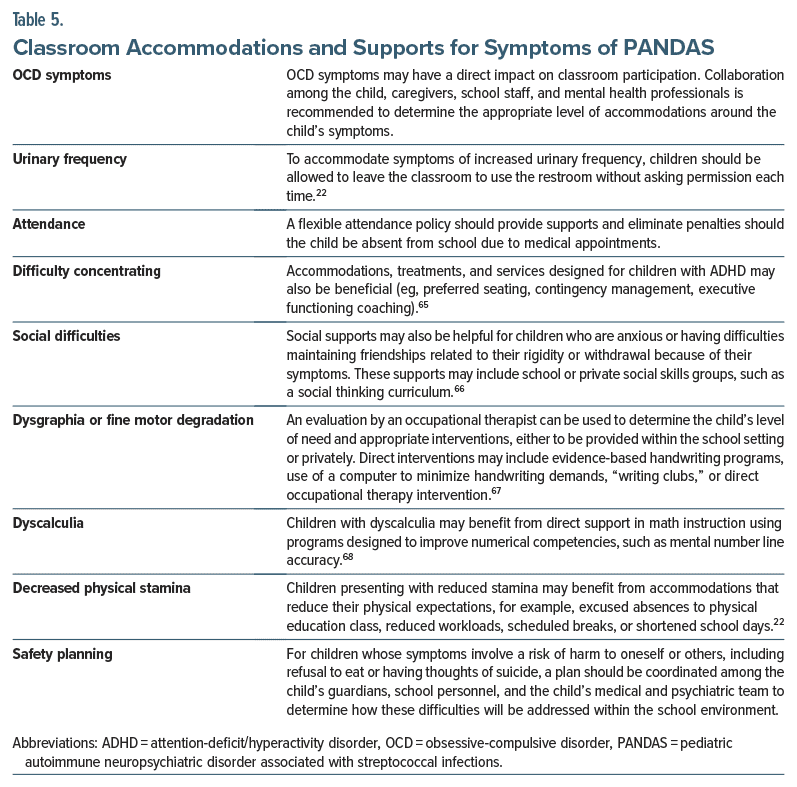
Many children with PANDAS have some degree of functional impairment due to the myriad symptoms that can adversely impact functioning at school, in the home, and in social settings.50 Therefore, children with PANDAS may require some level of school-based accommodation or interventions. To facilitate the administration of school based accommodations or services, children may benefit from working with their school-based team to implement a 504 plan (section 504 of the Rehabilitation Act) or an individualized education plan. Academic difficulties can occur when a child is absent from school due to medical appointments or secondary symptoms, such as school avoidance, poor attention, and dysgraphia. Physicians can help their patients receive school-based accommodations or support by providing a doctor’s note specifying the patient’s diagnosis and the recommended accommodations, services, or testing. Other accommodations or direct services should be considered to address specific symptoms (Table 5).
Why Is the Diagnosis of PANDAS Controversial?
Despite decades of research, clinical criteria for a definitive PANDAS diagnosis have yet to be established. The sheer diversity of symptoms combined with the improbability of obtaining a GAS test around the onset of neuropsychiatric symptoms poses a challenge in establishing specific diagnostic criteria. Parents may compensate for their child’s new neuropsychiatric symptoms and therefore not seek psychiatric care at symptom onset. Additionally, it is possible that a child had a strep infection in the absence of a documented positive GAS test. Furthermore, some studies may not accurately account for the temporal association with a GAS infection. Once established, reliable biomarkers will assist in confirming a PANDAS diagnosis.69
What Tools Can Be Used in the Primary Care Setting to Assess for OCD and Tic Severity?
There are a host of reliable and valid measures to assess for OCD and tic severity. The Children’s Yale Brown Obsessive-Compulsive Scale70 has parent-report and child-report questionnaires that assess the severity of obsessions and compulsions. For tics, the Parent Tic Questionnaire71 can be used to screen for the presence and severity of motor and vocal tics. Questionnaires designed to be completed by children and/or their parents may help to screen for the presence of ancillary symptoms of PANDAS. The Screen for Child Anxiety Related Emotional Disorders72 has parent- and child-report versions and screens for symptoms of anxiety, including separation. To assess for eating disturbances, including an ARFID, children can complete the Eating Disorders in Youth Questionnaire.73 For input from teachers as well as parents, the National Initiative for Children’s Healthcare Quality Vanderbilt Assessment,74 an assessment for ADHD, can also be used to screen for symptoms related to anxiety, conduct problems, attentional weaknesses, and hyperactivity in children 6–12 years of age.
CONCLUSION
PANDAS is a disorder of acute onset with OCD and/or tics in children following a GAS infection; other behavioral or cognitive symptoms, including irritability, and separation anxiety are also apparent. Awareness by health care providers, teachers, and parents will facilitate timely screening, recognition, and appropriate treatment, thereby mitigating undue suffering and impaired academic and social functioning.
Article Information
Published Online: May 23, 2024. https://doi.org/10.4088/PCC.23f03662
© 2024 Physicians Postgraduate Press, Inc.
Submitted: October 24, 2023; accepted December 27, 2023.
To Cite: O’Dor SL, Kuhn AJ, Williams KA, et al. Diagnosing and treating pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections. Prim Care Companion CNS Disord. 2024;26(3):23f03662.
Author Affiliations: Department of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts (O’Dor, Kuhn, Williams, Guerette, Stern); Department of Psychiatry, Harvard Medical School, Boston, Massachusetts (O’Dor, Williams, Stern); Veterans Affairs Boston/Harvard South Shore, Brockton, Massachusetts (Kuhn); Department of Pediatric Gastroenterology, Massachusetts General Hospital, Boston, Massachusetts (Guerette).
Corresponding Author: Sarah L. O’Dor, PhD, Department of Psychiatry, Massachusetts General Hospital, Yawkey Outpatient Center, Suite 6A, 55 Fruit St, Boston, MA 02114 ([email protected]).
Relevant Financial Relationships: Dr O’Dor has received grant funding from International OCD Foundation. Dr Williams has served on the advisory boards of Pfizer and Octapharma and has received grant funding from PANDAS Network, International OCD Foundation, and Fidelity Biosciences Research Initiative. Drs Kuhn, Stern, and Ms Guerette report no relevant financial relationships.
Funding/Support: None.
ORCID: Sarah L. O’Dor: https://orcid.org/0000-0001-6141-1266
Clinical Points
- Pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections (PANDAS) is a disorder of acute onset with obsessive-compulsive disorder and/or tics in children following a group A streptococcal infection, often with other behavioral or cognitive symptoms, including irritability and separation anxiety.
- Symptoms of PANDAS overlap with those of many psychiatric and medical conditions; most children experience a relapsing and remitting course, and some have persistent difficulties that last through childhood and into their adolescence.
- Since PANDAS is a diagnosis of exclusion, a thorough psychiatric and medical evaluation is necessary to make the correct diagnosis and institute appropriate treatment.
- Treatment of PANDAS should involve a 3-pronged approach: psychiatric/behavioral treatment, immunomodulatory therapies, and antimicrobial treatment.
References (74)

- Swedo SE, Leonard HL, Garvey M, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998;155(2):264–271. PubMed CrossRef
- Swedo SE, Leckman JF, Rose NR. From research subgroup to clinical syndrome: modifying the PANDAS criteria to describe PANS (pediatric acute-onset neuropsychiatric syndrome). Pediatr Ther. 2012;2(2):113.
- Bernstein GA, Victor AM, Pipal AJ, et al. Comparison of clinical characteristics of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections and childhood obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2010;20(4):333–340. PubMed CrossRef
- Cardoso F. Chapter 14–Sydenham’s chorea. In: Weiner WJ, Tolosa E, eds. Handbook of Clinical Neurology. Elsevier; 2011:221–229. Vol 100.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Association; 2013.
- Punukollu M, Mushet N, Linney M, et al. Neuropsychiatric manifestations of Sydenham’s chorea: a systematic review. Dev Med Child Neurol. 2016;58(1):16–28. PubMed CrossRef
- Comer JS, Kendall PC, Franklin ME, et al. Obsessing/worrying about the overlap between obsessive-compulsive disorder and generalized anxiety disorder in youth. Clin Psychol Rev. 2004;24(6):663–683. PubMed CrossRef
- Storch EA, Jones AM, Lack CW, et al. Rage attacks in pediatric obsessive compulsive disorder: phenomenology and clinical correlates. J Am Acad Child Adolesc Psychiatry. 2012;51(6):582–592. PubMed CrossRef
- Berman NC, Shaw AM, Curley EE, et al. Emotion regulation and obsessive compulsive phenomena in youth. J Obsessive Compuls Relat Disord. 2018;19:44–49.
- Banaschewski T, Jennen-Steinmetz C, Brandeis D, et al. Neuropsychological correlates of emotional lability in children with ADHD. J Child Psychol Psychiatry. 2012;53(11):1139–1148. PubMed CrossRef
- Conte G, Valente F, Fioriello F, et al. Rage attacks in Tourette syndrome and chronic tic disorder: a systematic review. Neurosci Biobehav Rev. 2020;119:21–36. PubMed CrossRef
- Jaspers-Fayer F, Lin SY, Belschner L, et al. A case-control study of sleep disturbances in pediatric obsessive-compulsive disorder. J Anxiety Disord. 2018;55:1–7. PubMed CrossRef
- Shen C, Luo Q, Chamberlain SR, et al. What is the link between attention-deficit/ hyperactivity disorder and sleep disturbance? A multimodal examination of longitudinal relationships and brain structure using large-scale population-based cohorts. Biol Psychiatry. 2020;88(6):459–469. PubMed CrossRef
- Ravizza L, Maina G, Bogetto F. Episodic and chronic obsessive-compulsive disorder. Depress Anxiety. 1997;6(4):154–158. PubMed CrossRef
- Chang K, Frankovich J, Cooperstock M, et al. Clinical evaluation of youth with pediatric acute-onset neuropsychiatric syndrome (PANS): recommendations from the 2013 PANS consensus conference. J Child Adolesc Psychopharmacol. 2015;25(1):3–13. PubMed CrossRef
- Swedo SE, Frankovich J, Murphy TK. Overview of treatment of pediatric acute onset neuropsychiatric syndrome. J Child Adolesc Psychopharmacol. 2017;27(7):562–565. PubMed CrossRef
- Murphy ML, Pichichero ME. Prospective identification and treatment of children with pediatric autoimmune neuropsychiatric disorder associated with group A streptococcal infection (PANDAS). Arch Pediatr Adolesc Med. 2002;156(4):356–361. PubMed CrossRef
- Kurlan R, Johnson D, Kaplan EL, et al. Streptococcal infection and exacerbations of childhood tics and obsessive-compulsive symptoms: a prospective blinded cohort study. Pediatrics. 2008;121(6):1188–1197. PubMed CrossRef
- Frankovich J, Thienemann M, Rana S, et al. Five youth with pediatric acute-onset neuropsychiatric syndrome of differing etiologies. J Child Adolesc Psychopharmacol. 2015;25(1):31–37. PubMed CrossRef
- Leon J, Hommer R, Grant P, et al. Longitudinal outcomes of children with pediatric autoimmune neuropsychiatric disorder associated with streptococcal infections (PANDAS). Eur Child Adolesc Psychiatry. 2018;27(5):637–643. PubMed CrossRef
- Radonjić NV, Hess JL, Rovira P, et al. Structural brain imaging studies offer clues about the effects of the shared genetic etiology among neuropsychiatric disorders. Mol Psychiatry. 2021;26(6):2101–2110. PubMed
- Thienemann M, Murphy T, Leckman J, et al. Clinical management of pediatric acute-onset neuropsychiatric syndrome: Part I—psychiatric and behavioral interventions. J Child Adolesc Psychopharmacol. 2017;27(7):566–573. PubMed CrossRef
- Öst LG, Riise EN, Wergeland GJ, et al. Cognitive behavioral and pharmacological treatments of OCD in children: a systematic review and meta-analysis. J Anxiety Disord. 2016;43:58–69. PubMed
- Storch EA, Murphy TK, Geffken GR, et al. Cognitive-behavioral therapy for PANDAS-related obsessive-compulsive disorder: findings from a preliminary waitlist controlled open trial. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1171–1178. PubMed CrossRef
- Nadeau JM, Jordan C, Selles RR, et al. A pilot trial of cognitive-behavioral therapy augmentation of antibiotic treatment in youth with pediatric acute-onset neuropsychiatric syndrome-related obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2015;25(4):337–343. PubMed CrossRef
- Geller DA, Biederman J, Stewart SE, et al. Which SSRI? A meta-analysis of pharmacotherapy trials in pediatric obsessive-compulsive disorder. Am J Psychiatry. 2003;160(11):1919–1928. PubMed CrossRef
- Pediatric OCD Treatment Study POTS Team. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292(16):1969–1976. PubMed CrossRef
- Fitzgerald KD, Stewart CM, Tawile V, et al. Risperidone augmentation of serotonin reuptake inhibitor treatment of pediatric obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 1999;9(2):115–123. PubMed CrossRef
- Ercan ES, Ardic UA, Ercan E, et al. A promising preliminary study of aripiprazole for treatment-resistant childhood obsessive-compulsive disorder. J Child Adolesc Psychopharmacol. 2015;25(7):580–584. PubMed CrossRef
- Akyol Ardic U, Ercan ES, Kutlu A, et al. Successful treatment response with aripiprazole augmentation of SSRIs in refractory obsessive-compulsive disorder in childhood. Child Psychiatry Hum Dev. 2017;48(5):699–704. PubMed CrossRef
- Shou S, Li Y, Fan G, et al. The efficacy of cognitive behavioral therapy for tic disorder: a meta-analysis and a literature review. Front Psychol. 2022;13:851250. PubMed CrossRef
- McGuire JF, Piacentini J, Brennan EA, et al. A meta-analysis of behavior therapy for Tourette syndrome. J Psychiatr Res. 2014;50:106–112. PubMed CrossRef
- Budman CL. The role of atypical antipsychotics for treatment of Tourette’s syndrome: an overview. Drugs. 2014;74(11):1177–1193. PubMed CrossRef
- Caye A, Swanson JM, Coghill D, et al. Treatment strategies for ADHD: an evidence-based guide to select optimal treatment. Mol Psychiatry. 2019;24(3):390–408. PubMed CrossRef
- Frankovich J, Swedo S, Murphy T, et al. Clinical management of pediatric acute onset neuropsychiatric syndrome: Part II—use of immunomodulatory therapies. J Child Adolesc Psychopharmacol. 2017;27(7):574–593. PubMed CrossRef
- Brown KD, Farmer C, Freeman GM Jr, et al. Effect of early and prophylactic nonsteroidal anti-inflammatory drugs on flare duration in pediatric acute-onset neuropsychiatric syndrome: an observational study of patients followed by an academic community-based pediatric acute-onset neuropsychiatric syndrome clinic. J Child Adolesc Psychopharmacol. 2017;27(7):619–628. PubMed CrossRef
- Perlmutter SJ, Leitman SF, Garvey MA, et al. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet. 1999;354(9185):1153–1158. PubMed CrossRef
- Williams KA, Swedo SE, Farmer CA, et al. Randomized, controlled trial of intravenous immunoglobulin for pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections. J Am Acad Child Adolesc Psychiatry. 2016;55(10):860–867.e2. PubMed CrossRef
- Hajjari P, Oldmark MH, Fernell E, et al. Paediatric acute-onset neuropsychiatric syndrome (PANS) and intravenous immunoglobulin (IVIG): comprehensive open label trial in ten children. BMC Psychiatry. 2022;22(1):535. PubMed
- Gerber MA, Baltimore RS, Eaton CB, et al. Prevention of rheumatic fever and diagnosis and treatment of acute streptococcal pharyngitis: a scientific statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease in the Young, the Interdisciplinary Council on Functional Genomics and Translational Biology, and the Interdisciplinary Council on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2009;119(11):1541–1551. PubMed CrossRef
- Cooperstock MS, Swedo SE, Pasternack MS, et al. Clinical management of pediatric acute-onset neuropsychiatric syndrome: Part III—treatment and prevention of infections. J Child Adolesc Psychopharmacol. 2017;27(7):594–606. PubMed CrossRef
- Farhood Z, Ong AA, Discolo CM. PANDAS: a systematic review of treatment options. Int J Pediatr Otorhinolaryngol. 2016;89:149–153. PubMed CrossRef
- Cocuzza S, Maniaci A, La Mantia I, et al. Obsessive-compulsive disorder in PANS/ PANDAS in children: in search of a qualified treatment—a systematic review and metanalysis. Children (Basel). 2022;9(2):155. PubMed
- Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis. 2012;55(10):e86–e102. PubMed CrossRef
- Bodner SM, Morshed SA, Peterson BS. The question of PANDAS in adults. Biol Psychiatry. 2001;49(9):807–810. PubMed CrossRef
- Krouse A, Li H, Krenzer JA, et al. Plasmapheresis, rituximab, and ceftriaxone provided lasting improvement for a 27-year-old adult male with pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections (PANDAS). Case Rep Psychiatry. 2021;2021:8697902. PubMed CrossRef
- Martinelli P, Ambrosetto G, Minguzzi E, et al. Late-onset PANDAS syndrome with abdominal muscle involvement. Eur Neurol. 2002;48(1):49–51. PubMed CrossRef
- Monasterio E, Mulder RT, Marshall TD. Obsessive-compulsive disorder in post streptococcal infection. Aust N Z J Psychiatry. 1998;32(4):579–581. PubMed CrossRef
- Murphy TK, Patel PD, McGuire JF, et al. Characterization of the pediatric acute onset neuropsychiatric syndrome phenotype. J Child Adolesc Psychopharmacol. 2015;25(1):14–25. PubMed CrossRef
- Calaprice D, Tona J, Parker-Athill EC, et al. A survey of pediatric acute-onset neuropsychiatric syndrome characteristics and course. J Child Adolesc Psychopharmacol. 2017;27(7):607–618. PubMed CrossRef
- Gabbay V, Coffey BJ, Babb JS, et al. Pediatric autoimmune neuropsychiatric disorders associated with streptococcus: comparison of diagnosis and treatment in the community and at a specialty clinic. Pediatrics. 2008;122(2):273–278. PubMed CrossRef
- Mataix-Cols D, Frans E, Perez-Vigil A, et al. A total-population multigenerational family clustering study of autoimmune diseases in obsessive-compulsive disorder and Tourette’s/chronic tic disorders. Mol Psychiatry. 2018;23(7):1652–1658. PubMed CrossRef
- Gromark C, Harris RA, Wickstrom R, et al. Establishing a pediatric acute-onset neuropsychiatric syndrome clinic: baseline clinical features of the pediatric acute onset neuropsychiatric syndrome cohort at Karolinska Institutet. J Child Adolesc Psychopharmacol. 2019;29(8):625–633. PubMed CrossRef
- Zohar AH. The epidemiology of obsessive-compulsive disorder in children and adolescents. Child Adolesc Psychiatr Clin N Am. 1999;8(3):445–460. PubMed
- Bitsko RH, Claussen AH, Lichstein J, et al. Mental health surveillance among children – United States, 2013–2019. MMWR Suppl. 2022;71(2):1–42.
- Knight T, Steeves T, Day L, et al. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol. 2012;47(2):77–90. PubMed CrossRef
- Orlovska S, Vestergaard CH, Bech BH, et al. Association of streptococcal throat infection with mental disorders: testing key aspects of the PANDAS hypothesis in a nationwide study. JAMA Psychiatry. 2017;74(7):740–746. PubMed
- Köhler-Forsberg O, Petersen L, Gasse C, et al. A nationwide study in Denmark of the association between treated infections and the subsequent risk of treated mental disorders in children and adolescents. JAMA Psychiatry. 2019;76(3):271–279. PubMed
- Xu J, Liu RJ, Fahey S, et al. Antibodies from children with PANDAS bind specifically to striatal cholinergic interneurons and alter their activity. Am J Psychiatry. 2021;178(1):48–64. PubMed
- Boedhoe PS, Schmaal L, Abe Y, et al. Distinct subcortical volume alterations in pediatric and adult OCD: a worldwide meta-and mega-analysis. Am J Psychiatry. 2017;174(1):60–69. PubMed
- Wan X, Zhang S, Wang W, et al. Gray matter abnormalities in Tourette syndrome: a meta-analysis of voxel-based morphometry studies. Transl Psychiatry. 2021;11(1):287. PubMed CrossRef
- Giedd JN, Rapoport JL, Garvey MA, et al. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am J Psychiatry. 2000;157(2):281–283. PubMed CrossRef
- Zheng J, Frankovich J, McKenna ES, et al. Association of pediatric acute-onset neuropsychiatric syndrome with microstructural differences in brain regions detected via diffusion-weighted magnetic resonance imaging. JAMA Netw Open. 2020;3(5):e204063. PubMed CrossRef
- Cabrera B, Romero-Rebollar C, Jimenez-Angeles L, et al. Neuroanatomical features and its usefulness in classification of patients with PANDAS. CNS Spectr. 2019;24(5):533–543. PubMed CrossRef
- Fabiano GA, Pyle K. Best practices in school mental health for attention-deficit/ hyperactivity disorder: a framework for intervention. School Ment Health. 2019;11:72–91.
- Kuypers LM. The Zones of Regulation: A Curriculum Designed to Foster Self Regulation and Emotional Control. Think Social Publishing; 2011.
- Chung PJ, Patel DR, Nizami I. Disorder of written expression and dysgraphia: definition, diagnosis, and management. Transl Pediatr. 2020;9(suppl 1):S46–S54. PubMed CrossRef
- Moeller K, Fischer U, Cress U, et al. Diagnostics and intervention in developmental dyscalculia: current issues and novel perspectives. In: Breznitz Z, Rubinsten O, Molfese VJ, eds, et al. Reading, Writing, Mathematics and the Developing Brain: Listening to Many Voices. Springer; 2012:233–275.
- Murphy TK, Gerardi DM, Parker-Athill EC. The PANDAS controversy: why (and how) is it still unsettled? Curr Dev Disord Rep. 2014;1:236–244.
- Storch EA, Murphy TK, Adkins JW, et al. The children[R8S2Q1M7]s Yale-Brown Obsessive-Compulsive Scale: psychometric properties of child-and parent-report formats. J Anxiety Disord. 2006;20(8):1055–1070. PubMed CrossRef
- Chang S, Himle MB, Tucker BT, et al. Initial psychometric properties of a brief parent-report instrument for assessing tic severity in children with chronic tic disorders. Child Fam Behav Ther. 2009;31(3):181–191.
- Birmaher B, Brent DA, Chiappetta L, et al. Psychometric properties of the screen for child anxiety related emotional disorders (SCARED): a replication study. J Am Acad Child Adolesc Psychiatry. 1999;38(10):1230–1236. PubMed CrossRef
- Hilbert A, van Dyck Z. Eating Disorders in Youth-Questionnaire. University of Leipzig; 2016.
- American Academy of Pediatrics and National Initiative for Children’s Healthcare Quality. NICHQ Vanderbilt Assessment Scale. NICHQ; 2002.
This PDF is free for all visitors!
Save
Cite