ABSTRACT
Objective: The current nomenclature for atypical antipsychotics does not indicate that they are used to treat nonpsychotic conditions (eg, bipolar disorder, major depressive disorder), which could have negative implications for both health care providers (HCPs) and patients. The objective of this study was to evaluate how the atypical antipsychotic class name affects HCPs who treat bipolar disorder and patients who receive the diagnosis.
Methods: Nationwide surveys of primary care and psychiatric HCPs (n = 200) and patients with bipolar disorder (n = 200) were conducted to assess perspectives regarding current atypical antipsychotic nomenclature. HCP opinion about a change in class name was also evaluated. The self-administered electronic surveys were completed by HCPs from May 22, 2020, to June 1, 2020, and by patients from August 25, 2020, to September 7, 2020.
Results: Compared with the mood stabilizer class name, the atypical antipsychotic name elicited stronger negative feelings from both HCPs and patients. Most HCPs avoided bringing up the atypical antipsychotic name with patients (72%), often due to fear of negative reactions. Despite being approved for bipolar mania and depression, only 48% and 39% of HCPs indicated that atypical antipsychotics were appropriate for these disorders, respectively. If an appropriate alternative for the term atypical antipsychotic was available for bipolar disorder, 71% of HCPs said they were likely to change their class-related behaviors. Most patients had never heard of the atypical antipsychotic class name (69%). Significantly more patients had negative reactions (eg, worry, fear, confusion) to the idea of their HCP prescribing an atypical antipsychotic versus a mood stabilizer (25% vs 6%). Compared with mood stabilizers, patients were less likely to take an atypical antipsychotic immediately as prescribed.
Conclusions: Significantly more HCPs and patients had negative reactions to atypical antipsychotic nomenclature compared with mood stabilizer nomenclature for treating bipolar disorder. The broad descriptive atypical antipsychotic class name may not support the standard of care for the treatment of bipolar disorder.
Prim Care Companion CNS Disord 2023;25(1):22m03331
To cite: Mattingly G, Matthews-Hayes T, Patel MD, et al. Do we need a new nomenclature for atypical antipsychotics? A survey of health care professionals and patients. Prim Care Companion CNS Disord. 2023;25(1):22m03331.
To share: https://doi.org/10.4088/PCC.22m03331
© 2023 Physicians Postgraduate Press, Inc.
aWashington University School of Medicine, St Louis, Missouri
bSeaside Behavioral Health, Virginia Beach, Virginia
cAbbVie, Madison, New Jersey
dDepartment of Psychiatry, University of California, San Diego, California
*Corresponding author: Greg Mattingly, MD, Washington University School of Medicine, 4801 Weldon Spring Pkwy, Ste 300, St Charles, MO 63304 ([email protected]).
For pharmacologic class nomenclature to be useful, it should reflect current scientific knowledge, inform clinical decision-making, and enhance patient understanding.1,2 Contrary to this guidance, however, the current approach to nomenclature across drugs used to treat psychiatric disorders is not standardized, and different naming conventions can be confounding. For example, when an antiepileptic agent is used to treat bipolar disorder, it is referred to as a mood stabilizer, a name that is specific to this use.1 In other cases, nomenclature is based on the proposed mechanism of action, as with selective serotonin reuptake inhibitors (SSRIs) used to treat depression and anxiety disorders, or by chemical class name, as seen with benzodiazepines for anxiety.3 Further, the class of medications known as antipsychotics uses a name that is based on their initial indication for the treatment of psychosis. This convention is particularly perplexing in given that a heterogeneous new generation of agents, the so-called “atypical antipsychotics,” are indicated and frequently prescribed to treat multiple nonpsychotic disorders including mood disorders, irritability associated with autism, and major depressive disorder (MDD). Unfortunately, this outdated class name does not identify that individual atypical antipsychotics have unique receptor pharmacology, different biological effects, differing side effects, and different US Food and Drug Administration (FDA) indications.2–10 A “one size fits all” nomenclature may influence health care provider (HCP) perceptions and contribute to inappropriate use or lack of use for atypical antipsychotics across multiple psychiatric conditions.
For patients, the inexact atypical antipsychotic class name may also contribute to undesirable consequences such as confusion, stigma, and worry that may arise when a prescription does not appear to be relevant to a diagnosis.3,10 In bipolar disorder, for example, lengthy delays to correct diagnosis are common, and patients with a long treatment history may not be sure that atypical antipsychotics are suitable for their diagnosis, especially if the class is perceived as being more accurate for schizophrenia than for bipolar disorder.11 Negative perceptions of antipsychotic medications combined with the stigma of mental health treatment in general may put patients at a high risk for nonadherence and subsequent poor outcomes.11 Since poor HCP communication has been cited as a major barrier to a good patient/provider relationship,12 it follows that clear dialogue may be key to successful atypical antipsychotic use in bipolar disorder.
To evaluate how the atypical antipsychotic class name affects HCPs who treat bipolar disorder and patients who are diagnosed with it, we conducted a nationwide electronic survey in each group of interest. Since mood stabilizers are another commonly prescribed treatment for bipolar disorder, implicit HCP and patient reactions to both class names were evaluated to assess the impact these nomenclatures have on attitudes and behaviors.
METHODS
Self-administered electronic surveys were completed by HCPs (May 22, 2020, to June 1, 2020) and patients with bipolar disorder (August 25, 2020, to September 7, 2020) to gauge their reactions to current atypical antipsychotic nomenclature; HCP opinion about a change in class name was also evaluated. Participants were identified via existing databases. Invitations were sent to potentially eligible HCPs and a random selection of patients with self-identified bipolar disorder who had opted to participate in surveys.
The first section of each 10-minute survey screened for eligibility. After meeting criteria, participants completed the main section of the survey, which consisted of mostly close-ended questions (eg, yes/no, multiple choice, ranking) about the class names used in bipolar disorder.
In open-ended questions, patients were asked to describe their initial reactions to the atypical antipsychotic and mood stabilizer class names, and HCPs were asked to type in their reactions to each class name and what they thought their patients’ reactions would be. Some questions were asked twice in random order, once for the atypical antipsychotic class name and once for the mood stabilizer class name. All data were self-reported. Responses were anonymous, and no identifiable information was collected during the survey.
The representative sample of HCPs was stratified by primary care (primary care practitioners [PCPs], general nurse practitioners [NPs], physician assistants [PAs]) and psychiatric specialty (psychiatrists, psychiatric NPs, and PAs). HCPs were included if they had completed residency in the United States, practiced in their specialty for less than 30 years, and worked at least 75% of the time in clinical practice, with at least 50% of their practice in private practice, outpatient treatment center, hospital or clinic, or community mental health center. Per month, psychiatric HCPs were required to see at least 15 patients with bipolar disorder and 15 patients with MDD; PCPs were required to see at least 5 patients with bipolar disorder and 10 patients with MDD.
Patients were included if they were 18 to 64 years of age, did not work in industry-specific agencies or settings (eg, public relations/advertising, pharmaceutical product manufacturer/distributer, any job in the medical field), had connected with a provider about bipolar disorder in the past 12 months, and were currently taking or willing to take prescription medication for bipolar disorder. Patients were categorized by bipolar illness characteristics including recent or not recent diagnosis (< 5 or ≥ 5 years ago) and past/current atypical antipsychotic use (been prescribed or never prescribed). Class use experience was derived from a list of medications; patients selected all medications (current or past) they had been prescribed for bipolar disorder, and their answers were auto coded by class. The list included the brand and generic names of 12 atypical antipsychotics (eg, cariprazine, aripiprazole, quetiapine), 3 mood stabilizers (lamotrigine, divalproex sodium, lithium), and 15 other medications (eg, escitalopram, haloperidol, olanzapine/fluoxetine). Familiarity with the atypical antipsychotic and mood stabilizer class names (ie, knowing at least a little bit about them) or lack of familiarity was classified as stated by the patient.
Statistical Methods
Findings were estimated by descriptive statistics, with significant between-group differences determined at a 95% confidence interval on groups with a minimum base of n = 20 for HCPs or n = 30 for patients. Collected data were analyzed using IBM SPSS Data Collection Professional/Dimensions software.
RESULTS
HCP Survey Outcomes: Disposition and Sample Characteristics
A total of 634 HCPs were screened; 319 HCPs were excluded based on screening qualifications, and 76 left the survey before completing it in full. An additional 24 HCPs were not included because the sample size goal for their specialty had been met, and 10 were not included because the total sample size for the study was met. The survey was completed by 205 HCPs; 5 surveys were removed for quality control issues, and 200 completed interviews were retained for evaluation (PCP = 100, primary care NP/PA = 30, psychiatrist = 50; psychiatric NP/PA = 20). The average monthly caseload of adult patients was 49 patients with bipolar disorder (PCP = 33, psychiatric = 79) and 96 patients with depression (PCP = 78, psychiatric = 130). The average number of prescriptions written per month was 35 for atypical antipsychotics (PCP = 19, psychiatric = 65) and 27 for mood stabilizers (PCP = 15, psychiatric = 49).
Impact of Atypical Antipsychotic Nomenclature on HCP Behavior
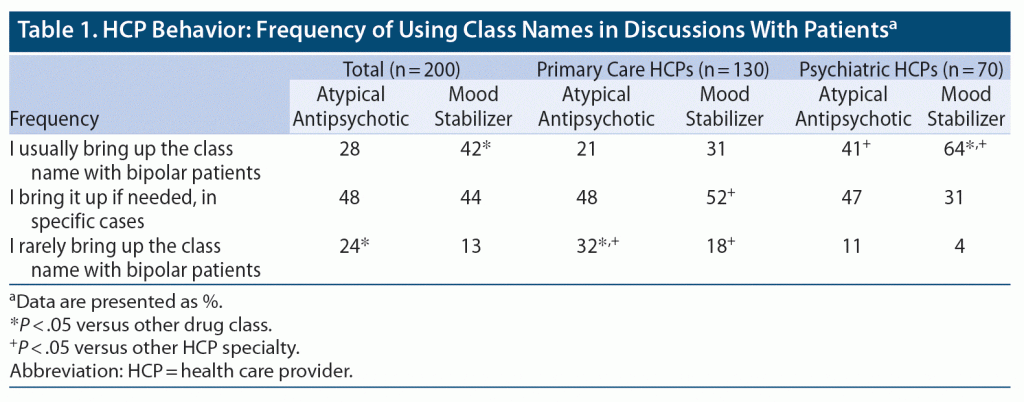
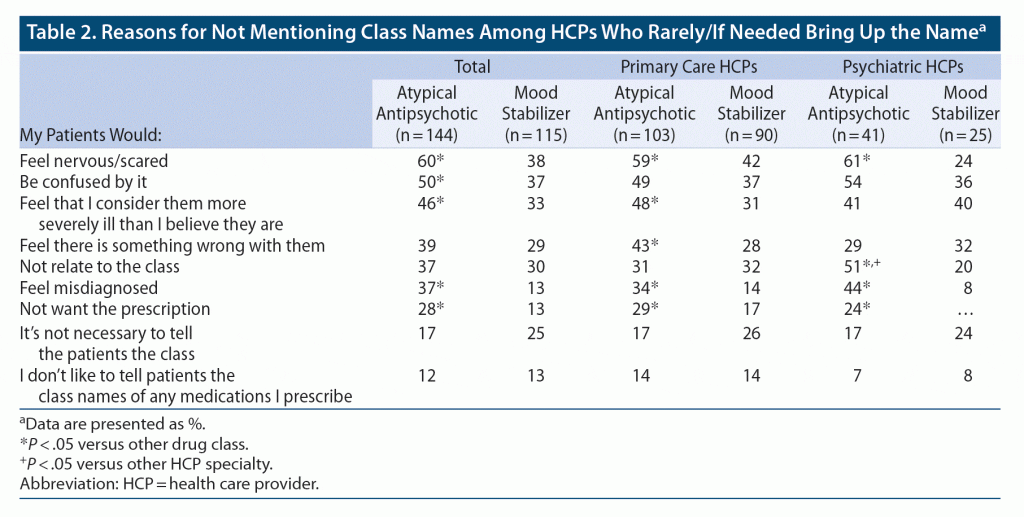
When asked about the use of class names in discussions with patients who have bipolar disorder, HCPs were significantly more likely to say they bring up the mood stabilizer name than the atypical antipsychotic name (P < .05), with 72% of HCPs bringing up the atypical antipsychotic name rarely or only as needed (Table 1). Among HCPs who only rarely mention atypical antipsychotics, about half believed that bringing it up would make their patients feel nervous, confused, or more severely ill (Table 2).
HCP Perspective: Appropriateness of Atypical Antipsychotic Drug Class by Indication
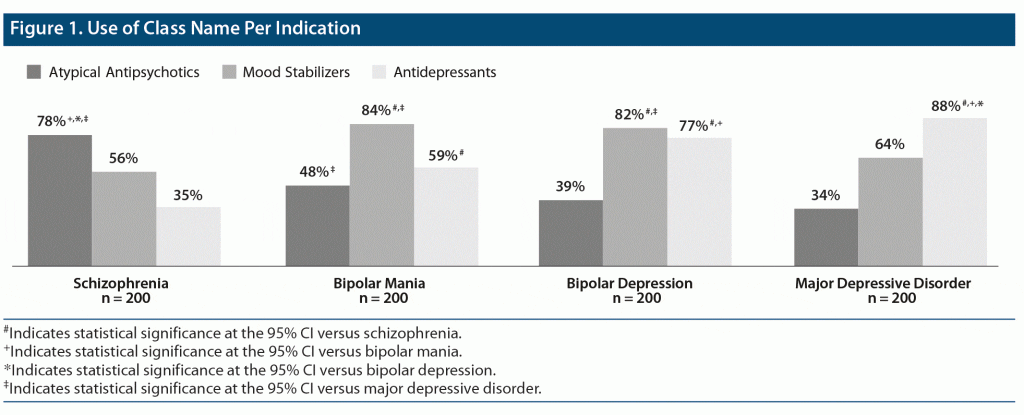
When asked how well they felt the atypical antipsychotic, mood stabilizer, and antidepressant class names described their use in schizophrenia, bipolar mania, bipolar depression, and MDD (1 [Does not describe at all] to 7 [Describes extremely well]), HCPs were significantly more likely to say that the atypical antipsychotic class name was appropriate for use in schizophrenia compared with bipolar disorder (mania and depression) or MDD (P < .05) (Figure 1). Despite having approved indications in bipolar mania and depression, only 48% and 39% of HCPs, respectively, indicated that the atypical antipsychotic class name was appropriate for use in these disorders. There were no significant differences between primary care and psychiatric HCPs regarding the appropriateness of atypical antipsychotics for the bipolar indications (data not shown). Despite not having FDA approval for monotherapy treatment of bipolar mania or depression, 59% and 77% of HCPs, respectively, still thought that the antidepressant class name was appropriate for use in these disorders.
HCP Reaction: Changing the Atypical Antipsychotic Class Name
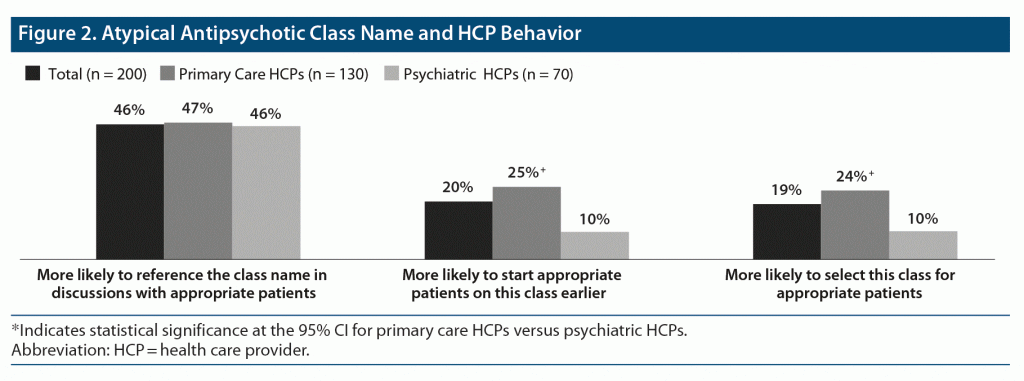
When HCPs were asked how, if at all, they would expect their behavior to change if the atypical antipsychotic class name was changed to an appropriate alternative for the treatment of bipolar disorder, approximately half said they would be more likely to reference the class name when having discussions with their patients (Figure 2). Additionally, one-quarter of primary care HCPs reported that they would be more likely to start their patients on an atypical antipsychotic earlier or to select an atypical antipsychotic for appropriate patients. In total, 71% of HCPs said they were likely to change their behavior.
Additionally, 82% of HCPs believed that their patients’ illness-related behavior would change if the atypical antipsychotic class name was changed. More than a third of HCPs indicated that patients would be more likely to start their medication (44%), to have confidence in the medication’s efficacy for their symptoms (38%), and to adhere to their medication’s regimen (36%).
Patient Survey Outcomes: Disposition and Sample Characteristics
A total of 515 patients were screened; 249 were excluded for not meeting screening qualifications, 53 left the survey before completing it, and 1 patient was not included because the sample goal was reached. The survey was completed by 212 patients, with 12 surveys removed for quality control issues and 200 completed interviews retained for evaluation. Among included patients, 67 (34%) patients reported a recent diagnosis (< 5 years ago), 132 (66%) patients reported a not recent diagnosis (≥ 5 years ago), and 1 patient (< 1%) did not recall their diagnostic timeframe. Most patients (184 [92%]) were currently taking a medication for bipolar disorder (average current medications = 1.5). Atypical antipsychotic and mood stabilizer use, which was derived by patients selecting the medications they had been prescribed from a list of medications, indicated that atypical antipsychotics were used by 140 (70%) patients (current use = 108 [54%], previous use = 32 [16%]) and mood stabilizers were used by 102 (51%) patients (current use = 58 [29%], previous use = 44 [22%]). One (0.05%) patient reported receiving a previous atypical antipsychotic prescription that was never taken.
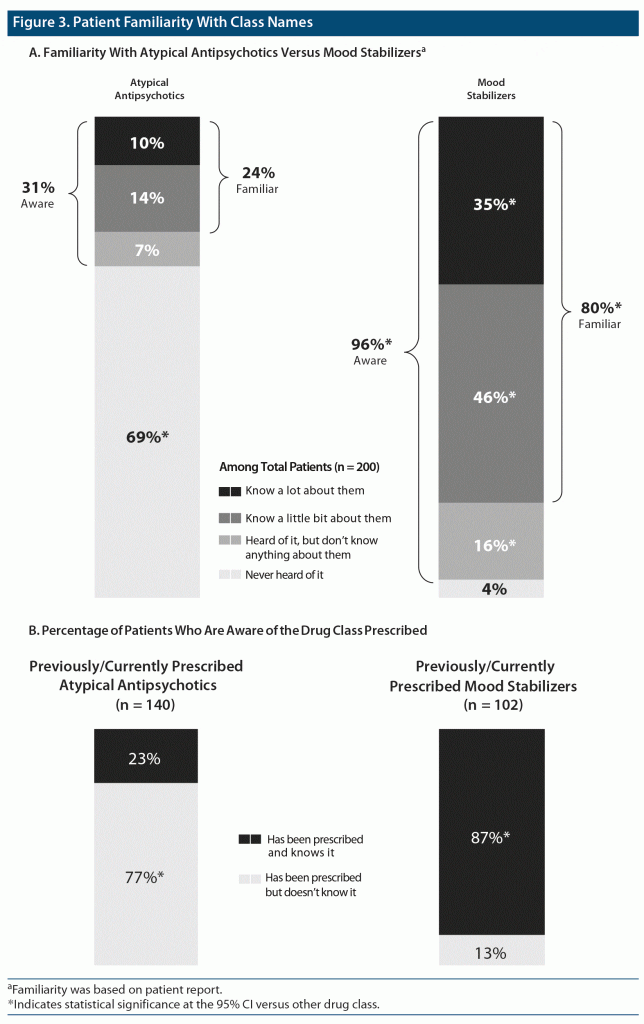
Patient Familiarity With the Atypical Antipsychotic Class Name
To determine familiarity with the class names, patients were asked if they had ever heard of the atypical antipsychotic or mood stabilizer name before taking the survey, and if they had, they indicated their level of familiarity (ie, heard of them but do not know anything about it, know a little bit about them, or know a lot about them). Almost 70% of patients stated that they had never heard of the atypical antipsychotic class name, with significantly fewer patients familiar with the atypical antipsychotic class than the mood stabilizer class (Figure 3A). Patients who were not treated by a psychiatric HCP were less likely to have heard of atypical antipsychotics (19% vs 36%). Although 70% of patients indicated current or previous antipsychotic use by selecting from a medication list, when patients were asked if they had they had ever been prescribed an atypical antipsychotic, more than three-quarters of those who previously indicated atypical antipsychotic use contradictorily reported that they had never been prescribed a medication from this class. Awareness of the drug class prescribed was much greater among patients with mood stabilizer prescriptions, with only 13% of patients unaware of it (Figure 3B).
Patient Reactions to the Atypical Antipsychotic Class Name
In an open-ended question (asked 1 time for each drug class), significantly more patients had a strong negative initial reaction to the idea of their HCP telling them that they were prescribing an atypical antipsychotic (25%) compared with a mood stabilizer (6%) (P < .05). Negative reactions included general worry, worry about side effects, and feeling crazy, with the most commonly expressed negative feelings significantly more likely to be reported in association with atypical antipsychotics versus mood stabilizers (unsure/mixed: 29% vs 20%, crazy/psychotic: 22% vs 5%, worried/afraid: 10% vs 2%, and hate/dislike: 8% vs 2%, P < .05 each). Positive feelings were significantly more likely to be associated with mood stabilizers versus atypical antipsychotics (enjoy/love: 42% vs 19%, happy/excited/interested: 22% vs 4%, P < .05 each). Patients who were unfamiliar with the atypical antipsychotic class name were significantly more likely to have a negative reaction than patients who were familiar (unsure/mixed: 34% vs 14%, worried/afraid: 13% vs 0%, P < .05 each).
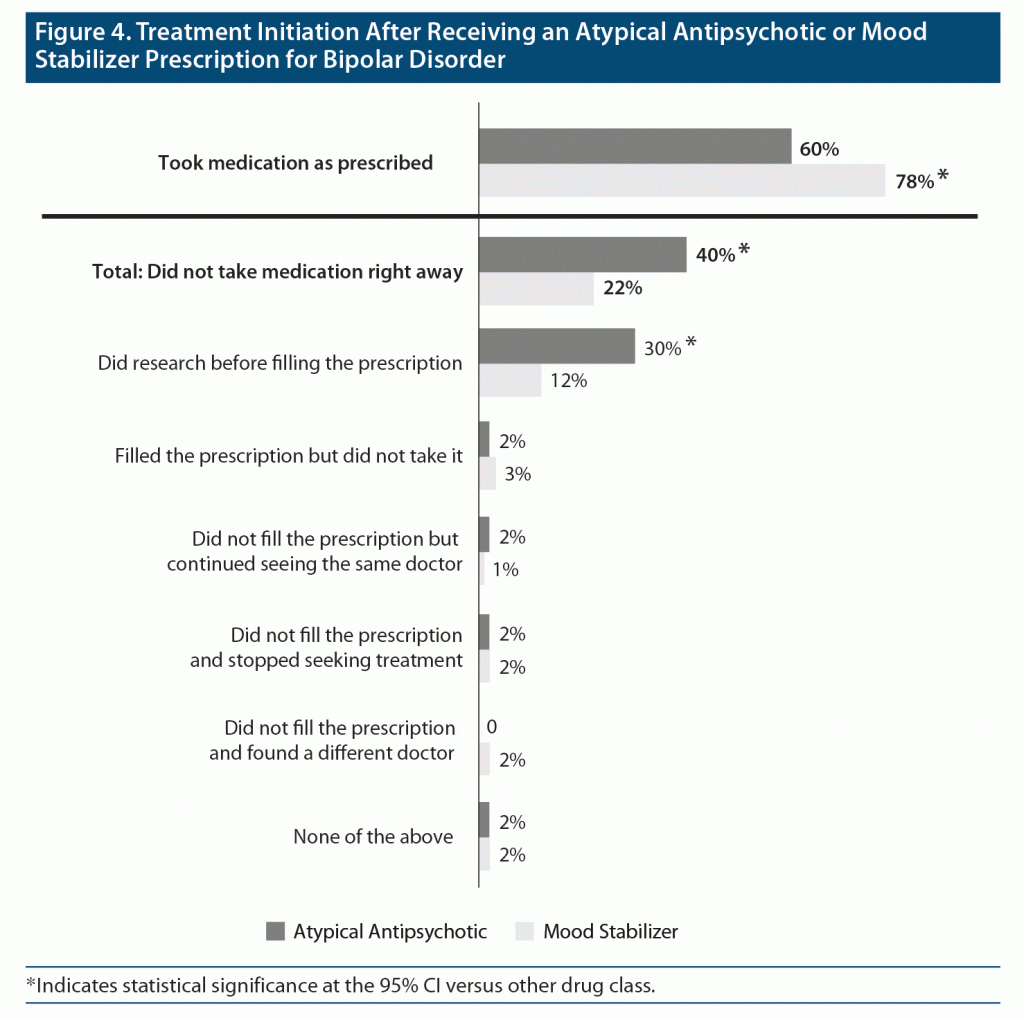
Treatment Initialization After Receiving a Prescription for Bipolar Disorder
When patients were asked about their experiences after receiving a prescription for bipolar disorder medication, significantly fewer patients reported taking their medication right away (as prescribed) if the medication was an atypical antipsychotic versus a mood stabilizer (Figure 4). Significantly more patients who were prescribed an atypical antipsychotic reported researching the medication before filling the prescription.
DISCUSSION
Results of the surveys indicated that compared with the mood stabilizer class name, the atypical antipsychotic class name provoked stronger negative feelings for HCPs and patients across a variety of survey questions. Most HCPs either rarely bring up the atypical antipsychotics class or only bring it up if needed, with discussions avoided out of concern that using the class name would cause their patients to have a negative reaction (eg, feeling nervous, scared, confused, misdiagnosed). Importantly, HCP concerns were supported by patient responses that indicated that the term atypical antipsychotic caused them to feel “unsure, crazy, or worried about psychosis.” Further, since negative reactions were significantly more likely to be reported by patients who were unfamiliar with the atypical antipsychotic class name than by patients who were familiar with it, HCP reluctance to discuss the class name appears to contribute to patient confusion, noncompliance, and worry. The survey also showed that negative feelings and lack of information may lead to delayed treatment, with nearly twice as many patients reporting that they delayed starting a medication labeled as an atypical antipsychotic versus a medication labeled as a mood stabilizer (40% vs 22%) when it was prescribed for the treatment of bipolar disorder, which can obviously impact treatment outcomes negatively.
Beyond these implicit perceptions, class name also greatly influenced treatment choices across indications, as seen in results showing that HCPs were significantly more likely to indicate that atypical antipsychotics had a potential use in schizophrenia than in bipolar disorder or other mood disorders. Although most atypical antipsychotics are approved for the treatment of bipolar mania, and 5 are approved for bipolar depression (ie, cariprazine, quetiapine, lurasidone, lumateperone, olanzapine/fluoxetine combination), less than half of the HCPs surveyed indicated that there was potential use for the atypical antipsychotic class name in these indications. Of additional concern, more than half of HCPs incorrectly reported that the antidepressant class name had potential use in bipolar mania, while three-quarters reported potential use in bipolar depression even though antidepressants are not a recommended treatment option.13 Since antidepressants are not FDA approved as monotherapy treatment for either bipolar mania or depression, it appears that non–evidence-based factors may play a role in prescribing decisions in bipolar disorder.
While atypical antipsychotics are a heterogeneous group of drugs with diverse efficacy, tolerability, and safety profiles, compared with conventional agents, they have a much improved therapeutic index (ie, the ratio of the relative benefit divided by the probability of harm) and have advanced the pharmacotherapy of bipolar disorder across a broad range of presenting symptoms.14 However, despite being considered a first-line treatment for bipolar disorder,13 both HCPs and patients in our survey expressed reticence about the atypical antipsychotic class name, which suggests that inadequate information and negative attitudes may lead to delay of appropriate care with these highly effective agents in bipolar disorder. Would changing the class name help? According to our survey, 71% of HCPs indicated that they would change their clinical behavior if there was an appropriate alternative to the term atypical antipsychotic for the treatment of bipolar disorder, with almost half saying they would reference the class name in discussion with patients. However, since a new name for atypical antipsychotics in bipolar disorder is not a short-term eventuality, assessing patient attitudes toward their medication and employing strategies to refute negative perceptions have been shown to help maintain treatment adherence and improve long-term outcomes in patients with bipolar disorder.15 Further, although our survey only inquired about the impact of the atypical antipsychotic class name as it applied to use in bipolar disorder, the HCP and patient perceptions reported here may have similar implications for atypical antipsychotic use in other nonpsychotic indications, such as MDD, irritability in autism, or generalized anxiety disorder as well.
In cases in which medications have multiple uses, a descriptive nomenclature that readily informs, as seen when antiepileptic drugs are classified as mood stabilizers for the treatment of bipolar disorder, may be advantageous to HCPs and patients alike. Results of our nationwide surveys indicated that the outdated and imprecise atypical antipsychotic nomenclature may not support the standard of care for the treatment of patients with bipolar disorder. HCPs across primary care and psychiatric specialties and patients with bipolar disorder had negative associations with the atypical antipsychotic class name compared with other classes of medications used for mood disorders. Further, most HCPs avoided bringing up the atypical antipsychotic class name when discussing bipolar disorder treatment with their patients, which likely contributes to a widespread lack of familiarity with the drug class and negative attitudes for patients. Education about the approved use of atypical antipsychotics in bipolar disorder, along with better HCP and patient interactions surrounding medication choice, may help improve negative perceptions of the atypical antipsychotic class and enhance its acceptability for the treatment of patients with bipolar I disorder. Based on these data, considerations should be made to limit the use of broad descriptive class nomenclatures that are specific to a single indication since many psychiatric medications are approved for use and frequently prescribed across multiple indications.
Submitted: June 1, 2022; accepted August 16, 2022.
Published online: February 23, 2023.
Author contributions: All authors participated in the writing, editing, and critical revision for intellectual content and approval of the final version of this manuscript. All authors met ICMJE authorship criteria and agree to be accountable for all aspects of the work.
Relevant financial relationships: Dr Mattingly has served as a consultant to Akili, Alkermes, Allergan (now AbbVie), Axsome, Ironshore, Intracellular, Janssen, Lundbeck, Neos Therapeutics, Otsuka, Purdue, Rhodes, Sage, Sunovion, Takeda, and Teva; received research grants from Akili, Alkermes, Allergan (now AbbVie), Axsome, Boehringer, Janssen, Lundbeck, Medgenics, NLS-1 Pharma AG, Otsuka, Reckitt Benckiser, Roche, Sage, Sunovion, Supernus, Takeda, and Teva; and received speaker/promotional honoraria from Alkermes, Allergan (now AbbVie), Ironshore, Janssen, Lundbeck, Otsuka, Sunovion, and Takeda. Dr Matthews-Hayes has received speaker fees from AbbVie, Neurocrine, and Otsuka; served on advisory boards or as a consultant for AbbVie and Neurocrine; and has also been a paid consultant for Psychiatry Times and Psych Congress Bipolar Disease State CME, sponsored by Intra-Cellular Therapies, Inc. Dr Patel was an employee of AbbVie at the time of the study and may hold stock. Dr Kramer is an employee of AbbVie and may hold stock. Dr Stahl has served as a consultant to Acadia, Alkermes, Allergan, Arbor, AstraZeneca, Axovant, Biogen, Biopharma, Celgene, Forest, Forum, Genomind, Innovative Science Solutions, Intra-Cellular Therapies, Jazz, Lundbeck, Merck, Otsuka, PamLabs, Servier, Shire, Sunovion, Takeda, and Teva; is a board member of Genomind; has served on speakers bureaus for Forum, Lundbeck, Otsuka, Perrigo, Servier, Sunovion, and Takeda; and has received research and/or grant support from Acadia, Avanir, Braeburn, Eli Lilly, Intra-Cellular Therapies, Ironshore, ISSWSH, Neurocrine, Otsuka, Shire, Sunovion, and TMS NeuroHealth Centers.
Funding/support: This manuscript was supported by funding from AbbVie.
Role of the sponsor: AbbVie funded this study and participated in the study design, research, analysis, data collection, interpretation of data, review, and approval of the publication. All authors had access to relevant data and participated in the drafting, review, and approval of this publication. No honoraria or payments were made for authorship.
Previous presentations: Presented at the 2020 Neuroscience Education Institute Virtual Poster Library; January 21, 2021 • Presented at the 2021 College of Psychiatric and Neurologic Pharmacists Annual Meeting; Virtual; April 19–23, 2021 • Presented at the 2021 American Association of Nurse Practitioners Annual Conference; Virtual; June 15–August 31, 2021 • Presented at the 2021 American Psychiatric Nurses Association Annual Conference; Virtual; October 13–16, 2021.
Acknowledgments: Writing and editorial assistance were provided by Carol Brown, MS, ELS, of Prescott Medical Communications Group, Chicago, Illinois, which was funded by AbbVie. Project development assistance was provided by Mary Keever, MMR, BS, of Material (formerly Lieberman Research Worldwide), Charlotte, North Carolina. Carol Brown and Mary Keever have no conflicts of interest to disclose.
Clinical Points
- The current nomenclature for atypical antipsychotics does not convey that they are frequently prescribed to treat nonpsychotic indications (eg, bipolar I mania, bipolar I depression, major depressive disorder).
- When treating bipolar I disorder, health care providers were reluctant to discuss atypical antipsychotics with their patients; lack of familiarity and stigma contributed to patients’ negative perceptions about this class of medication.
- Education about approved medications for bipolar I mania and bipolar I depression and better provider/patient communication surrounding medication choice could enhance atypical antipsychotic acceptability for treating bipolar I disorder.
References (15)

- US Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER). Guidance for Industry and Review Staff; Labeling for Human Prescription Drug and Biological Products — Determining Established Pharmacologic Class for Use in the Highlights of Prescribing Information; Good Review Practice. Accessed January 15, 2023. https://www.fda.gov/media/77834/download
- Zohar J, Nutt DJ, Kupfer DJ, et al. A proposal for an updated neuropsychopharmacological nomenclature. Eur Neuropsychopharmacol. 2014;24(7):1005–1014. PubMed CrossRef
- Minami F, Zohar J, Suzuki T, et al. Discrepancies between nomenclature and indications of psychotropics. Pharmacopsychiatry. 2019;52(4):175–179. PubMed CrossRef
- Caraci F, Enna SJ, Zohar J, et al. A new nomenclature for classifying psychotropic drugs. Br J Clin Pharmacol. 2017;83(8):1614–1616. PubMed CrossRef
- Howland RH. A proposed system for classifying psychotropic drugs. J Psychosoc Nurs Ment Health Serv. 2014;52(12):13–15. PubMed CrossRef
- Stahl SM. Classifying psychotropic drugs by mode of action and not by target disorder. CNS Spectr. 2013;18(3):113–117. PubMed CrossRef
- Uchida H. Neuroscience-based Nomenclature: what is it, why is it needed, and what comes next? Psychiatry Clin Neurosci. 2018;72(2):50–51. PubMed CrossRef
- Uchida H, Fleischhacker W, Juckel G, et al. Naming for psychotropic drugs: dilemma and challenge. Pharmacopsychiatry. 2017;50(1):1–2. PubMed CrossRef
- Zohar J, Allgulander C. Antipsychotics in anxiety disorders: an oxymoron or a reflection of non-adequate nomenclature? Eur Neuropsychopharmacol. 2011;21(6):427–428. PubMed CrossRef
- Zohar J, Stahl S, Moller HJ, et al. A review of the current nomenclature for psychotropic agents and an introduction to the neuroscience-based nomenclature. Eur Neuropsychopharmacol. 2015;25(12):2318–2325. PubMed CrossRef
- Sajatovic M, Jenkins JH. Is antipsychotic medication stigmatizing for people with mental illness? Int Rev Psychiatry. 2007;19(2):107–112. PubMed CrossRef
- Blixen C, Perzynski AT, Bukach A, et al. Patients’ perceptions of barriers to self-managing bipolar disorder: a qualitative study. Int J Soc Psychiatry. 2016;62(7):635–644. PubMed CrossRef
- Yatham LN, Kennedy SH, Parikh SV, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord. 2018;20(2):97–170. PubMed CrossRef
- Liauw SS, McIntyre RS. Atypical antipsychotic tolerability and switching strategies in bipolar disorder. Expert Opin Pharmacother. 2010;11(17):2827–2837. PubMed CrossRef
- Sajatovic M, DiBiasi F, Legacy SN. Attitudes toward antipsychotic treatment among patients with bipolar disorders and their clinicians: a systematic review. Neuropsychiatr Dis Treat. 2017;13:2285–2296. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite