Three Months of Treatment With Esketamine:
Effects on Depression, Insomnia, and Weight
Resistant depression can be very difficult to manage, as many patients may have already tried multiple psychotropic medications and psychotherapy without substantial results. Spravato (esketamine), an intranasal form of ketamine, may provide a treatment alternative. We describe a case of resistant depression successfully treated with this medication.
Case Report
Ms A is a 55-year-old white woman with a long history of depression and anxiety that had previously been treated with duloxetine, escitalopram, fluoxetine, and bupropion. Her depression symptoms were positive for insomnia and decreased energy, concentration, interests, motivation, libido, and self-esteem. Her Beck Depression Inventory (BDI)1 score was 38 at the time of intake, despite taking fluoxetine 60 mg and duloxetine 120 mg daily. She was tapered off duloxetine and given propranolol 10 mg each morning and each afternoon for her anxiety, while continuing fluoxetine 60 mg/day.
Spravato was approved by the US Food and Drug Administration for treatment-resistant depression in March 2019. Ms A began Spravato in early May 2019. She complained of feeling exhausted for 24 hours after the first treatment and had nausea for which she was given ondansetron as needed. Her only ongoing complaints during treatment have been those of feeling "high," slight dissociation, and "wobbly legs." Her blood pressure has remained stable throughout treatment. She was titrated up to a dose of 84 mg. As of this writing, she has completed 3 months of treatment and is in the maintenance phase of bimonthly treatments.
Ms A has had weekly monitoring of her mood, weight, and insomnia using the BDI, the 9-item Patient Health Questionnaire (PHQ-9),2 the Insomnia Severity Index (ISI),3 and the Pittsburgh Sleep Quality Index (PSQI).4 The BDI is a 21-item scale that evaluates depressive symptoms for the previous week. Interpretations are as follows: 1-10 = normal, 11-16 = mild mood disturbance, 17-20 = borderline depression, 21-30 = moderate depression, 31-40 = severe depression, and > 40 = extreme depression. The PHQ-9 consists of 9 questions evaluating symptoms based on the previous 2 weeks. A score of 0-4 = minimal depression, 5-9 = mild symptoms, 10-14 indicates moderate impairment, 15-19 is moderately severe, and 20-27 represents severe depression. The ISI has 7 questions that the patient completes about sleep habits over the prior 2 weeks. The ISI evaluates the severity of nighttime and daytime components of insomnia, with 0-7 = no clinical insomnia, 8-14 = mild insomnia, 15-21 = moderate severity clinical insomnia, and 22-28 = severe clinical insomnia. The PSQI measures sleep quality over the past month in the areas of sleep latency, quality, habitual sleep efficiency, daytime functioning, use of medications, and sleep duration. A total score ≥ 5 is suggestive of poor sleep quality.
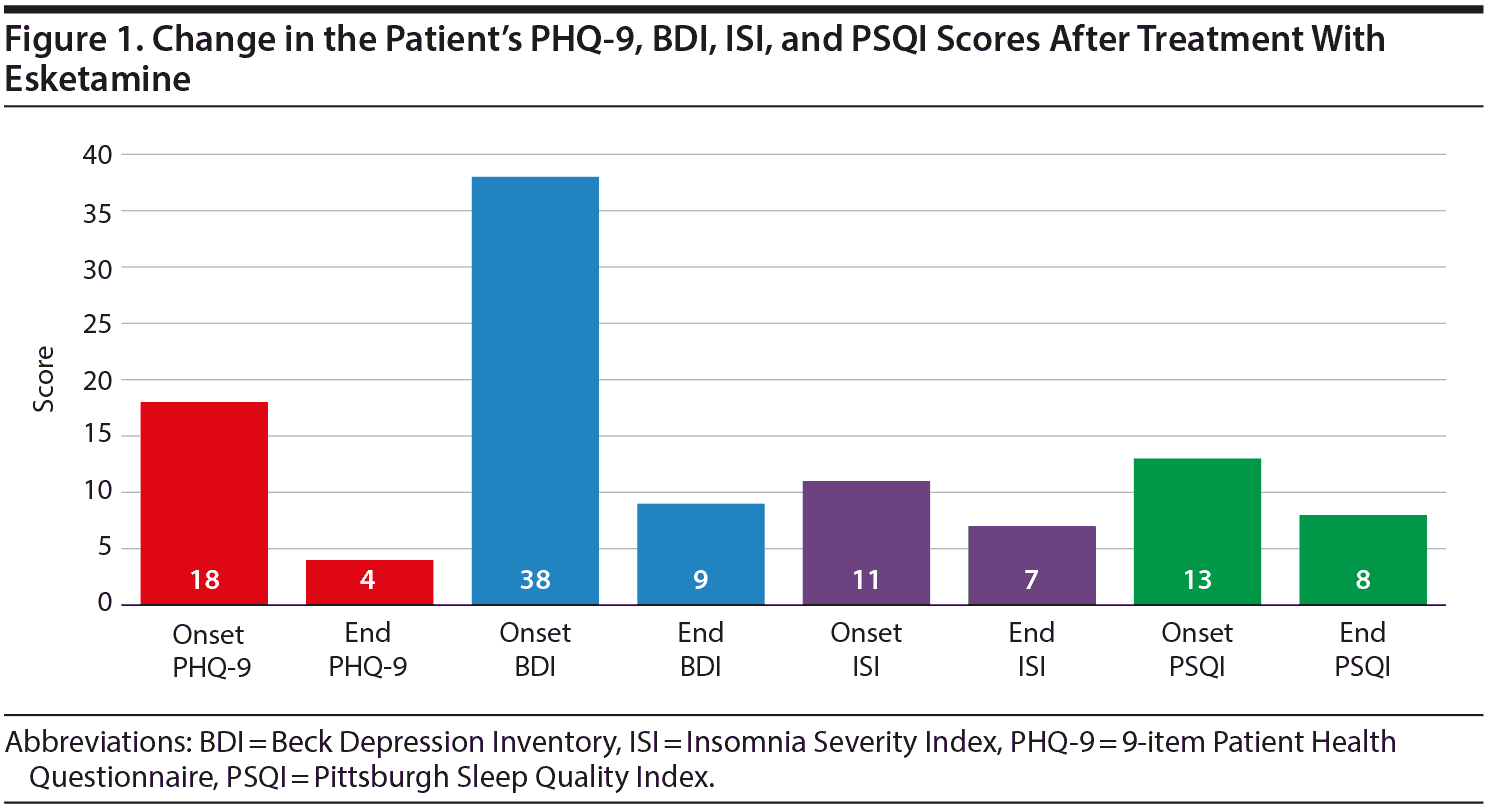
With treatment, Ms A’s PHQ-9 score has decreased from 18 to 4 (Figure 1). Her BDI score at intake was 38 and currently is 9. Her ISI score has decreased from 11 to 7. Her PSQI score has decreased from 13 to 8, and one would expect continued improvement, as this scale is based on symptoms over the previous month. Her weight has had no significant change, as her starting weight was 122 lb and her current weight is 120 lb. This finding is important to note, as many antidepressant treatments may cause weight gain. Also of significance is how rapidly Ms A demonstrated response, with a decrease in her BDI score from 38 to 19 and a decrease in her PHQ-9 score from 18 to 12 within the first 2 weeks of treatment. In summary, this patient with resistant depression and complaints of insomnia demonstrated dramatic improvement of her depression, had improvement in her insomnia, and had no change in weight with 3 months of esketamine treatment.
Published online: July 9, 2020.
Potential conflicts of interest: Dr Stultz is a speaker with Jazz Pharmaceuticals and is on the advisory board of Harmony Biosciences, both with narcolepsy medications. Mr Burns previously owned Johnson and Johnson stock, which he has since sold. Drs Pawlowska-Wajswol & Walton; Mss Stanley and Osburn; and Mssr Gills and Moomaw report no conflicts of interest related to the subject of this report.
Funding/support: None.
Patient consent: Permission was received from the patient to publish this case report, and information has been de-identified to protect anonymity.
REFERENCES
1.Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4(6):561-571. PubMed CrossRef
2.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. PubMed CrossRef
3.Morin CM, Belleville G, Bélanger L, et al. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601-608. PubMed CrossRef
4.Buysse DJ, Reynolds CF 3rd, Monk TH, et al. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. PubMed CrossRef
aStultz Sleep & Behavioral Health, Barboursville, West Virginia
*Corresponding author: Debra J. Stultz, MD, Stultz Sleep & Behavioral Health, 6171 Childers Rd, Barboursville, WV 25504 ([email protected]).
Prim Care Companion CNS Disord 2020;22(4):19l02555
To cite: Stultz DJ, Stanley N, Gills T, et al. Three months of treatment with esketamine: effects on depression, insomnia, and weight. Prim Care Companion CNS Disord. 2020;22(4):19l02555.
To share: https://doi.org/10.4088/PCC.19l02555
© Copyright 2020 Physicians Postgraduate Press, Inc.
Please sign in or purchase this PDF for $40.00.
Save
Cite