LESSONS LEARNED AT THE INTERFACE OF MEDICINE AND PSYCHIATRY
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord 2023;25(3):22f03305
To cite: Paudel S, Donovan AL, Petriceks A, et al. Drug-induced abnormal involuntary movements: prevalence and treatment. Prim Care Companion CNS Disord. 2023;25(3):22f03305.
To share: https://doi.org/10.4088/PCC.22f03305
© 2023 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts
‡All authors contributed equally to the work.
*Corresponding author: Shreedhar Paudel, MD, MPH, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, 55 Fruit St, Boston, MA 02114 ([email protected]).
Have you ever wondered how often abnormal involuntary movements (AIMs) develop following use of medications? Have you been unsure about which aspects of the history, physical examination, and laboratory tests are most likely to yield meaningful information about the etiology and treatment of drug-induced abnormal movements? Have you been uncertain about how (and why) you should treat AIMs? If so, then the following case vignette and discussion should prove useful.
CASE VIGNETTE
Ms A, a 23-year-old woman, was brought to the emergency department (ED) by police because of increasingly disruptive behaviors in her apartment building over the past week. She was disheveled, agitated, and yelling obscenities. She described hearing neighbors accuse her of poisoning their pets; moreover, she believed that they were “bugging” her apartment and watching her through her TV. Her medical history was notable for having systemic lupus erythematosus and chronic kidney disease. Although she had an episode of depression during college, she had not received psychiatric treatment for the past 2 years.
Ms A screamed loudly and made threatening gestures when the ED physician attempted to perform a physical examination. Feeling unsafe, the physician called for the assistance of hospital security officers to ensure his own safety, that of Ms A, and others in the ED. Intramuscular (IM) haloperidol (5 mg) and lorazepam (1 mg) were administered, and she calmed down over the next hour. However, a bilateral coarse tremor developed in her hands, her arms became increasingly stiff/rigid, her eyes appeared to roll upward, and she developed torticollis, which terrified her. Other than tachycardia, her vital signs were stable, including a normal oxygen saturation and temperature.
Ms A was given IM benztropine 1 mg. Over the next 30 minutes, her tremor, rigidity, eye movement deviation, and torticollis gradually resolved. She relaxed and allowed her blood to be drawn. All laboratory testing, including a comprehensive metabolic panel, liver function tests, thyroid function tests, and serum and urine toxicology screens, were within normal limits. A physical and neurologic examination revealed no additional abnormalities. The ED staff subsequently modified their treatment plan.
DISCUSSION
What Types of Involuntary Movements Exist, and How Can They Be Conceptualized?
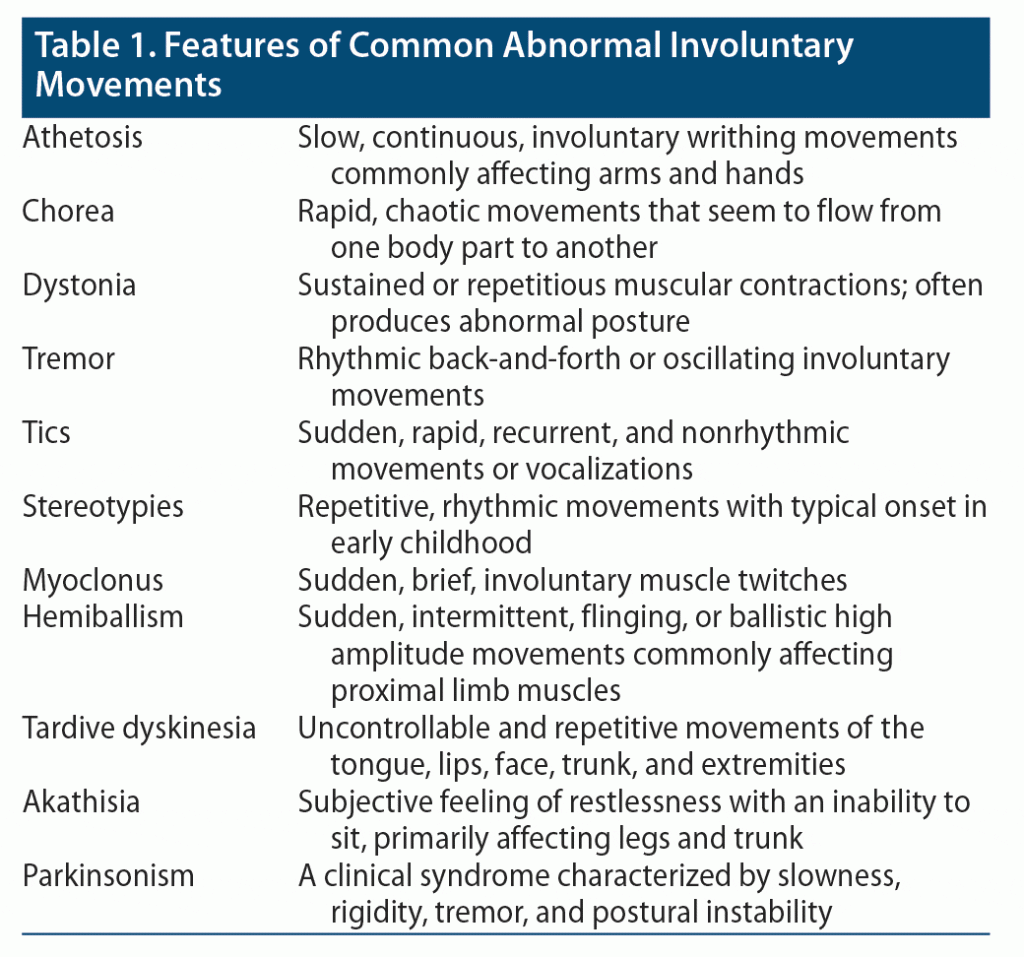
AIMs comprise a group of uncontrolled and unintended movements that may manifest as tremor, tics, myoclonus, chorea, athetosis, dystonia, akathisia, tardive dyskinesia (TD), stereotypies, hemiballism, or parkinsonism. Clinical features are provided in Table 1.1 Involuntary movements can be conceptualized according to their location and distribution. For example, focal movements are manifest in one anatomic region, while multifocal movements involve multiple regions. Furthermore, generalized movements affect the entire body. In clinical practice, involuntary movements are often divided into 2 broad categories: hypokinetic or hyperkinetic disorders. Hypokinetic disorders are characterized by diminished movements and a paucity of movements (eg, Parkinsonian disorders). Hyperkinetic disorders are manifest by unwanted or excessive movements (eg, tremor, tics, myoclonus). Many hyperkinetic disorders comprise a combination of hyperkinesias. Of note, a subset of movement disorders can be caused by psychological factors, hence referred to as psychogenic movement disorders.2
Which Aspects of the Patient’s History May Influence the Differential Diagnosis and Workup?
Obtaining a complete psychiatric history from someone who has developed abnormal muscle movements is crucial to refining the differential diagnosis and mapping out a treatment plan. Details of the location and nature of the abnormal movements, their onset and progression, and their aggravating and relieving factors are essential. For instance, athetosis and hyperkinetic movement disorders tend to affect the face, neck, and extremities,1 while TD is largely localized to the head, neck, and torso.3 Those with these conditions might also have rhythmic movements (such as tremors),4 while nonrhythmic movements occur in tics,5 and stereotypic movements occur in TD and myoclonus.3,6 Knowledge of the current and past medication history also facilitates making the diagnosis of medication-induced movement disorders (eg, akathisia, extrapyramidal symptoms [EPS], TD, athetosis, and tremors). For instance, recent use of a dopamine-blocking antipsychotic medication might be the cause of akathisia or EPS,7 while use of lithium, antiepileptic drugs (AEDs), or antidepressants can cause prominent tremors.8 In addition, knowledge of the medical history guides formulation of the differential diagnosis. For example, a recent history of an infection (eg, tuberculosis or syphilis) can cause dystonia,9 encephalitis can cause multiple movement disorders including dystonia and akathisia,10 and rheumatic fever and herpes simplex can cause chorea.11 Similarly, a history of an autoimmune disease (such as systemic lupus erythematosus) or an endocrine disorder (like hyperthyroidism) can be the cause of chorea and tremor, respectively. Another important aspect of the history is a review of the neurologic and neurodevelopmental history. A history of a seizure disorder might provide insight into the causes of stereotypic movements. A history of a recent stroke might contribute to athetosis, while a traumatic brain injury or Parkinson’s disease (PD) can cause akathisia or tremors. Similarly, patients with autism spectrum disorder often have stereotypic movements, while motor tics are often comorbid with attention-deficit/hyperactivity disorder (ADHD), Tourette syndrome, or obsessive-compulsive disorder (OCD). Another important part of the history is the family history of inherited and genetic disorders (like Huntington’s disease and Wilson’s disease) that affect the basal ganglia and typically present with abnormal movements (including chorea, athetosis, and dystonia). Similarly, a substance use history that includes prior manifestations of intoxication or withdrawal should be obtained.
Which Aspects of the Physical Examination Should Be Assessed in Those With Abnormal Involuntary Movements?
Like the historical components described previously, a detailed physical examination is paramount to diagnosing a movement disorder. Based on the components obtained in the history, the examination can be tailored to certain bodily systems. Determination of vital signs might provide evidence of infection (with fever) or tachycardia (as seen in hyperthyroidism). Similarly, correlates of intoxication or withdrawal from substances might be apparent during the head-to-toe examination. A detailed mental status examination is the standard psychiatric examination.12 Rumination, worry, obsessions, and impaired executive functioning can be associated with tremors or tics. These features might suggest an anxiety disorder, OCD, or ADHD as the cause of involuntary muscle movements. Similarly, a detailed neurologic examination will guide the diagnosis of multiple neurologic conditions and the nature of the movement disorders.13 Major components of the examination include an examination of the cranial nerves; a detailed motor examination that includes assessment of coordination, gait, and reflexes; and a detailed sensory examination. The value of the examination can be 2-fold: establishing the nature of the movement disorder (eg, chorea, athetosis, dystonia, tremors, TD, EPS) and pointing to the cause of the disorders (eg, PD, stroke, basal ganglia disease).
Which Laboratory Tests Should Be Assessed In a Patient With Abnormal Involuntary Movements?
Many movement disorders can be diagnosed by the history and a physical examination.14 However, laboratory testing is often indicated to assess treatable causes of abnormal movements. Electrolytes should be checked, since abnormalities, especially hypomagnesemia and hypocalcemia, can cause abnormal movements.15 Uremia can cause movement abnormalities, necessitating the screening of blood urea nitrogen and creatinine.16 Liver function tests are also indicated, as some hepatic diseases including Wilson’s disease and hepatic encephalopathy are associated with abnormal movements.17 Given that hyperthyroidism is associated with abnormal movements, thyroid screening is also important.18 A toxicology screen is also useful, as some substances (eg, stimulants) can induce abnormal movements.19
Associated symptoms may indicate the need for further laboratory testing. For example, muscular rigidity in the setting of fever and a change in the mental status should prompt immediate consideration of neuroleptic malignant syndrome. In this setting, complete blood count and creatine phosphokinase testing are indicated. If the clinical history suggests an acute stroke, head imaging is also indicated.
How Can Abnormal Involuntary Movements Be Treated?
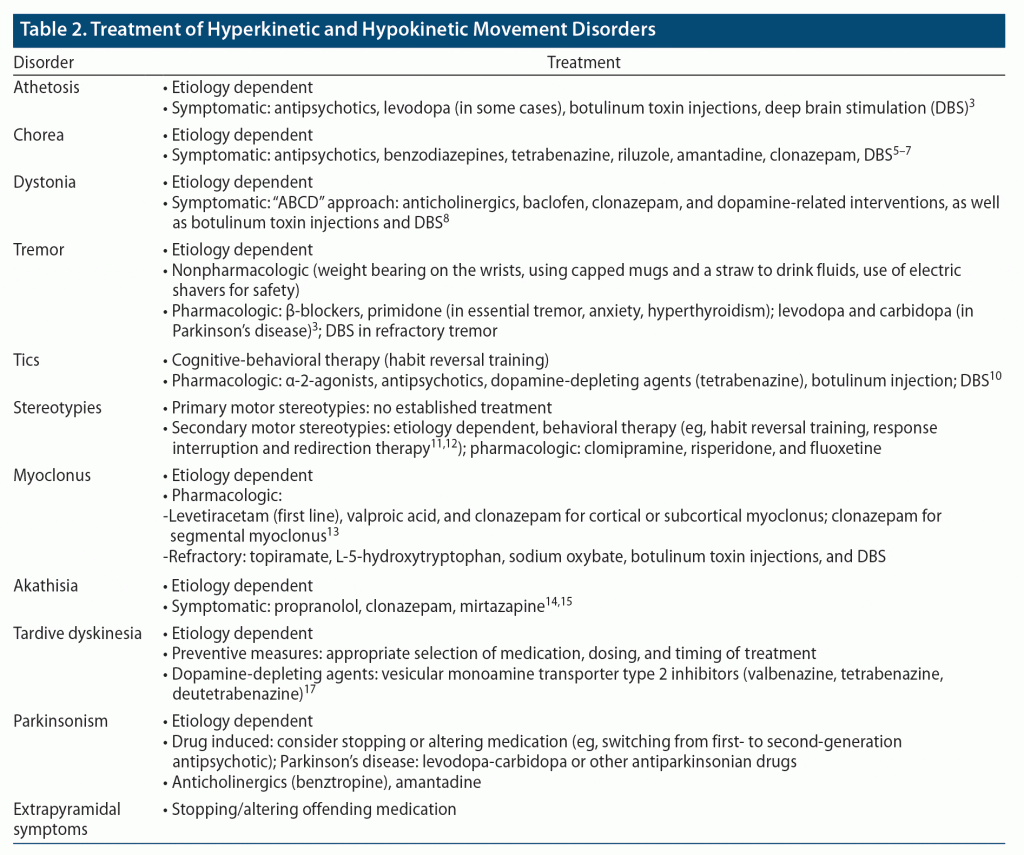
Treatment of AIMs is based on the underlying etiology (eg, metabolic, drug induced). However, symptomatic treatments are indicated to reduce motoric and psychological symptoms that impair social function and worsen quality of life. Recommended treatments for each type of AIM are summarized in Table 21,20–30 and described below. Some of these treatments are within the scope of practice of primary care physicians; others will require referral to specialists.
