ABSTRACT
Objective: To determine if there was a significant increase in cessation rates over a 6-month time frame with a pharmacist-managed smoking cessation clinic.
Methods: This single-center, prospective study recruited veterans via ICD-10 diagnosis codes for nicotine/tobacco dependence to participate in a clinical pharmacy practitioner (CPP)–run smoking cessation clinic. To aid cessation efforts, participants were offered pharmacotherapy, behavioral modifications, and frequent follow-up appointments. Once a participant demonstrated tobacco-free success for 6 months, the veteran was eligible for discharge from the clinic. Patient visits were conducted between December 2020 and October 2021. Data were analyzed using intention-to-treat principle.
Results: A total of 54 participants were screened, of which 29 met the inclusion criteria. Eleven participants withdrew from the study, as they were no longer willing to quit smoking. Thirty-eight percent (n = 11) of the enrolled participants achieved complete tobacco cessation by the end of the study, with 31% (n = 9) achieving the primary endpoint of tobacco cessation for 6 months. Seventeen percent (n = 5) of participants maintained ≥ 50% reduction in tobacco use. One participant increased the amount of tobacco used since the initial visit.
Conclusions: Providing pharmacotherapy, behavioral counseling, and frequent follow-up visits may result in higher numbers of tobacco-free patients. Expanding pharmacist-run smoking cessation clinics may result in an increase in cessation rates in the veteran population.
Prim Care Companion CNS Disord 2023;25(1):22m03308
To cite: Patel KA, Noormohammadi A, Attia EM. Evaluation of tobacco cessation rates among veterans in a pharmacist-run smoking cessation clinic. Prim Care Companion CNS Disord. 2023;25(1):22m03308.
To share: https://doi.org/10.4088/PCC.22m03308
© 2023 Physicians Postgraduate Press, Inc.
aMichael E. DeBakey VA Medical Center, Houston, Texas
bLake Jackson VA Clinic, Lake Jackson, Texas
*Corresponding author: Khushbu A. Patel, PharmD, 2002 Holcombe Blvd Houston, TX 77030 ([email protected]).
Cigarette smoking is one of the leading preventable causes of death and disease in the United States. It leads to over 480,000 deaths annually, with 10% of deaths resulting from secondhand smoke.1 This statistic amounts to 1 in 5 deaths annually or 1,300 deaths every day.1 If the ongoing trend of smoking is maintained worldwide, more than 8 million people per year are estimated to die from tobacco-related diseases by 2030.2 In 2015, 68% (22.7 million) of adult smokers reported that they wanted to quit smoking.3 In 2018, more than half of adult smokers said that they made an attempt to quit in the last year; however, fewer than 1 in 10 adult cigarette smokers actually succeeded in quitting each year.4
Pharmacists are some of the most accessible health care professionals who, as midlevel practitioners at some institutions, may aid patients by offering tobacco cessation services. The US public health guidelines for treating tobacco dependence recommend a combination of tobacco cessation counseling and pharmacotherapy to maximize the effectiveness of cessation efforts.5 Several studies have documented pharmacist involvement in smoking cessation clinics in efforts to increase cessation rates. In 2004, Dent et al6 published a study conducted at a Veterans Affairs Medical Center (VAMC) smoking cessation clinic in Missoula, Montana that implemented a transtheoretical model of change. This model is designed to promote change through 5 stages: precontemplation, contemplation, preparation, action, and maintenance. The implementation of this model, along with hands-on involvement of a health care provider in cessation efforts, supports the role of clinical pharmacists in a smoking cessation clinic and identifies outcomes associated with success.6 At the Lake Jackson community-based outpatient clinic (CBOC), which is a part of the Michael E. DeBakey VAMC (MEDVAMC) in Houston, Texas, a smoking cessation clinic has been implemented that provides pharmacotherapy and behavioral modifications to patients; however, its success in improving veteran cessation rates has yet to be evaluated.
METHODS
This study was approved by the MEDVAMC Research and Development Committee and the Baylor College of Medicine Institutional Review Board. It was a single-center, prospective study that recruited participants via ICD-10 diagnosis codes for nicotine/tobacco dependence to participate in a clinical pharmacy practitioner (CPP)–run smoking cessation clinic. The smoking cessation clinic was part of a primary care clinic located at the Lake Jackson VA outpatient clinic. A national primary care dashboard was used to generate a list of local patients who had active ICD-10 diagnosis codes for nicotine/tobacco dependence. Thereafter, the primary care CPP reviewed the list and called participants to assess current nicotine use and participants’ willingness to quit. If the participant was willing to quit and met study inclusion criteria, then he/she was enrolled in the smoking cessation clinic.
Veterans aged ≥ 18 years who were receiving medical care at MEDVAMC or geographically surrounding CBOCs were included in the study. Participants were eligible for enrollment in the study regardless of the nicotine product used (ie, cigarettes, smokeless tobacco, e-cigarettes/vapes). Marijuana use was not assessed when recruiting participants. Study candidates who were not willing to participate in virtual follow-up appointments were excluded for 2 reasons. The first reason is that the study was conducted during the coronavirus disease 2019 pandemic when most CPP’s were teleworking, and in-person visits were prioritized based on necessity or emergency. The second reason is that the smoking cessation clinic was located at the Lake Jackson VA outpatient clinic. Not all participants recruited in the study received care at this specific CBOC; therefore, it would not be feasible to have participants drive 2 or more hours solely for their smoking cessation clinic visit. Patient enrollment took place between December 2020 and January 2021.
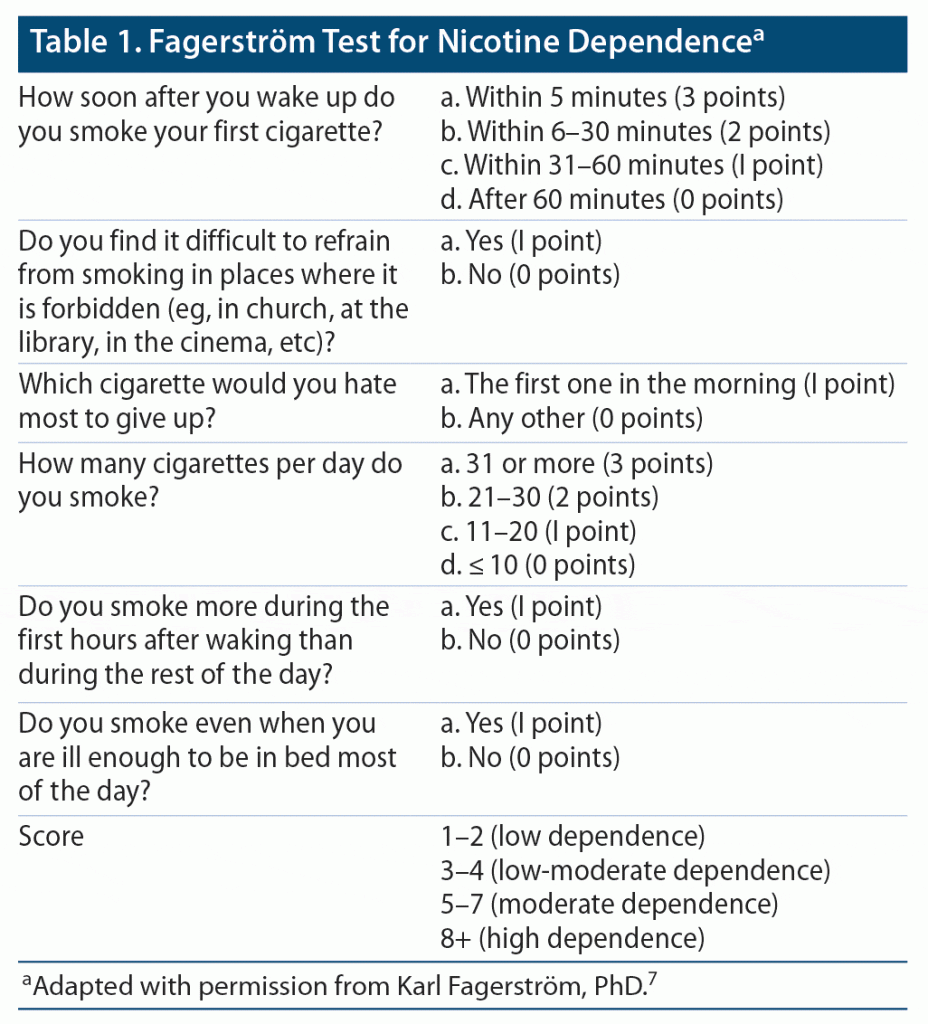
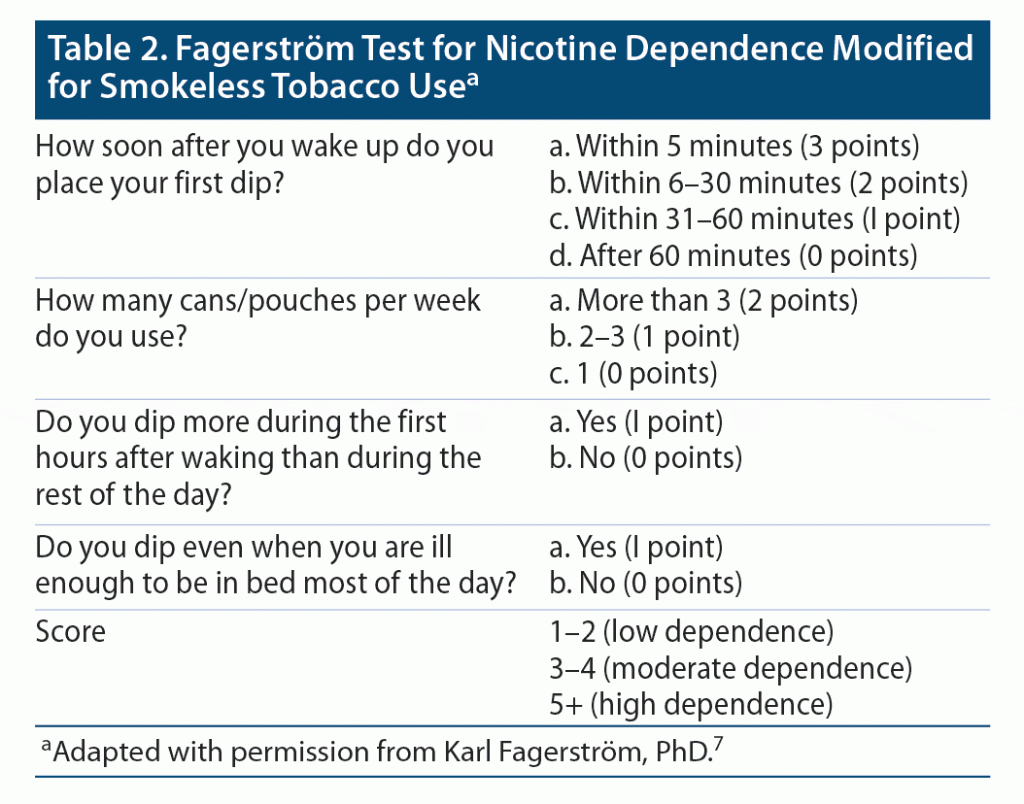
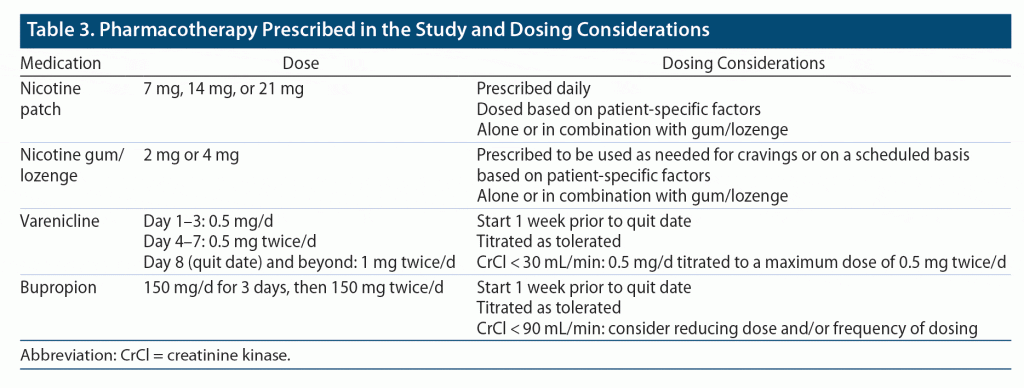
During the initial meeting, the CPP assessed the participants’ degree of tobacco use, willingness to quit, and obstacles hindering the participant from quitting. Participants’ degree of tobacco dependence was determined by using the Fagerström Test for Nicotine Dependence7 (Table 1). The Fagerström test contains several questions to evaluate the quantity of cigarette consumption, the urge to use, as well as dependence. The test was administered verbally to patients (via phone or video call). Many variations of this test are available, including a version that may be modified for those who report use of smokeless tobacco. The version used in this study was modified internally within the pharmacotherapy clinic specifically for smokeless tobacco (Table 2). Based on participants’ responses and scores, they were categorized as having low, moderate, or high dependence. The degree of dependence, along with frequency of tobacco use and previous quit attempts/trials, was used to determine appropriate pharmacotherapy for smoking cessation. Additionally, the CPP initiated pharmacologic management tailored toward each individual by assessing drug-drug interactions and contraindications. Pharmacotherapy prescribed during the study, as well as dosing considerations, are included in Table 3. All data obtained from clinic visits were documented in the participants’ electronic medical record.
The CPP also discussed possible behavioral modifications and assisted the participant in choosing a target quit date. A virtual follow-up appointment was scheduled 1 week after the patient’s target quit date to assess current smoking status after starting therapy, to monitor for potential adverse reactions, to make changes in therapy if needed, and to discuss additional behavioral modifications. Additional frequent follow-up appointments were made on a case-by-case basis. The CPP was available Monday through Friday from 8:00 AM to 4:30 PM, except on federal holidays when the clinic was closed. Participants were given the CPP’s office phone number and extension if they needed to reach them during normal office hours.
Clinic visits were conducted between December 2020 and October 2021. Once a participant demonstrated that they were tobacco free for 6 months after their quit date, they were eligible for discharge from the clinic. Data collection was completed in October 2021. Participants who were still enrolled in the smoking cessation clinic at this time continued to receive care with the CPP for continuity of care; however, endpoints met after October 2021 were not included in the final analysis and results of the study.
In July 2021, Pfizer, the manufacturer of varenicline, voluntarily recalled several lots of the medication due to levels of a nitrosamine impurity, called N-nitroso-varenicline, that were found to be higher than the US Food and Drug Administration’s acceptable intake. As a result, study participants who were prescribed varenicline were switched to an alternative medication for tobacco cessation. Participants’ prescriptions were replaced with bupropion if they were a candidate. Those who were not appropriate candidates for bupropion were switched back to nicotine replacement therapy (NRT) even despite past failures with NRT due to lack of other options. Specifically for veteran patients receiving mental health care, their providers were consulted prior to initiation of bupropion due to its dual use as an antidepressant, as well as its ability to increase anxiety. Participants with a history of anxiety, seizures, anorexia/bulimia, or severe liver disease and those at risk for alcohol withdrawal or with previous failed attempt with bupropion were not candidates for bupropion. Data were analyzed using the intention-to-treat principle. Statistical analyses were not conducted, as there was no comparator group.
RESULTS
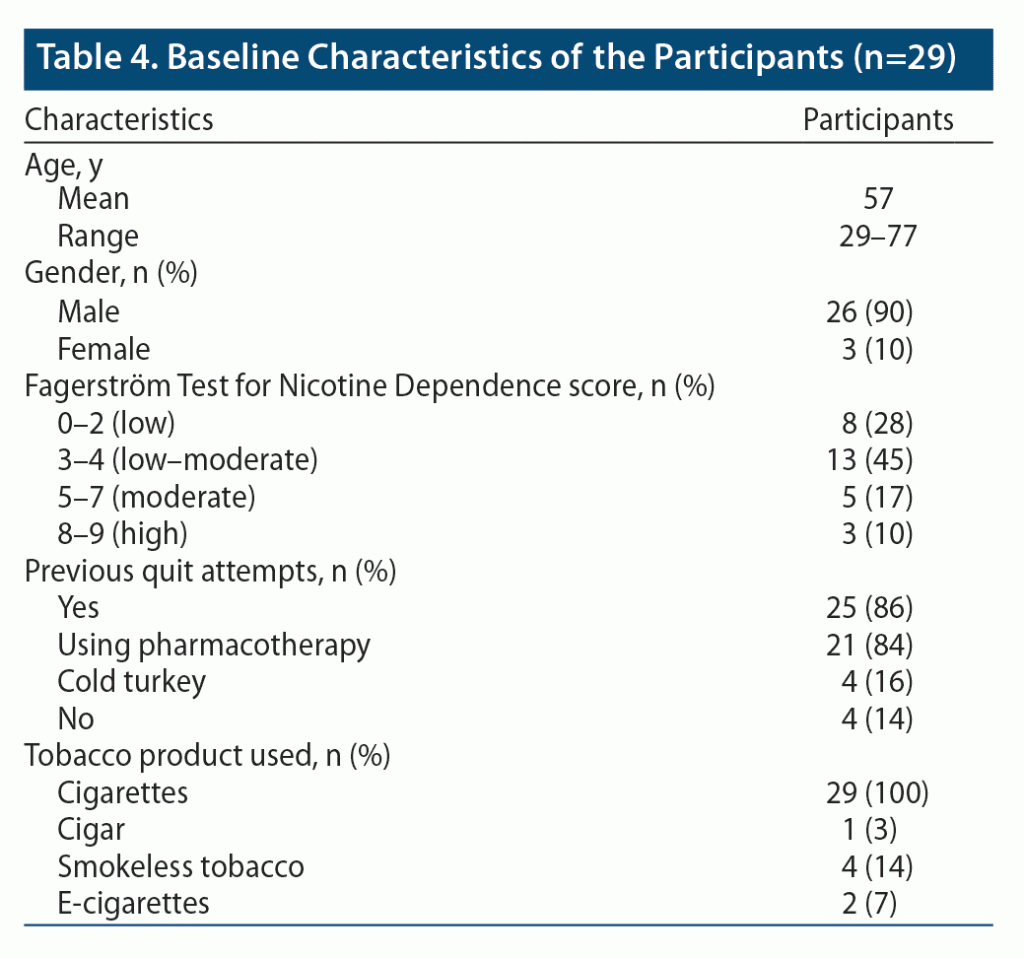
The mean age of participants in the study was 57 years. A majority of the participants were male, which highly represents the veteran population. A majority (72%, n = 21) of the participants had attempted to quit smoking in the past, often with the use of pharmacotherapy. All participants enrolled in the study smoked cigarettes; however, some reported using more than one tobacco-containing product (smokeless tobacco, e-cigarettes, cigars). A full breakdown of baseline characteristics can be found in Table 4.
A total of 54 participants were screened for this study, of which 25 were excluded. Participants were excluded if they were not a veteran, were no longer using tobacco, or were not willing to quit smoking at the time. A total of 29 participants were enrolled in the study, of which 11 withdrew, as they were no longer ready or willing to quit using tobacco at the time. Nine of the 11 participants, including one who was incarcerated, withdrew after starting pharmacotherapy, and 2 withdrew before starting.
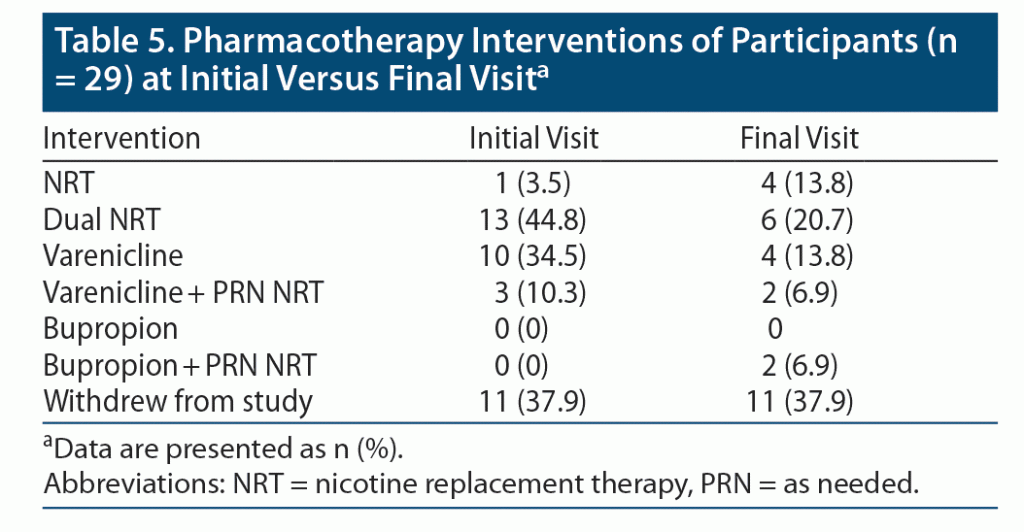
At the initial visit, 45% (n = 13) of participants were started on dual NRT; however, at subsequent visits, only 20% (n = 6) remained on dual NRT with many being switched to varenicline. The most common reason for switching to varenicline was failure with NRT, followed by adverse effects from NRT. A few months after the study began, varenicline was recalled due to the presence of unacceptable N-nitroso-varenicline levels. As a result, those who were still on varenicline at the time of the recall were switched to either NRT (monotherapy or dual therapy) or bupropion based on patient-specific factors mentioned previously. A total of 6 participants were affected by the recall, of which one dropped out of the study after being switched to bupropion. This participant reported lack of motivation once he switched to bupropion, which caused him to stop taking his medications for tobacco cessation. Comparing data between initial and subsequent visits, as seen in Table 5, frequent follow-up visits allowed the CPP to evaluate and modify therapy regularly. As a result, medication regimens were tailored to participant-specific factors quickly to ensure that each participant was on a medication regimen that would maximize their cessation efforts.
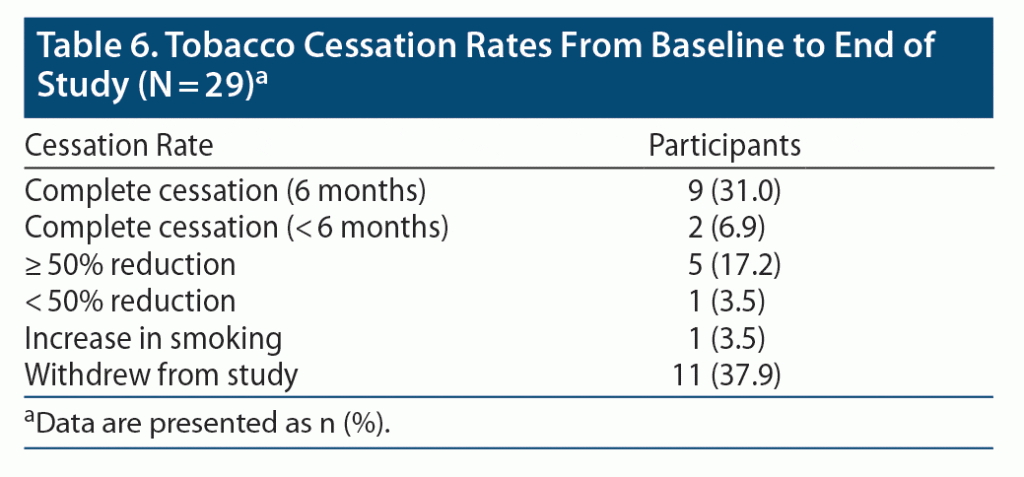
As seen in Table 6, by the end of the study, 38% (n = 11) of participants enrolled in the study achieved complete tobacco cessation, with 31% (n = 9) achieving the primary endpoint of tobacco cessation for 6 months. Seventeen percent (n = 5) of participants maintained ≥ 50% reduction in tobacco use. One participant increased the amount of tobacco used since the initial visit. This participant reported making progress in his cessation efforts; however, due to family problems and stress, the participant relapsed and started smoking more compared to his initial visit.
DISCUSSION
This study demonstrates the impact pharmacists can have on tobacco cessation rates. By the end of the study, over one-third of the enrolled participants had achieved complete cessation, which also accounts for the 38% (n = 11) of participants who withdrew from the study. Of the participants who remained enrolled in the study until completion, 61% (n = 11) achieved complete cessation, with 50% (n = 9) achieving complete cessation for 6 months. These tobacco cessation rates are higher in comparison to those reported in the study completed at the Missoula VAMC.6
This study has several strengths and limitations. One strength is that it was a prospective study design. Additionally, one CPP conducted all smoking cessation clinic visits, which allowed for continuity of care. This helped participants develop a comfortable and trusting relationship with their pharmacist, which ultimately allowed the CPP to assist participants on their journey to cessation. Building a sense of trust is an advantage, as it allows participants to be more comfortable disclosing pertinent information with their providers, which could potentially produce better outcomes. However, this can also be seen as a limitation due to lack of generalizability with just one CPP making interventions. Another advantage of this study is the routine follow-up visits that were offered, each based on participant-specific factors. Providing consistent follow-ups permitted the CPP to make changes more frequently and to account for adverse effects, contraindications, and participant preferences. These follow-ups also served as motivation for participants to make progress in their cessation efforts. Finally, this study was analyzed using an intention-to-treat analysis and thus accounted for all participants enrolled in the study regardless of completion status.
Some limitations include that it was a single-center study with a small sample size. Furthermore, this study had only 3 women; therefore, we could not explore significant differences based on sex. Another limitation included the recall of varenicline, which caused an interruption in many of the participants’ (n = 6) cessation journeys, as therapy was switched from varenicline to other agents (ie, NRT or bupropion) despite previous trials and failures on those agents. Finally, a noteworthy limitation is that this study took place during a severe winter storm, which resulted in various setbacks and cessation relapses in some participants. The storm resulted in many participants losing water and electricity for over a week, along with some losing their homes due to flooding. These uncontrollable stressors led many participants to relapse back to previous smoking habits. Additionally, many roads were closed, resulting in delays in medication deliveries and causing participants to go several days without their medications, including those prescribed as part of this study.
CONCLUSION
Providing pharmacotherapy and behavioral counseling, as well as frequent follow-up visits, may result in higher numbers of patients who achieve tobacco abstinence. Frequent follow-up appointments allowed CPPs to make changes to medication regimens as needed and avoid patients’ self-discontinuation of pharmacotherapy due to intolerance or adverse effects of medications. Expanding pharmacist-run smoking cessation clinics across VA primary care clinics and CBOCs may result in an increase in cessation rates in the veteran population.
Submitted: April 20, 2022; accepted August 8, 2022.
Published online: February 23, 2023.
Relevant financial relationships: The authors report no financial or other relationship relevant to the subject of this article.
Funding/support: None.
Previous presentations: Poster presented at the annual American Society of Health System Pharmacists Midyear Clinical Meeting and Exhibition Virtual Conference, December 2020 and December 2021 and the VA/Indian Health Service Pharmacy Resident Virtual Poster Session; Houston, Texas; March 30, 2022. Preliminary results presented at the Texas Society of Health System Pharmacists ALCALDE 2021 Virtual Southwest Leadership Conference, May 6, 2021. Final results presented at the Texas Society of Health System Pharmacists 2022 ALCALDE Southwest Leadership Conference; Round Rock, Texas; May 12, 2022.
Clinical Points
- Providing pharmacotherapy and behavioral counseling, as well as frequent follow-up visits, may result in higher numbers of tobacco abstinence.
- Expanding pharmacist-run smoking cessation clinics across all Veterans Affairs patient-aligned care team clinics and community-based outpatient clinics may result in an increase in cessation rates in the veteran population.
References (7)

- US Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; August 5, 2014.
- World Health Organization. WHO Report on the Global Tobacco Epidemic, 2011. Geneva: World Health Organization; 2011.
- Babb S, Malarcher A, Schauer G, et al. Quitting smoking among adults - United States, 2000–2015. MMWR Morb Mortal Wkly Rep. 2017;65(52):1457–1464. PubMed CrossRef
- Creamer MR, Wang TW, Babb S, et al. Tobacco product use and cessation indicators among adults - United States, 2018. MMWR Morb Mortal Wkly Rep. 2019;68(45):1013–1019. PubMed CrossRef
- Fiore MC, Jaén CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. Clinical Practice Guideline. Rockville, MD: US Department of Health and Human Services. Public Health Service. Accessed August 5, 2020. https://www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/TreatingTobaccoUseandDependence-2008Update.pdf
- Dent LA, Scott JG, Lewis E. Pharmacist-managed tobacco cessation program in Veterans Health Administration community-based outpatient clinic. J Am Pharm Assoc (2003). 2004;44(6):700–714, quiz 714–715. PubMed CrossRef
- Heatherton TF, Kozlowski LT, Frecker RC, et al. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite