ABSTRACT
Objective: Early response to selective serotonin reuptake inhibitors (SSRIs) at 4 weeks may predict response at 12 weeks. The objective of this study was to examine if early response to SSRIs can reliably indicate future response to the same drug, while simultaneously comparing 2 medications frequently used to treat obsessive-compulsive disorder (OCD): sertraline and fluvoxamine.
Methods: This was a randomized, open-label prospective study of 50 drug-naive patients with OCD (per DSM-5 criteria) conducted from January 2019 to November 2020 in the outpatient department of a tertiary care teaching hospital in North India. Patients were randomly assigned to receive either sertraline or fluvoxamine after assessing the severity of illness with the Yale-Brown Obsessive-Compulsive Scale (YBOCS). Following the induction phase of treatment for 2 weeks, patients were assessed for early response, defined as ≥ 20% reduction in YBOCS score after 4 weeks of reaching the minimum therapeutic dose of each drug. The final YBOCS assessment to evaluate response was conducted at 12 weeks.
Results: A total of 60% (n = 30) of patients with OCD showed early response at week 4 with a mean dose of sertraline 95 mg and fluvoxamine 102 mg/d. Both drugs showed significant reduction in YBOCS score at week 12. However, fluvoxamine fared significantly better than sertraline (P = .012).
Conclusions: The study results show that early response can predict future response to the drug with a good sensitivity and specificity.
Trial Registration: Clinical Trial Registry of India: CTRI/2020/01/022890
Prim Care Companion CNS Disord 2022;24(2):21m03065
To cite: Brar J, Sidana A, Chauhan N, et al. Early improvement as a predictor of treatment response in patients with obsessive-compulsive disorder: a 12-week randomized trial of sertraline and fluvoxamine. Prim Care Companion CNS Disord. 2022;24(2):21m03065.
To share: https://doi.org/10.4088/PCC.21m03065
© Copyright 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Government Medical College and Hospital, Chandigarh, India
*Corresponding author: Ajeet Sidana, MD, Department of Psychiatry, Government Medical College and Hospital, Level-5, Block-D, Sector-32, Chandigarh 160030, India ([email protected]).
Obsessive-compulsive disorder (OCD) is a common neuropsychiatric disorder that follows a chronic course with fluctuations in the severity of symptoms. The lifetime prevalence of OCD is 1%–3%.1 The phenomenology of OCD is heterogenous, characterized by presence of obsessions and compulsions. Obsessions are defined as repetitive, intrusive, unwanted, absurd, and distressing thoughts, images, or urges that are considered to be ones’ own but are involuntary. Compulsions are defined as behaviors (overt) or mental acts (covert) that are not pleasurable but simply aimed at reducing the anxiety associated with the obsessions or to prevent a feared consequence.
Abnormalities in a range of neurotransmitter systems have been shown to contribute to OCD. These include serotonin, dopamine, glutamate, and γ-aminobutyric acid (GABA) neurotransmitters. This understanding has led to the use of selective serotonin reuptake inhibitors (SSRIs) for treatment of OCD, which are efficacious and well tolerated. Literature suggests that approximately 40%–70% of patients show an adequate response to a first trial of SSRI with a remission rate of 10%–40%.2 The rate of response to a subsequent trial of SSRI decreases to 0%–20%.3 An adequate number of placebo-controlled studies have shown that all SSRIs are more efficacious than placebo for OCD.4,5 However, the literature does not solve the decision-making dilemma of which SSRI to try first.
Also, the adequate duration of an OCD treatment trial is currently agreed to be 12 weeks.6 Given the impact of OCD on quality of life and its economic burden, attempts should be made to revise the recommended minimum treatment duration before a change in treatment strategy is considered for favorable outcomes.
A prospective study7 from Brazil with 128 participants found that early improvement, defined as a ≥ 20% reduction in baseline Yale-Brown Obsessive-Compulsive Scale (YBOCS) score at 4 weeks, predicted response at 12 weeks with 75.6% sensitivity and 61.9% specificity. Early response was the strongest predictor of response at 12 weeks when compared with sociodemographic and other clinical variables.7 However, Indian literature in this regard is lacking.
Therefore, the objective of this study was to examine the time course of early response to SSRIs and whether this early response can reliably indicate future response to the same drug while simultaneously comparing 2 medications frequently used to treat OCD: sertraline and fluvoxamine.
METHODS
This was a randomized, open-label, prospective, intent-to-treat study comparing sertraline and fluvoxamine in patients with OCD. The study was conducted from January 2019 to November 2020 in the outpatient department of the department of psychiatry at a tertiary care teaching hospital in North India. Drug-naive patients with OCD, diagnosed per DSM-5 criteria,8 visiting the walk-in clinic of the department of psychiatry for the first time were approached.
A total of 50 patients aged 18–55 years who consented to participate were enrolled in the study. Patients with depression, anxiety, or any other comorbid psychiatric illness of diagnosable severity; comorbid intellectual disability; comorbid neurologic disorder; organic brain syndrome; dementia; epilepsy; or comorbid substance dependence (except caffeine and nicotine) were excluded from the study, as were actively suicidal patients with unstable medical/surgical illnesses and pregnant and lactating women. Written informed consent was taken from all participants.
Procedure
Sociodemographic and clinical details were recorded via a semistructured form. Severity of illness was assessed using the YBOCS.9 Baseline laboratory investigations including hemogram, renal function tests, liver function tests, serum electrolytes, and electrocardiogram were conducted to rule out underlying medical illnesses. Participants were then randomly assigned to either the sertraline or fluvoxamine group as per a computer-generated random table number in the ratio of 1:1. Participants in both groups were started on an initial dose of 50 mg/d. The initial 2 weeks were considered an induction period, during which doses were escalated to obtain at least a therapeutic dose at the end of 2 weeks, based on dose stratification categories used in previous meta-analytic studies.10,11 Participants were then observed for the next 4 weeks and were assessed for “early response,” defined as ≥ 20% reduction in YBOCS score based on a previous study.7 Concomitant medication for anxiety (ie, etizolam 0.25–0.5 mg/d) was coprescribed only during the induction phase if required and an attempt was made to gradually taper down and stop the medication before assessment for early response. Following this assessment at week 6, the dose of respective drug was escalated further by 50 mg every 4–7 days, guided by tolerability to maximum tolerable dose until week 8. Thereafter, participants were followed up at the end of 8 weeks and 12 weeks. The final clinical assessment at 12 weeks evaluated the response guided by the operational definitions of response (35% or more reduction in their YBOCS scores), partial response (≥ 25% but < 35% reduction in YBOCS scores), and nonresponse (< 25% reduction in YBOCS scores).12 Psychoeducation sessions were provided by the researcher at the time of patient intake as part of usual management protocol. Any patient requiring behavioral therapy was listed for the same and to be initiated only after 12 weeks of pharmacologic treatment by the other clinical psychologist (not a part of the study). Efforts were made to provide free samples of medications to patients who had affordability issues. Patients would be at liberty to discontinue the ongoing medication at any point, and those who developed any serious side effects were dropped from the study and managed according to established treatment guidelines.
The study was approved by the institutional ethics committee. The confidentiality of patient information was maintained, and principles of the Declaration of Helsinki13 and Indian Medical Council of Research14 were adhered to. The study was registered with the Clinical Trial Registry of India: CTRI/2020/01/022890.
Statistical Analysis
The statistical analysis was performed using the Statistical Package for the Social Sciences, version 16.0.15 Descriptive statistics and independent sample t test were used for analyzing and comparing sociodemographic variables between the 2 groups. Paired samples t test was used to examine difference between pre- and posttreatment clinical variables. χ2 test was used to compare response rates between the 2 drugs and to calculate sensitivity and specificity. The homogeneity of variance and covariance preassumptions was computed using Levene test. A P value of .05 was considered significant and .01 highly significant.
RESULTS
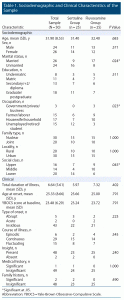
A total of 50 patients with OCD were included and equally randomly assigned to the sertraline or fluvoxamine groups. The sociodemographic and clinical characteristics of participants in both groups were comparable as shown in Table 1. All participants completed the study period of 12 weeks.
Participants in both groups had similar sociodemographic profiles except for a significant difference on marital status, occupation, and social class (see Table 1). The mean baseline ± SD YBOCS score of 23.48 ± 6.29 indicated moderate severity of illness. In the context of the clinical variables, both drug groups were comparable at baseline (see Table 1).
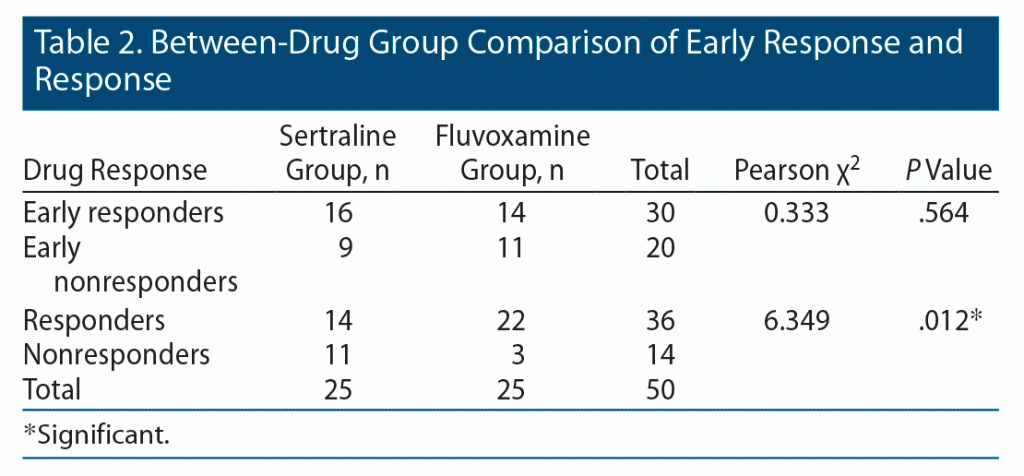
The number of early responders in both drug groups were comparable as shown in Table 2. The average minimum dose of sertraline and fluvoxamine achieved at the end of the induction period was 95 mg/d and 102 mg/d, respectively. However, the number of responders was significantly higher in the fluvoxamine group compared to the sertraline group at 12 weeks (Pearson χ2 = 6.349, P = .012). The mean maximum tolerable therapeutic doses of sertraline and fluvoxamine were 196 mg/d and 284 mg/d, respectively.
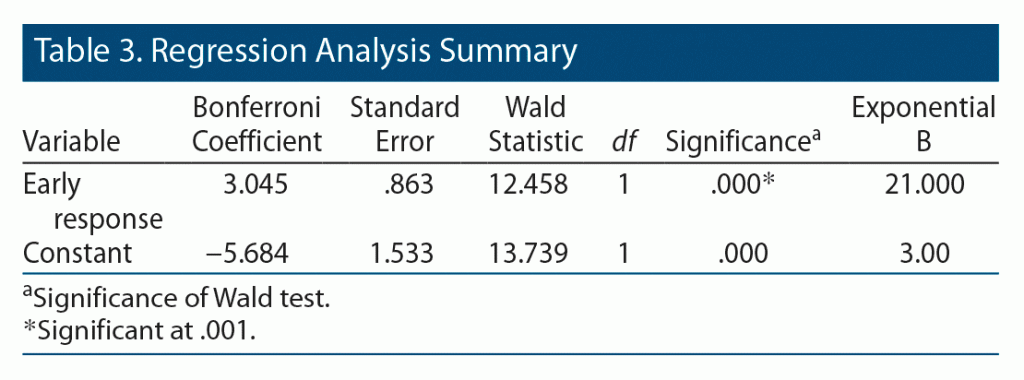
Of the total sample, 60% (n = 30) of patients showed early response at 4 weeks, defined as ≥ 20% reduction in YBOCS score. Of those responders, 93.3% showed a response, defined as ≥ 35% reduction in YBOCS score at the end of 12 weeks (Pearson χ2 = 16.93, P = .000). This early response predicted response at 12 weeks with 77.8% sensitivity and 85.7% specificity. Furthermore, 40% (n = 20) of patients did not show an early response. Of those patients, 8 showed a response at the end of 12 weeks. No significant difference was found between early responders and nonresponders regarding sociodemographic and clinical characteristics. The results of the regression analysis revealed a significant relationship between early response to treatment and response at 12 weeks (Table 3).
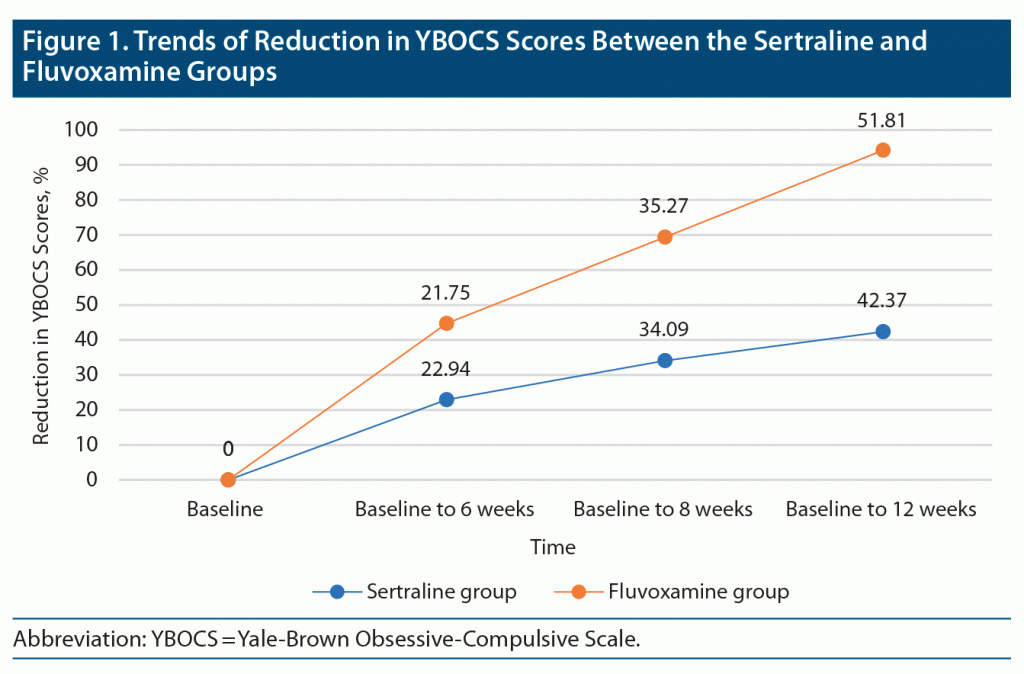
The mean YBOCS score (N = 50) after 12 weeks of treatment was 12.88, which showed a statistically significant reduction in the severity score (P = .000). However, the reduction in mean YBOCS scores across 12 weeks in both drug groups (sertraline group = 13.80, fluvoxamine group = 11.96) was statistically insignificant (P = .443). Figure 1 presents the percentage mean reduction in the YBOCS scores at various time intervals for both drug groups. The differences observed in the mean reductions in YBOCS scores between the 2 groups were found to be statistically insignificant at all assessment points. Also, both medications had similar side effect profiles, as shown in Table 4.
In keeping with the commonly available brands of the 2 medications, the mean per month cost of sertraline was 1,284 Indian rupees (US$ 17.22) and of fluvoxamine was 2,790 Indian rupees (US$ 37.41).
DISCUSSION
The present study assessed the time course of response to pharmacologic treatment in patients with OCD and whether an early improvement can predict future response to the treatment. Early response, defined as ≥ 20% reduction in YBOCS score, was observed earliest at 4 weeks after achieving the minimum therapeutic dose, contrary to the commonly held notion of delayed response in OCD and consistent with previous meta-analytic studies.16 This study clearly shows that early response to treatment can predict OCD treatment response with a notable sensitivity and specificity. This finding is consistent with previous studies.7,16 These results suggest that the concept of early response should be given due consideration while treatment-related decisions are made. Further, a small number of patients who did not show early response did show a response at 12 weeks, which substantiates the findings of a meta-analysis11 that higher doses of SSRI improve treatment response.
Additionally, the study results show that sertraline and fluvoxamine are equally efficacious in reducing symptom severity and are equally well tolerable. These findings are well substantiated by previous studies.5,17 The remarkable finding of the current study is that a significant number of participants tried on fluvoxamine culminate as responders, when compared with those in the sertraline group, suggesting a possible better response rate with fluvoxamine. Therefore, the findings of the index study suggest that fluvoxamine may be considered prior to sertraline when initiating treatment in drug-naive patients with OCD. This is significant when considering that patients who do not respond to a first SSRI trial are less likely to respond to subsequent trials, when compared with treatment-naive patients.3
However, there might be affordability issues with fluvoxamine. But, keeping in mind the direct and indirect economic costs of OCD,18 it might be justified to start with fluvoxamine, as the chances of a favorable treatment outcome are higher with fluvoxamine.
The stringent criteria of including drug-naive patients, excluding patients with comorbid depression, and avoiding use of neuroleptic medications and formal behavioral therapy accentuates the strength of the study. However, a small sample size and coincidental inclusion of more patients with moderate severity of illness are limitations of the index study.
CONCLUSION
The study findings conclude that early response to a SSRI trial in OCD can assuredly predict future response to that drug with good sensitivity and specificity. Only a small percentage of early nonresponders showed response at 12 weeks. Also, both sertraline and fluvoxamine were found to be equally efficacious, but fluvoxamine fared better if response rate is considered. Therefore, it might be safe to say that a long drug trial of 12 weeks may not be warranted to see a response in all patients with OCD if the patient does not show early improvement with 4 weeks of minimum therapeutic dose of an SSRI. The results of this study indicate the need for further research with a larger sample size so a revision in current understanding can be made for better therapeutic outcomes in patients with OCD.
Submitted: July 3, 2021; accepted October 14, 2021.
Published online: March 31, 2022.
Relevant financial relationships: None.
Funding/support: None.
Previous presentation: Presented at the 45th Annual Conference of Indian Psychiatric Society-North Zone; January 23–24, 2021; Chandigarh, India.
Clinical Points
- Almost two-thirds of drug-naive patients with obsessive-compulsive disorder show early response with sertraline and fluvoxamine.
- Early response predicts response at 12 weeks, with 77.8% sensitivity and 85.7% specificity.
- Both fluvoxamine and sertraline are equally effective and lead to significant reduction in Yale-Brown Obsessive-Compulsive Scale scores at 12 weeks.
References (18)

- Ruscio AM, Stein DJ, Chiu WT, et al. The epidemiology of obsessive-compulsive disorder in the National Comorbidity Survey replication. Mol Psychiatry. 2010;15(1):53–63. PubMed CrossRef
- Skapinakis P, Caldwell DM, Hollingworth W, et al. Pharmacological and psychotherapeutic interventions for management of obsessive-compulsive disorder in adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2016;3(8):730–739. PubMed CrossRef
- Koran L, Saxena S. Issues and strategies in treating refractory obsessive-compulsive disorder. CNS Spectr. 2000;5(4):24–31. CrossRef
- Bandelow B, Zohar J, Hollander E, et al; WFSBP Task Force on Treatment Guidelines for Anxiety, Obsessive-Compulsive and Post-Traumatic Stress Disorders. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders–first revision. World J Biol Psychiatry. 2008;9(4):248–312. PubMed CrossRef
- Soomro GM, Altman D, Rajagopal S, et al. Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev. 2008;2008(1):CD001765. PubMed CrossRef
- Koran LM, Hanna GL, Hollander E, et al; American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry. 2007;164(suppl):5–53. PubMed
- da Conceição Costa DL, Shavitt RG, Castro Cesar RC, et al. Can early improvement be an indicator of treatment response in obsessive-compulsive disorder? Implications for early-treatment decision-making. J Psychiatr Res. 2013;47(11):1700–1707. PubMed CrossRef
- Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
- Goodman WK, Price LH, Rasmussen SA, et al. The Yale-Brown Obsessive-Compulsive Scale, I: development, use, and reliability. Arch Gen Psychiatry. 1989;46(11):1006–1011. PubMed CrossRef
- Bollini P, Pampallona S, Tibaldi G, et al. Effectiveness of antidepressants: meta-analysis of dose-effect relationships in randomized clinical trials. Br J Psychiatry. 1999;174(4):297–303. PubMed CrossRef
- Bloch MH, McGuire J, Landeros-Weisenberger A, et al. Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry. 2010;15(8):850–855. PubMed CrossRef
- Mataix-Cols D, Fernández de la Cruz L, Nordsletten AE, et al. Towards an international expert consensus for defining treatment response, remission, recovery and relapse in obsessive-compulsive disorder. World Psychiatry. 2016;15(1):80–81. PubMed CrossRef
- Williams JR. The Declaration of Helsinki and public health. Bull World Health Organ. 2008;86(8):650–652. PubMed CrossRef
- Ananthakrishnan N, Shanthi AK. ICMR’s ethical guidelines for biomedical research on human participants: need for clarification. Indian J Med Ethics. 2012;9(3):207–209. PubMed CrossRef
- SPSS for Windows. Version 16.0. Chicago: SPSS Inc; 2007.
- Issari Y, Jakubovski E, Bartley CA, et al. Early onset of response with selective serotonin reuptake inhibitors in obsessive-compulsive disorder: a meta-analysis. J Clin Psychiatry. 2016;77(5):e605–e611. PubMed CrossRef
- Rajagopalan R. A comparative study of efficacy and tolerability of fluvoxamine and sertraline in treatment of obsessive-compulsive disorder. Int J Pharm Pharm Sci. 2013;5(2):629–632.
- DuPont RL, Rice DP, Shiraki S, et al. Economic costs of obsessive-compulsive disorder. Med Interface. 1995;8(4):102–109. PubMed
Please sign in or purchase this PDF for $40.
Save
Cite