Athetosis. Treatment of athetosis includes use of dopamine-depleting agents (such as antipsychotic medications, eg, fluphenazine). Although dopamine agonists (eg, levodopa) may suppress athetosis, their long-term use may be limited due to side effects.1 Other treatments include injection of botulinum toxin (BoNT; eg, onabotulinumtoxinA [Botox] or rimabotulinumtoxinB [Myobloc]), which may relieve athetosis temporarily but may need to be repeated at 3-month intervals given the short duration of effect, and deep brain stimulation.1,31
Chorea. Antipsychotic medications have also been helpful for the treatment of chorea; second-generation antipsychotics (SGAs) (eg, olanzapine or risperidone) are preferred over first-generation antipsychotics (FGAs) (eg, haloperidol) given their lower risk of inducing EPS.21 These medications can also help with co-occurring neuropsychiatric symptoms like psychosis.22 In addition, vesicular monoamine transporter 2 (VMAT-2) inhibitors (eg, tetrabenazine, deutetrabenazine), benzothiazoles (eg, riluzole), and amantadine have also decreased chorea.21 Benzodiazepines (eg, clonazepam), which are often used to treat comorbid anxiety in patients with chorea, have also decreased motor symptoms.1,22 Several small studies22 have found that deep brain stimulation helps to reduce motoric dysfunction in those with refractory chorea, since it has fewer side effects than pharmacologic therapies. Larger randomized controlled studies investigating the long-term efficacy and safety of deep brain stimulation are underway.
Dystonia. Symptomatic treatment of dystonia includes use of oral medications, BoNT injections, and deep brain stimulation. Treatment with oral medications is based on the “ABCD” approach: anticholinergics (primarily trihexyphenidyl), GABAergic medications such as baclofen (a muscle relaxant) or clonazepam (a benzodiazepine), and dopamine-related interventions.23 While counterintuitive, both dopaminergic (eg, levodopa) and dopamine-depleting agents (eg, tetrabenazine, antipsychotics) have decreased dystonia. Among these, tetrabenazine and related compounds are rarely used as first-line agents unless they are indicated for treatment of TD. Other than for dopa-responsive dystonia, where small doses of levodopa (≤ 300 mg/day) can dramatically improve dystonia, the effects of these oral medications tend to be modest and temporary. Conversely, deep brain stimulation that is directed at the function of the GPi can provide long-lasting dramatic improvement of dystonia that leads to significant improvement in the quality of life for more than 10 years.1 BoNT injections can also be rapidly effective in relieving focal dystonia and related pain, with efficacy lasting up to several months.
Tremor. Nonpharmacologic treatments (eg, weight bearing on the wrists, using capped mugs and a straw to drink fluids, use of electric shavers for safety) may be sufficient to manage mild cases of tremor, especially essential tremor, or they may be used to augment pharmacologic options.1 These include use of β-blockers, like propranolol, and an AED, like primidone, which alone or in combination with propranolol can reduce essential tremor as well as tremor associated with hyperadrenergic states such as hyperthyroidism and anxiety. Of note, since anxiety can precipitate and worsen almost all types of tremors, its treatment will help to decrease them. Levodopa and carbidopa can be effective in PD-related resting tremor. In refractory tremor cases, deep brain stimulation that is directed at the ventral intermediate nucleus of the thalamus can dramatically suppress tremor, while other therapies including focused ultrasound and gamma knife therapy are under investigation.1,32
Tic. Nonpharmacologic interventions like habit reversal training, a type of cognitive-behavioral therapy (CBT), may reduce tics or delay their onset.1 Pharmacologic approaches include use of α-adrenergic agonists (eg, clonidine and guanfacine) that can suppress tics, although their mechanism of action remains unknown. Dopamine receptor antagonists, such as the antipsychotics (eg, haloperidol, fluphenazine, and pimozide), can suppress both vocal and motor tics in approximately 80% of patients, with haloperidol and fluphenazine having received US Food and Drug Administration (FDA) approval for use in Tourette syndrome. Similarly, the dopamine-depleting medications, like tetrabenazine, can also reduce tics. For refractory and particularly disabling tics, BoNT injections may be temporarily effective, while deep brain stimulation can suppress motor and vocal tics; however, the most appropriate electrode placement is under investigation.24
Stereotypies. While most stereotypies tend to cause mild, if any, physical or psychological distress and do not require treatment, those that are more bothersome can respond to behavioral therapies such as habit reversal training and response interruption and redirection therapy when applied consistently.25,26 While there are no known pharmacologic treatments for primary stereotypies, secondary stereotypies, especially when they are associated with self-injurious behavior, can be alleviated via use of psychotropics such as clomipramine (a tricyclic antidepressant), risperidone (an atypical antipsychotic), and fluoxetine (a selective serotonin reuptake inhibitor [SSRI]).25
Myoclonus. Pharmacologic agents including the AEDs (levetiracetam, valproic acid) and the benzodiazepine clonazepam are first-line treatments for cortical or subcortical myoclonus.27 Clonazepam is also a first-line treatment for segmental and spinal myoclonus that is otherwise largely unresponsive to AEDs. Other second-line agents that target specific types of refractory myoclonus include topiramate, L-5-hydroxytryptophan, and sodium oxybate. BoNT injection can treat localized spinal myoclonus, while deep brain stimulation that targets the GPi bilaterally can be effective in refractory myoclonus dystonia. Of note, treatment for functional (or psychogenic) myoclonus entails a multimodal and multidisciplinary approach that includes use of psychotropics and physical and occupational therapy.27
Akathisia. The first step in treating medication-induced akathisia should be management of the offending agent. In the case of non–antipsychotic medications, the offending agent should be discontinued immediately, especially in the case of acute akathisia. However, when antipsychotic medications are etiologic, options include dose reduction, switching from a higher-potency antipsychotic (eg, a FGA) to a lower-potency one (eg, a SGA), or switching to clozapine or quetiapine, which are less likely to cause akathisia.28,30 In addition to addressing the underlying etiology, first-line treatments for akathisia include use of non-selective β-blockers (eg, propranolol) and benzodiazepines (eg, clonazepam). 5-hydroxytryptamine2A antagonists (5-HT2A), such as mirtazapine or trazodone, are second-line treatments for akathisia, especially when it is induced by an FGA. It is important to note that clonidine is not recommended for treatment of akathisia due to its significant side effects (eg, hypotension, sedation). Anticholinergics are also not recommended due to their lack of efficacy for akathisia as well as concerns for significant side effects (eg, delirium, cognitive impairment).28,30
Parkinsonism. The first steps in addressing drug-induced parkinsonism are like those for akathisia: management of the offending medication via discontinuation, dose reduction, or switching to another agent.30 Anticholinergics (eg, benztropine) have historically been considered a first-line treatment for parkinsonism. However, given the significant side effects associated with prolonged use, they are not currently recommended for prophylactic use; rather, they are introduced only when parkinsonism exists and are limited to short-term use. Amantadine is preferred over anticholinergics due to its relatively safer side effect profile, especially in those who are prone to experiencing anticholinergic side effects, like the elderly or those taking SGAs such as clozapine.30
Tardive dyskinesia. In contrast to akathisia or parkinsonism, management of the TD-causing agent (eg, an antipsychotic) or withdrawal from, dose reduction of, or switching to a different medication may not improve TD and may even worsen it.30,33 VMAT-2 inhibitors such as valbenazine or deutetrabenazine, also known as dopamine-depleting agents, are currently the mainstay of TD treatment. Tetrabenazine is an earlier VMAT-2 inhibitor; however, it is used as an agent of last resort due to its risk of significant side effects (eg, depression and suicidal ideation). In cases in which withdrawal of an antipsychotic does not reduce TD and when a VMAT-2 inhibitor or other agents are not used, some evidence indicates that switching to clozapine monotherapy can treat or reduce antipsychotic-induced TD. Amantadine may also be used to treat TD; however, evidence supporting its use is still weak.
Co-occurring psychological symptoms. Concomitant use of psychotropic medications may be indicated to target psychological symptoms that co-occur with AIMs. These agents include antidepressants (eg, SSRIs) for treatment of depression, a short course of benzodiazepines (eg, clonazepam) for treatment of anxiety, an SGA (eg, clozapine, quetiapine, or olanzapine) for treatment of psychosis, and AEDs (eg, valproic acid) that may be useful in the long-term management of aggression and irritability.22
How Often Do Extrapyramidal Symptoms Develop After Use of Dopamine-Blocking Agents or in Those With Neurologic/Neuropsychiatric Conditions?
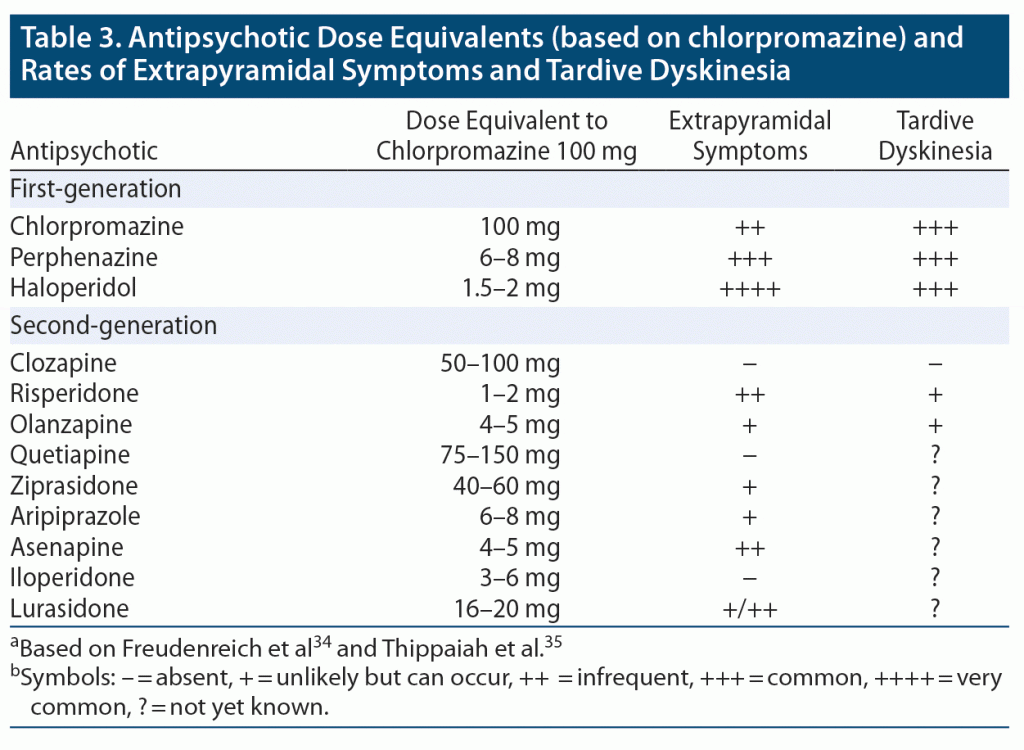
EPS (including akathisia, dystonia, parkinsonism, and TD) can develop with use of all antipsychotic agents; however, they are more prominent with FGAs, with haloperidol being the most likely to induce EPS, followed by perphenazine and chlorpromazine. All 3 FGAs are equally likely to cause TD, with an incidence of TD from FGAs being 4%–8% per year (about 3 times the annual risk of that caused by SGAs) (Table 3).34,35
Among SGAs, risperidone, asenapine, and lurasidone are more likely to induce EPS than are clozapine, quetiapine, and iloperidone. TD has been reported less frequently in association with risperidone and olanzapine than with FGAs, while data are lacking for the other SGAs. EPS tends to appear early after use of antipsychotics, while TD, as its name implies, is present later in treatment. Table 3 presents FGAs and SGAs with equivalent chlorpromazine doses and the relative frequency of EPS and TD.34,35
EPS is also common in certain neuropsychiatric disorders, such as Alzheimer’s disease (AD), dementia with Lewy bodies, and PD with and without dementia. The overall presence of EPS in these disorders has been proportionally linked with a higher illness severity and related morbidity.36,37 The reported incidence of EPS in AD varies from 12% in mild stages, up to 92% in more advanced stages of the disease.38 The presence or absence of EPS also determines the AD phenotypes: “cognitive/pure” and “cognitive/motor,” with the latter phenotype showing a more rapid disease progression. EPS has been reported to be more frequent in individuals with PD with dementia and dementia with Lewy bodies than in those with PD without dementia. The presence of EPS has been correlated with a higher degree of cognitive impairment.36 While the severity of parkinsonism can be especially high in PD with dementia, this has not been correlated with a more protracted course of the disease.
Case Vignette: What Happened to Ms A?
Ms A was admitted to the hospital medicine service with ongoing consultation from the rheumatology and psychiatry departments. She was diagnosed with an acute dystonic reaction due to administration of haloperidol, and an adverse reaction warning was entered into her electronic medical record. She was started on oral benztropine 1 mg 3 times/day for 3 days as prophylaxis against the return of acute dystonia. Ms A was also diagnosed with a substance-induced mood disorder (bipolar disorder with psychotic features triggered by use of corticosteroids). She was prescribed oral lorazepam 0.5 mg 3 times/day to treat her manic symptoms (eg, increased energy, decreased need for sleep, irritability, rapid speech). The dose of methylprednisolone was rapidly tapered and discontinued. Over the next several days, Ms A’s mania and psychotic symptoms resolved; her dystonia did not recur. Ms A was discharged from the hospital with outpatient rheumatology follow-up.
CONCLUSION
Clinicians are often faced with the task of categorizing the nature of AIMs and establishing their etiology (via a detailed history, physical/neurologic examination, and laboratory testing). Since treatment is predicated on etiology, a thorough approach to the problem facilitates implementation of effective treatment and minimizes adverse side effects that often accompany drug therapies.
Submitted: April 13, 2022; accepted September 27, 2022.
Published online: May 11, 2023.
Relevant financial relationships: Dr Vyas has received research salary support from Nestlé Purina PetCare Company. Drs Paudel, Donovan, Van Alphen, and Stern and Mr Petriceks report no relevant financial relationships.
Funding/support: None.
Clinical Points
- Involuntary movements are nonvolitional, abnormal, unintended movements that may manifest as tremors, tics, myoclonus, chorea, athetosis, dystonia, akathisia, tardive dyskinesia, stereotypies, or hemiballism.
- Involuntary movements can be categorized according to their location and distribution or by their neuroanatomical basis and can also be labeled as hyperkinetic or hypokinetic.
- Major components of the physical examination include an examination of the cranial nerves; a detailed motor examination that includes assessment of coordination, gait, and reflexes; and a detailed sensory examination.
- Treatment of involuntary movements is based on their underlying etiology; however, symptomatic treatments are indicated to reduce motoric and psychological symptoms that impair social function and worsen quality of life.
References (38)

- Kaufman DM, Geyer HL, Milstein MJ. Involuntary movement disorders. In: Kaufman’s Clinical Neurology for Psychiatrists. 2017:389–447. doi:10.1016/b978-0-323-41559-0.00018-6
- Peckham EL, Hallett M. Psychogenic movement disorders. Neurol Clin. 2009;27(3):801–819, vii. PubMed CrossRef
- Waln O, Jankovic J. An update on tardive dyskinesia: from phenomenology to treatment. Tremor Other Hyperkinet Mov (N Y). 2013;3:tre-03-161-4138-1. PubMed CrossRef
- Sanger TD, Chen D, Fehlings DL, et al. Definition and classification of hyperkinetic movements in childhood. Mov Disord. 2010;25(11):1538–1549. PubMed CrossRef
- Ueda K, Black KJ. A comprehensive review of tic disorders in children. J Clin Med. 2021;10(11):2479. PubMed CrossRef
- Kojovic M, Cordivari C, Bhatia K. Myoclonic disorders: a practical approach for diagnosis and treatment. Ther Adv Neurol Disord. 2011;4(1):47–62. PubMed CrossRef
- D’Souza RS, Hooten WM. Extrapyramidal symptoms. In: StatPearls [Internet]. Treasure Island, Florida: StatPearls Publishing; 2022.
- Shin HW, Chung SJ. Drug-induced parkinsonism. J Clin Neurol. 2012;8(1):15–21. PubMed CrossRef
- Jinnah HA, Factor SA. Diagnosis and treatment of dystonia. Neurol Clin. 2015;33(1):77–100. PubMed CrossRef
- Jhunjhunwala K, Netravathi M, Pal PK. Movement disorders of probable infectious origin. Ann Indian Acad Neurol. 2014;17(3):292–297. PubMed CrossRef
- Baizabal-Carvallo JF, Cardoso F. Chorea in children: etiology, diagnostic approach and management. J Neural Transm (Vienna). 2020;127(10):1323–1342. PubMed CrossRef
- Voss RM, Das JM. Mental status examination. In: StatPearls [Internet]. Treasure Island, Florida: StatPearls Publishing; 2021.
- Benjamin S, Lauterbach MD. The neurological examination adapted for neuropsychiatry. CNS Spectr. 2018;23(3):219–227. PubMed CrossRef
- Flaherty AW, Ivkovic A. Movement disorders. In: Stern TA, Fava M, Wilens TE, et al, eds. Massachusetts General Hospital Comprehensive Clinical Psychiatry. 2nd ed. London: Elsevier; 2016.
- Espay AJ. Neurologic complications of electrolyte disturbances and acid-base balance. Handb Clin Neurol. 2014;119:365–382. PubMed CrossRef
- Rao AR, Kumar P, Gunasekaran V, et al. Reversible chorea secondary to uremia in an older adult. Aging Med (Milton). 2019;2(2):118–120. PubMed CrossRef
- Mulroy E, Baschieri F, Magrinelli F, et al. Movement disorders and liver disease. Mov Disord Clin Pract (Hoboken). 2021;8(6):828–842. PubMed CrossRef
- Delhasse S, Debove I, Arnold-Kunz G, et al. Erratic movement disorders disclosing Graves’ disease and paralleling thyroid function but not autoantibody levels. J Int Med Res. 2019;47(3):1378–1386. PubMed CrossRef
- Freudenreich O, Flaherty AW. Patients with abnormal movements. In: Stern TA, Freudenreich O, Smith FA, et al, eds. Massachusetts General Hospital Handbook of General Hospital Psychiatry. 7th ed. London: Elsevier; 2018.
- Bhidayasiri R, Truong DD. Chorea and related disorders. Postgrad Med J. 2004;80(947):527–534. PubMed CrossRef
- Burgunder JM. Recent advances in the management of choreas. Ther Adv Neurol Disord. 2013;6(2):117–127. PubMed CrossRef
- Gibson JS, Claassen DO. State-of-the-art pharmacological approaches to reduce chorea in Huntington’s disease. Expert Opin Pharmacother. 2021;22(8):1015–1024. PubMed CrossRef
- Termsarasab P, Thammongkolchai T, Frucht SJ. Medical treatment of dystonia. J Clin Mov Disord. 2016;3(1):19. PubMed CrossRef
- Swain JE, Scahill L, Lombroso PJ, et al. Tourette syndrome and tic disorders: a decade of progress. J Am Acad Child Adolesc Psychiatry. 2007;46(8):947–968. PubMed CrossRef
- Katherine M. Stereotypic movement disorders. Semin Pediatr Neurol. 2018;25:19–24. PubMed CrossRef
- Sheehey PH, Wells JC. Using response interruption and redirection to reduce vocal stereotypy. Interv Sch Clin. 2018;53(3):171–176. CrossRef
- Levy A, Chen R. Myoclonus: pathophysiology and treatment options. Curr Treat Options Neurol. 2016;18(5):21. PubMed CrossRef
- Zareifopoulos N, Katsaraki M, Stratos P, et al. Pathophysiology and management of akathisia 70 years after the introduction of the chlorpromazine, the first antipsychotic. Eur Rev Med Pharmacol Sci. 2021;25(14):4746–4756. PubMed
- Blaisdell GD. Akathisia: a comprehensive review and treatment summary. Pharmacopsychiatry. 1994;27(4):139–146. PubMed CrossRef
- Stahl SM, Sy S, Maguire GA. How and when to treat the most common adverse effects of antipsychotics: Expert review from research to clinical practice. Acta Psychiatr Scand. 2021;143(2):172–180. PubMed CrossRef
- Miller F. Spasticity, Spasticity, dystonia, and athetosis management in the upper extremity in cerebral palsy. In: Miller F, Bachrach S, Lennon N, et al, eds. Cerebral Palsy. Cham: Springer; 2020.
- Armstrong MJ, Okun MS. Diagnosis and treatment of parkinson disease: a review. JAMA. 2020;323(6):548–560. PubMed CrossRef
- Bhidayasiri R, Jitkritsadakul O, Friedman JH, et al. Updating the recommendations for treatment of tardive syndromes: a systematic review of new evidence and practical treatment algorithm. J Neurol Sci. 2018;389:67–75. PubMed CrossRef
- Freudenreich O, Goff D, Henderson DC. Antipsychotic drugs. In: Stern TA, Fava M, Wilens TE, et al, eds. Massachusetts General Hospital Comprehensive Clinical Psychiatry. 2nd ed. London: Elsevier; 2016:475–488.e6.
- Thippaiah SM, Fargason RE, Birur B. Switching antipsychotics: a guide to dose equivalents. Curr Psychiatr. 2021;20(4):13. CrossRef
- Burn DJ, Rowan EN, Minett T, et al. Extrapyramidal features in Parkinson’s disease with and without dementia and dementia with Lewy bodies: a cross-sectional comparative study. Mov Disord. 2003;18(8):884–889. PubMed CrossRef
- Tsolaki M, Kokarida K, Iakovidou V, et al. Extrapyramidal symptoms and signs in Alzheimer’s disease: prevalence and correlation with the first symptom. Am J Alzheimers Dis Other Demen. 2001;16(5):268–278. PubMed CrossRef
- Peralta V, Cuesta MJ. Motor abnormalities: from neurodevelopmental to neurodegenerative through “functional” (neuro)psychiatric disorders. Schizophr Bull. 2017;43(5):956–971. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite