LESSONS LEARNED AT THE INTERFACE OF MEDICINE AND PSYCHIATRY
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord 2022;24(6):21f03154
To cite: Petriceks AH, Stern TA. Diagnosis and management of hypertensive emergency in a patient with generalized anxiety disorder. Prim Care Companion CNS Disord. 2022;24(6):21f03154.
To share: https://doi.org/10.4088/PCC.21f03154
© 2022 Physicians Postgraduate Press, Inc.
aHarvard Medical School, Boston, Massachusetts
bDepartment of Psychiatry, Massachusetts General Hospital, Boston, Massachusetts
*Corresponding author: Aldis H. Petriceks, BA, Harvard Medical School, 25 Shattuck St, Boston, MA 02115 ([email protected]).
Have you ever wondered what signs and symptoms determine whether a patient has a hypertensive emergency? Have you been uncertain as to which conditions or circumstances might have contributed to, or caused, life-threatening hypertension? Have you wondered which neuropsychiatric manifestations can arise during a hypertensive crisis? Have you been unsure about how best to treat a hypertensive crisis acutely and over the longer term? If you have, the following case vignette and discussion should prove useful.
CASE VIGNETTE
Mr A, a 50-year-old man with a history of type 2 diabetes mellitus (on metformin and glyburide), generalized anxiety disorder (on sertraline), and poorly controlled hypertension (on metoprolol and lisinopril), presented to the emergency department with an altered mental status.
On the evening of admission, Mr A finished dinner and sat down on his couch to watch television. He had been extremely stressed by his high-pressure finance job and had just told his wife he worried he would have a panic attack “one of these days.” His stress and long working hours contributed to being disorganized at home, and he episodically missed doses of his medications for anxiety and blood pressure (BP) control. One hour after dinner, his wife found him lethargic, difficult to rouse, confused, and complaining semi-intelligibly about a headache. She called 911 for assistance. When the emergency medical services arrived, they found him on the couch. There was no incontinence, emesis, or tongue laceration. He was barely communicative and appeared to have difficulty breathing.
Mr A was taken to the emergency department, where his BP was 220/136 mm Hg on an automated BP cuff placed over his right arm. His oxygen saturation was 93% on room air, and his body mass index (BMI) was 33 kg/m2. He had similar BP readings on his left arm and right thigh. His wife reported that he had no history of seizures and was not taking any new medications. He was oriented to person but not to time or place, and a chest x-ray revealed evidence of bilateral basilar pulmonary edema. Cardiac auscultation revealed an S3 sound over the apex. There was evidence of lower extremity edema, as well as jugular venous distension. An electrocardiogram (ECG) showed sinus tachycardia and evidence of left ventricular hypertrophy, but there were no ST segment changes. A head computed tomography (CT) scan without contrast showed no signs of infarction or hemorrhage. Ocular findings were negative for retinopathy.
Given his history, vital signs, and findings on the physical examination, the resident caring for Mr A suspected a hypertensive emergency, as this diagnosis would explain his cardiac and pulmonary symptoms, as well as his altered mental status. However, he was uncertain about the etiology and whether Mr A’s history of anxiety and recent increase in stress levels may have precipitated this episode. The resident initiated emergency care for Mr A and deferred an exploration of etiology until he had stabilized. An arterial catheter was placed in the radial artery for more accurate BP measurement, and treatment options were considered.
DISCUSSION
What Is a Hypertensive Emergency?
Hypertensive emergency (previously labeled as a hypertensive crisis) is a subset of acute severe hypertension, which is defined as an elevated systolic BP ≥ 180 mm Hg and a diastolic BP ≥ 120 mm Hg.1,2 What distinguishes a hypertensive emergency from hypertensive urgency—the other subset of acute severe hypertension—is evidence of end-organ damage.2 In other words, if a patient presents with a BP ≥ 180/≥120 mm Hg and shows signs of end-organ damage, they are likely suffering from a hypertensive emergency. If a patient presents with the same BP but does not show signs of end-organ damage, they are likely suffering from hypertensive urgency. In addition to these definitions, it is important to note, as did Peixoto,2 that both “absolute blood-pressure level and the pace of rise determine the risk of acute hypertension-mediated target-organ damage.”(p1,843) As such, it may be helpful for clinicians not to fixate too heavily on hard cutoff BP measurements and to attend closely to signs, symptoms, and other laboratory values and findings, perhaps especially in older adults.
What Are the Signs and Symptoms of a Hypertensive Emergency?
The foundational sign of hypertensive emergency is a BP ≥ 180/≥ 120 mm Hg. Beyond this, patients with a hypertensive emergency may present with a wide variety of signs and symptoms of end-organ damage, depending on the organ systems affected.
One organ system often affected is the cardiovascular system.3 As a result of the hypertensive emergency, the patient may experience myocardial ischemia, myocardial infarction (MI), heart failure, or an aortic dissection.3 Signs of myocardial ischemia may include substernal chest pain, tightness, or pressure, with or without exertion. Signs of an MI may include similar pain with or without radiation to the shoulder or an upper extremity, as well as nausea, vomiting, diaphoresis, and heart failure. Heart failure itself may manifest as shortness of breath, dyspnea with or without exertion, orthopnea, an S3 heart sound on cardiac auscultation, bilateral basilar crackles on lung auscultation, lower extremity edema, jugular venous distension, decreased appetite or early satiety, and hepatosplenomegaly. As such, it is important for clinicians to check for distension of the jugular veins, to percuss for liver size, to assess for hepatojugular reflux, and to examine the lower extremities for edema and ankle pulses. Aortic dissection may present with severe, sharp, “tearing” chest pain with radiation to the back, often unlike anything the patient has experienced before. Diffuse microvascular injury may also occur, leading to complications of retinopathy, damage to red blood cells and platelets, and acute kidney injury.2 Related to retinopathy, example findings of the fundoscopic examination in a hypertensive emergency were shown and described by Kumari et al,4 including soft exudates, hard exudates, macular star, and papilledema. Ocular findings may also assist in the diagnosis of cases of hypertensive crisis related to drug use, such as through the observation of miosis secondary to cocaine use.
The renal system is also susceptible to damage from a hypertensive emergency. When such damage does occur, it is known as acute hypertensive nephrosclerosis.1 Patients with acute hypertensive nephrosclerosis often have hematuria and an elevated serum creatinine level.1,5 Pathologically, the renal arterioles often show evidence of fibroid necrosis.1
Apart from the heart and kidneys, many of the most concerning consequences of a hypertensive emergency are found in the brain.6 Hypertensive encephalopathy, cerebral ischemia, and cerebral hemorrhage are of greatest concern. Hypertensive encephalopathy is distinguished by the onset of headache, lethargy or somnolence, an altered mental status, visual disturbances, or seizures as a result of failed autoregulation of cerebral BP.3 Ischemic stroke may present with the sudden onset of focal neurologic deficits (eg, hemiparesis, hemisensory loss, cortical signs) as well as hypodense lesions on a noncontrast computerized tomography (CT) scan.7 Intracranial hemorrhage may present with headache, vomiting, and an altered mental status that progresses over the course of minutes to hours. Patients with smaller hemorrhages may have minor symptoms or be asymptomatic without any clear indicators, although eventually evidence of progressive stroke-like neurologic deficits may arise. As with ischemic stroke, specific deficits will correlate with localization of the lesion. A noncontrast head CT will show evidence of an intracerebral hemorrhage almost immediately after the original insult. The most notable sign is a hyperdense lesion, possibly surrounded by edema or a shift of the brain. A magnetic resonance imaging scan of the brain will typically show hypointense lesions on T2-weighted imaging.
What Is the Mechanism Behind Hypertensive Encephalopathy?
While the systemic vasculature of the human body often experiences a wide range of arterial pressures, the brain typically maintains near-constant perfusion pressures through adaptive changes to its arteriolar tone. The perfusion pressure within the cerebral vasculature typically remains within 60–160 mm Hg.8 When the systemic mean arterial pressure (MAP) rises above this range, cerebral arteriolar vasoconstriction reduces blood flow to the brain, and the reverse occurs when the systemic MAP drops below 60–160 mm Hg.8
In a hypertensive emergency, however, cerebral BP rises far more quickly and to a far greater extent than can be accommodated by cerebral arteriolar vasoconstriction. This causes increased hydrostatic pressure and eventual movement of blood from the vasculature into the brain parenchyma.8 The entry of blood into the brain parenchyma then causes cerebral edema, which leads directly to the presentation of an altered mental status, headache, visual disturbances, and possible seizures that are seen with hypertensive encephalopathy.8,9
What Should the Evaluation of Acute Severe Hypertension Entail?
As highlighted in a review,2 the approach to acute severe hypertension is divided into 3 parts. First, the clinician should ensure that the BP readings are accurate. Second, the patient should be evaluated for potential contributors to acute severe hypertension and assess for signs and symptoms of end-organ damage. Third, treatment should be provided based on the findings of the first 2 steps.
Clinicians should assess for hypertension using the guidelines provided by the American Heart Association.10 This assessment entails BP measurements in both arms and the thigh.2,10 Auscultative and automated oscillometric measurements should be avoided in cases of suspected end-organ damage, given their susceptibility toward inaccurate readings.2 Placement of an arterial catheter should be used in these settings.
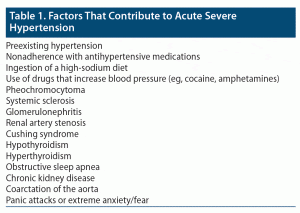
Once an accurate BP reading is obtained and found to be suggestive of acute severe hypertension, the clinician should evaluate for precipitating factors and evidence of end-organ damage (Table 1). Studies2,11 suggest that most patients who present with acute severe hypertension have a preexisting diagnosis of hypertension and are being managed for their condition. Many patients do not present with a clear precipitant; but, for those who do, nonadherence to antihypertensive medication is the most common cause.2,11,12 Patients may also present with acute severe hypertension because they have been eating high-sodium diets or because they have been using illicit drugs associated with an increased BP (eg, cocaine, amphetamines).2
In addition, patients may experience excessively high BPs as a result of underlying health conditions: those with a pheochromocytoma, systemic sclerosis, or glomerulonephritis are all at increased risk for acute severe hypertension. Other causes of secondary hypertension, such as renal artery stenosis, Cushing’s syndrome, hypothyroidism/hyperthyroidism, hyperaldosteronism, obstructive sleep apnea, chronic kidney disease, and coarctation of the aorta, may also contribute. A longitudinal study from Saguner et al12 suggests that higher grades of obesity may be associated with increased risk of hypertensive crisis. Finally, patients experiencing profound anxiety or panic—particularly those with underlying hypertension—may present with acute severe hypertension as a result of their psychological and emotional disturbance.
Clinicians should take these precipitating factors into account when evaluating patients for acute severe hypertension. A thorough history and physical should be performed, including the systems and target organs mentioned previously. However, before initiating treatment, clinicians should assess for evidence of end-organ damage, as this will distinguish between a patient suffering from hypertensive emergency or hypertensive urgency.13 It is important to note that the signs and symptoms of end-organ damage may present in isolation or in combination. Laboratory assessments may also provide important information. Standard practice in the evaluation of acute severe hypertension involves ordering a basic metabolic panel, a complete blood count, a urinalysis, an ECG, and troponin levels to assess for asymptomatic end-organ damage.2 If the findings from these assessments collectively indicate that a hypertensive emergency exists, clinicians should initiate timely treatment to prevent complications.
How Can Blood Pressure Be Lowered Safely in the Context of a Hypertensive Emergency?
Studies suggest that there is considerable heterogeneity in the treatment of hypertensive emergencies.14,15 This diversity of approaches likely reflects the wide range of clinical scenarios encountered by clinicians who treat patients with acute severe hypertension and end-organ damage. Depending on the BP readings, the organs implicated, and patient characteristics, clinicians may need to tailor their BP-lowering treatments to protect vital organs, without creating complications secondary to rapid correction of BP. Nevertheless, guidelines for a range of patient scenarios have been created.
As a general rule, patients with a hypertensive emergency should be admitted to the intensive care unit (ICU) and be treated with intravenous (IV) antihypertensive medications; however, the BP should not be lowered too rapidly, as many patients, especially older adults, will have adjusted to these elevated BPs and may suffer from ischemia to certain tissues and organs.2,16 In most cases, a reduction in the MAP of 10%–20% (though many sources suggest 15%–25%) over the first hour, and an additional 5%–15% over the remainder of the 24 hours after initiation of treatment, should suffice for effective and safe correction.1,6 Ideally, the patient will have a BP < 180/< 120 mm Hg after the first hour of treatment, unless initial BPs were far above these levels.1 A markedly larger reduction (eg, from 200 to 120 mm Hg systolic) would be associated with a greater risk for mortality.2,17 Such overcorrections may be reversed by holding antihypertensives and, if necessary, administering IV fluids or vasopressor therapy to slow the rate of correction.2
Labetalol, metoprolol, nitroglycerin, and hydralazine are the most used IV antihypertensives in hypertensive emergencies.14 However, use of hydralazine may lead to a greater risk of excessively rapid BP correction, and it is not generally recommended as an initial intervention.2,18 Beyond this, specific interventions are typically tailored to the clinical scenario. Peixoto2 outlined the general treatment approaches for given clinical scenarios in hypertensive emergency. Target BPs for various signs of end-organ damage were also addressed by Johnson et al.13
Neurologic complications represent a major complication of hypertensive emergencies. Clinicians managing hypertensive emergency complicated by hypertensive encephalopathy should aim for a maximum MAP reduction of 20% within the first hour.13 A diastolic BP of 100–120 mm Hg may also be used as a benchmark. From there, clinicians should aim to correct toward the patient’s baseline BP over the following 2 to 3 days.13 In patients who present with a hypertensive emergency complicated by an ischemic stroke, a reduction in the MAP between 15% and 20% should be the target.13 Patients with a hemorrhagic stroke may be managed slightly more aggressively, with clinicians aiming for a 20%–25% reduction within the first hour. In each of these scenarios, labetalol and nicardipine may be employed as first-line agents, while use of hydralazine should be avoided.2
Cardiovascular complications represent another category. In the setting of aortic dissection, systolic BP should be reduced to 100–120 mm Hg within the first hour.13 This may be achieved using either esmolol or labetalol, in addition to nicardipine.2 In the setting of suspected heart failure or myocardial ischemia or MI, clinicians should aim for a MAP between 60 and 100 mm Hg. The difference between these 2 scenarios is in the choice of antihypertensive. β-blockers (eg, metoprolol, esmolol) are often used in the management of acute coronary syndrome (ACS) in hypertensive emergencies, while the same medications are avoided in hypertensive emergency–associated heart failure.2 In this latter setting, nitroprusside with loop diuretics is more appropriate.2
As noted by Peixoto,2 the data guiding these target BPs are still limited, and various guidelines and sources often contradict one another or do not fully address the need (or lack thereof) for differential treatment of certain patient populations. For instance, Peixoto2 does not mention any need (or lack of need) for differential treatments for patients with obesity. Therefore, it is crucial for clinicians to collaborate and learn from colleagues who are experienced in managing hypertensive emergencies, as knowledge of a particular clinical setting paired with a particular MAP may be important for a given patient and their condition.
What Are the Complications of Overrapid Blood Pressure Correction or the Adverse Effects of Antihypertensive Medications Used in Hypertensive Emergency?
The primary danger of overrapid correction of the BP in hypertensive emergency is ischemia. In patients with long-standing hypertension who experience acute severe hypertension, the body undergoes several physiologic adaptations to mitigate potential tissue damage. This includes cerebral autoregulation, in which arteriolar constriction and reflex vasodilation allow the brain to maintain constant blood flow and thus prevent cerebral edema despite an increased MAP.2,19 These changes are profoundly adaptive in the setting of hypertension. However, when BPs drop rapidly—with, for instance, rapid administration of IV esmolol and nicardipine—the body is unable to compensate quickly enough to maintain a sufficient perfusion pressure. This leaves a wide range of tissues, most notably the brain and heart, vulnerable to ischemia.19 As such, patients who exhibit new alteration in mental status, neurologic signs, or signs and symptoms of myocardial ischemia or MI should be assessed for iatrogenic complications. Older adults may be especially prone to these effects, as their physiologic systems are often less capable of rapid change and adaptation.
Apart from the general effects associated with overrapid correction of hypertensive emergency, the antihypertensives used in this setting are associated with several adverse effects. Older adults are especially vulnerable to these effects. Nitroprusside, a nitrate metabolized into cyanide, is associated with cyanide toxicity, which may manifest as new-onset altered mental status, nausea, and abdominal pain.15 It is also contraindicated in pregnant patients.2 Nicardipine, a calcium-channel blocker, may be associated with headache, local phlebitis, and vomiting, as well as reflex tachycardia in the setting of an ACS.2,16 Labetalol and esmolol, β-blockers, may precipitate heart block and are contraindicated in patients with heart failure, bradycardia, preexisting heart block, and asthma or airway hyperreactivity.2,16 Hydralazine is associated with hypotension and reflex tachycardia, and its effects on BP are often unpredictable, making it less desirable as an initial intervention.2,16 Clonidine, an α-antagonist, may cause untoward sedation and rebound hypertension.16 Therefore, clinicians who treat patients with a hypertensive emergency should monitor for complications of overrapid correction, as well as the adverse effects of the medications they are using in the patient’s treatment.
How Does Anxiety Relate to Hypertension and Hypertensive Emergencies and How Might Clinicians Manage Anxiety in the Setting of Hypertension or Hypertensive Emergency?
Recent work suggests that there is a strong link between anxiety and hypertension and that a lack of awareness of this co-occurrence may contribute to the underrecognition of both conditions.20 This is not, of course, to suggest that anxiety causes hypertensive emergencies, but rather that the persistence of chronic anxiety and emotional distress can contribute to long-standing hypertension and an increased risk for acute severe hypertension. In addition, anxiety is associated with worse cardiovascular outcomes in patients who are at risk for serious cardiovascular disease.21 With nearly 1 in 5 adults suffering from anxiety, this relationship is of high importance for the long-term management of patients who have suffered from a hypertensive emergency.22
While anxiety does not appear to cause hypertensive emergencies, clinicians who have managed the acute dangers of such hypertensive episodes should assess for anxiety and other mood disorders. They may do so by taking the time to discuss the psychological and social difficulties with the patient once the BP has been stabilized. If the patient provides evidence of anxiety or a related mental disorder, a psychiatrist may be consulted. The goal of these assessments and conversations would be to improve long-term outcomes by addressing contributory factors to the patient’s underlying hypertension. Patients with mental health conditions likely related to their hypertension may benefit from psychotherapy, psychotropic medication, or some combination of the 2 treatments.20
What Are the Other Psychiatric Considerations to Keep in Mind With Regard to Hypertensive Crises?
There are several psychiatric conditions for the clinician to keep in mind when learning about, diagnosing, or treating hypertensive crises. One of these conditions is panic disorder. The literature suggests that patients may present with “episodic BP elevations” secondary to panic attacks and that appropriate therapy may “reduce the frequency and intensity” of such attacks.23 A case series described patients presenting with pseudopheochromocytoma, which is episodes of “severe, symptomatic paroxysmal hypertension” not originally thought by the patient to be “related to stress or emotional distress, initially discouraging consideration of a link to emotions.”24 In this cases series,24 appropriate psychiatric and medical care eliminated future episodes in 62% of patients.
Relatively less is known about the exact relationship between bipolar disorder and hypertensive crises. However, it is certainly reasonable to assume that if a patient’s psychiatric disorder leads to difficulty in taking their BP medications, they may be at increased risk for hypertensive crises. The same could be said for brief psychotic disorders and other psychotic states and conditions, whose associations with hypertensive crises might be better understood from the reverse relationship: hypertensive crises as a possible risk factor for psychotic behavior.25
There are, however, clear concepts to be aware of in the long-term management and prevention of hypertensive crises in patients with psychiatric conditions. One important consideration is patients who are taking a monoamine oxidase inhibitor (MAOI) medication. In these patients, it is important to avoid foods and drinks that are rich in tyramine, such as certain cured meats (eg, salami), aged cheeses, excess chocolate, and certain alcoholic drinks.26 When patients taking MAOIs ingest too much tyramine, they are at risk for hypertensive crises secondary to excess norepinephrine.26 In contrast, clinicians likely do not need to worry about the implications for patients with hypertension when prescribing the commonly prescribed class of selective serotonin reuptake inhibitors.27 Among the serotonin-norepinephrine reuptake inhibitors, venlafaxine may require further thought among prescribing clinicians, as it has been associated with increased risk for elevated BP in some patients.27,28 Antipsychotics may dispose to hypertension through the promotion of metabolic syndrome.27
Another consideration, given the association between preexisting hypertension and the development of acute hypertensive crises, is the link between psychiatric disorders and later diagnosis of hypertension.29 Because individuals with long-term psychiatric illness may be at higher risk of hypertension, it is important for their primary care providers to monitor BP closely over time. Mindfulness-based stress reduction programs may be beneficial in these patients, though further research is needed in this field.30 Sleep and exercise are important factors in BP management and should also be discussed during health care visits.31,32
What Happened to Mr A?
After his hypertensive emergency was diagnosed, Mr A was admitted to the medical ICU for treatment and monitoring. The resident determined that Mr A was suffering from a hypertensive encephalopathy and decompensated heart failure and started him on IV nitroprusside and furosemide. The resident and medical team monitored the BP closely over the next hour, which declined to 186/116 mm Hg. Satisfied with this reduction rate, and the absence of symptoms of overcorrection, they tapered the dosage of nitroprusside and furosemide. A repeat chest x-ray 3 hours later showed a reduction in the pulmonary edema, and the oxygen saturation rose to 96% on room air. By the following morning, Mr A felt moderately uncomfortable but was alert and oriented to person, place, and time and denied chest pain, shortness of breath, orthopnea, or headache. His BP was 156/100 mm Hg, and his IV antihypertensives were discontinued and replaced with oral medications.
During a conversation with Mr A and his wife, the resident asked what might have triggered this event. Mr A said, “I have no clue. I’ve been stressed, and I’ll admit it, on the brink of panic these last few weeks, but that’s not too different from a lot of other weeks at work. I’m not sure what happened, really.”
His wife, however, brought Mr A’s pillbox from home, which showed unused medications for the previous week. Surprised at first, Mr A realized that, due to increased stress at work, he had been leaving for work earlier and returning later than usual, which had disrupted his daily routine enough to make him forget to take his medications.
The resident told Mr A that missing BP medication doses is one of the more common causes of episodes like he had experienced and that it was probably a big factor. However, the resident said that the stress and anxiety was another factor. He explained that Mr A was not only feeling anxious and in a state of near panic, which will certainly increase BP levels, but that antianxiety medications were among those he had missed because of that stress. To prevent this from happening again, Mr A would need a reliable method of taking his medications, and the treatment team would help figure out how to reduce his daily stress, or at least help him respond to it differently.
Over multiple discussions, the medical team and patient decided that Mr A’s wife would help ensure that he took his daily medications and that he would work slightly fewer hours over the next few months, while engaging in psychotherapy for his anxiety and resuming sertraline. Mr A thanked the resident and medical team, noting how hopeful he felt about his physical and mental health moving forward.
Submitted: September 24, 2021; accepted March 2, 2022.
Published online: November 10, 2022.
Relevant financial relationships: None.
Funding/support: None.
Clinical Points
- Hypertensive emergency (previously labeled as a hypertensive crisis) is a subset of severe hypertension, which is an elevated systolic blood pressure (BP) ≥ 180 mm Hg and a diastolic BP ≥ 120 mm Hg.
- Evidence of end-organ damage distinguishes a hypertensive emergency from hypertensive urgency, the other subset of acute severe hypertension.
- The primary danger of overrapid correction of the BP in hypertensive emergency is ischemia.
- While anxiety does not appear to cause hypertensive emergencies, clinicians should assess for anxiety and other mood disorders, as they can contribute to elevated BP.
References (32)

- Elliot WJ, Varon J. Evaluation and treatment of hypertensive emergencies in adults. UpToDate website. 2020. Accessed September 14, 2022. https://www.uptodate.com/contents/evaluation-and-treatment-of-hypertensive-emergencies-in-adults
- Peixoto AJ. Acute severe hypertension. N Engl J Med. 2019;381(19):1843–1852. PubMed CrossRef
- Vaughan CJ, Delanty N. Hypertensive emergencies. Lancet. 2000;356(9227):411–417. PubMed CrossRef
- Kumari R, Kapil A, Grewal V, et al. Hypertensive emergency: a look into the eye. Postgrad Med J. 2021;98(e1):140389. PubMed CrossRef
- Elliott WJ, Weber RR, Nelson KS, et al. Renal and hemodynamic effects of intravenous fenoldopam versus nitroprusside in severe hypertension. Circulation. 1990;81(3):970–977. PubMed CrossRef
- Elliott WJ. Clinical features and management of selected hypertensive emergencies. J Clin Hypertens (Greenwich). 2004;6(10):587–592. PubMed CrossRef
- Lin MP, Liebeskind DS. Imaging of ischemic stroke. Continuum (Minneap Minn). 2016;22(5, Neuroimaging):1399–1423. PubMed CrossRef
- Potter T, Schaefer TJ. Hypertensive encephalopathy. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing; 2022.
- Strandgaard S, Paulson OB. Cerebral blood flow and its pathophysiology in hypertension. Am J Hypertens. 1989;2(6 Pt 1):486–492. PubMed CrossRef
- Muntner P, Shimbo D, Carey RM, et al. Measurement of blood pressure in humans: a scientific statement from the American Heart Association. Hypertension. 2019;73(5):e35–e66. PubMed CrossRef
- Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981–988. PubMed CrossRef
- Saguner AM, Dür S, Perrig M, et al. Risk factors promoting hypertensive crises: evidence from a longitudinal study. Am J Hypertens. 2010;23(7):775–780. PubMed CrossRef
- Johnson W, Nguyen ML, Patel R. Hypertension crisis in the emergency department. Cardiol Clin. 2012;30(4):533–543. PubMed CrossRef
- Katz JN, Gore JM, Amin A, et al; STAT Investigators. Practice patterns, outcomes, and end-organ dysfunction for patients with acute severe hypertension: the Studying the Treatment of Acute hyperTension (STAT) registry. Am Heart J. 2009;158(4):599–606.e1. PubMed CrossRef
- Mayer SA, Kurtz P, Wyman A, et al; STAT Investigators. Clinical practices, complications, and mortality in neurological patients with acute severe hypertension: the Studying the Treatment of Acute hyperTension registry. Crit Care Med. 2011;39(10):2330–2336. PubMed CrossRef
- Alshami A, Romero C, Avila A, et al. Management of hypertensive crises in the elderly. J Geriatr Cardiol. 2018;15(7):504–512. PubMed.
- Peacock F, Amin A, Granger CB, et al; Stat Investigators. Hypertensive heart failure: patient characteristics, treatment, and outcomes. Am J Emerg Med. 2011;29(8):855–862. PubMed CrossRef
- Campbell P, Baker WL, Bendel SD, et al. Intravenous hydralazine for blood pressure management in the hospitalized patient: its use is often unjustified. J Am Soc Hypertens. 2011;5(6):473–477. PubMed CrossRef
- Breu AC, Axon RN. Acute treatment of hypertensive urgency. J Hosp Med. 2018;13(12):860–862. PubMed CrossRef
- Hamam MS, Kunjummen E, Hussain MS, et al. Anxiety, depression, and pain: considerations in the treatment of patients with uncontrolled hypertension. Curr Hypertens Rep. 2020;22(12):106. PubMed CrossRef
- Roest AM, Martens EJ, de Jonge P, et al. Anxiety and risk of incident coronary heart disease: a meta-analysis. J Am Coll Cardiol. 2010;56(1):38–46. PubMed CrossRef
- Kessler RC, Chiu WT, Demler O, et al. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62(6):617–627. PubMed CrossRef
- White WB, Baker LH. Episodic hypertension secondary to panic disorder. Arch Intern Med. 1986;146(6):1129–1130. PubMed CrossRef
- Mann SJ. Severe paroxysmal hypertension (pseudopheochromocytoma). Curr Hypertens Rep. 2008;10(1):12–18. PubMed CrossRef
- Kim CH, Syed S. Hypertensive encephalopathy: a case of a male who bit off his fingers. Cureus. 2017;9(6):e1334. PubMed CrossRef
- Flockhart DA. Dietary restrictions and drug interactions with monoamine oxidase inhibitors: an update. J Clin Psychiatry. 2012;73(suppl 1):17–24. PubMed CrossRef
- Morreale MK, Wake LA. Psychiatric medications and hypertension. Curr Hypertens Rep. 2020;22(11):86. PubMed CrossRef
- Thase ME. Effects of venlafaxine on blood pressure: a meta-analysis of original data from 3744 depressed patients. J Clin Psychiatry. 1998;59(10):502–508. PubMed CrossRef
- Stein DJ, Aguilar-Gaxiola S, Alonso J, et al. Associations between mental disorders and subsequent onset of hypertension. Gen Hosp Psychiatry. 2014;36(2):142–149. PubMed CrossRef
- Hofmann SG, Asmundson GJG. Acceptance and mindfulness-based therapy: new wave or old hat? Clin Psychol Rev. 2008;28(1):1–16. PubMed CrossRef
- Calhoun DA, Harding SM. Sleep and hypertension. Chest. 2010;138(2):434–443. PubMed CrossRef
- Choudhury A, Lip GYH. Exercise and hypertension. J Hum Hypertens. 2005;19(8):585–587. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite