Objective: To identify patient-reported factors that influence medication treatment decisions among individuals with bipolar and unipolar depression.
Methods: The Depression and Bipolar Support Alliance (DBSA) conducted an online survey February 2016 to April 2016 asking participants about factors that influence treatment decisions (eg, starting and stopping specific medications).
Results: In total, 896 participants completed the survey (49.9% unipolar depression [n = 447] and 50.1% bipolar depression [n = 449]). The majority of respondents reported several previous medication trials. The most frequently reported factors impacting treatment decisions were side effects, doctor recommendations, cost, and how quickly the treatment will begin to work. The most common reason for changing treatments was ineffectiveness in the unipolar depression group and side effects in the bipolar depression group. Weight gain was the side effect that most commonly led respondents to discontinue a medication. When respondents currently using medications versus respondents not using medications were compared, doctor recommendations were more likely to be influential for those taking medications (P < .0001). Conversely, cost (P = .008) and impact on pregnancy/lactation (P = .045) were more likely to impact treatment decisions in participants not currently taking medications. Current medication use was associated with increased rates of perceived treatment effectiveness (P < .0001).
Conclusions: Side effects, doctor recommendations, cost, and rapidity of antidepressant effects were determined to be particularly important factors in making treatment decisions, with doctor recommendations being more influential for medication users and cost being more influential for participants not using medications. These findings highlight the importance of patient-centered factors in adjudicating treatment decisions.
ABSTRACT
Objective: To identify patient-reported factors that influence medication treatment decisions among individuals with bipolar and unipolar depression.
Methods: The Depression and Bipolar Support Alliance (DBSA) conducted an online survey February 2016 to April 2016 asking participants about factors that influence treatment decisions (eg, starting and stopping specific medications).
Results: In total, 896 participants completed the survey (49.9% unipolar depression [n = 447] and 50.1% bipolar depression [n = 449]). The majority of respondents reported several previous medication trials. The most frequently reported factors impacting treatment decisions were side effects, doctor recommendations, cost, and how quickly the treatment will begin to work. The most common reason for changing treatments was ineffectiveness in the unipolar depression group and side effects in the bipolar depression group. Weight gain was the side effect that most commonly led respondents to discontinue a medication. When respondents currently using medications versus respondents not using medications were compared, doctor recommendations were more likely to be influential for those taking medications (P < .0001). Conversely, cost (P = .008) and impact on pregnancy/lactation (P = .045) were more likely to impact treatment decisions in participants not currently taking medications. Current medication use was associated with increased rates of perceived treatment effectiveness (P < .0001).
Conclusions: Side effects, doctor recommendations, cost, and rapidity of antidepressant effects were determined to be particularly important factors in making treatment decisions, with doctor recommendations being more influential for medication users and cost being more influential for participants not using medications. These findings highlight the importance of patient-centered factors in adjudicating treatment decisions.
Prim Care Companion CNS Disord 2018;20(6):18m02340
To cite: Rosenblat JD, Simon GE, Sachs GS, et al. Factors that impact treatment decisions: results from an online survey of individuals with bipolar and unipolar depression. Prim Care Companion CNS Disord. 2018;20(6):18m02340.
To share: https://doi.org/10.4088/PCC.18m02340
© Copyright 2018 Physicians Postgraduate Press, Inc.
aMood Disorders Psychopharmacology Unit, Toronto Western Hospital, University Health Network, University of Toronto, Toronto, Ontario, Canada
bDepression and Bipolar Support Alliance, Chicago, Illinois
cKaiser Permanente Washington Health Research Insitute, Seattle, Washington
*Corresponding author: Roger S. McIntyre, MD, FRCPC, Mood Disorders Psychopharmacology Unit, University Health Network, University of Toronto, 399 Bathurst St, MP 9-325, Toronto, ON M5T 2S8, Canada ([email protected]).
Bipolar depression (BD) and major depressive disorder (MDD) are common and disabling disorders associated with significant functional impairments, morbidity, and mortality.1 Pharmacologic interventions for BD and MDD remain the mainstay of treatment for most affected individuals.2 Dozens of pharmacologic agents are currently approved for the treatment of MDD and BD. However, current treatments are often ineffective and poorly tolerated, with high rates of treatment discontinuation, treatment resistance, and relapses and recurrences of mood episodes.3,4 Consequently, the treatment of depression is characterized by multiple changes in selected agents and modalities of care in an attempt to achieve full remission of symptoms, functional recovery, and wellness.
All US Food and Drug Administration (FDA)-approved treatments for BD and MDD have demonstrated efficacy compared to placebo, as shown by replicated randomized controlled trials (RCTs).5,6 However, when comparing approved treatments, there is significant variability in numerous domains including, but not limited to, comparative efficacy, safety, side effect profiles, cost, availability, frequency of dosing, route of administration, and time to onset of antidepressant effects. These disparate factors all impact treatment decisions (eg, starting, changing, adding, or discontinuing specific medications); however, it is unclear to what extent these factors, from the consumer’s (ie, patient’s ) perspective, influence treatment (dis)continuation. Further, the focus of RCTs and treatment guidelines is primarily efficacy and safety rather than other patient-reported factors that may also be important to consumers when making treatment decisions.
When selecting a new treatment or proposing a change in the current treatment plan, patients and health care providers will typically engage in an informed consent discussion, evaluating the expected risks and benefits of the newly proposed treatment. The current standard of care for informed consent discussions includes summarizing efficacy and safety data, along with potential treatment alternatives. From a medical-legal perspective, establishing capacity and summarizing these factors is sufficient for initiating treatment.7 However, from a patient-centered perspective, there may be other factors that are of equal or greater importance in informing treatment decisions.8
Previous research9,10 on factors impacting treatment decisions have focused on prescriber perspectives rather than patient-consumers. Previous survey results10 have suggested that the most common factors influencing antidepressant selection for psychiatrists are the avoidance of specific side effects, consideration of comorbid psychiatric disorders, and the presence of specific clinical symptoms. Replicated evidence11 has also demonstrated improved outcomes (from the provider’s perspective) when using algorithm, measurement-based care to guide treatment decisions. Previous studies12 on patient preferences for treatment have focused on identifying preferred treatments rather than understanding the underlying reasons for these preferences.
Accordingly, the objective of the current study was to identify the most common patient-reported factors that influence medication treatment decisions for MDD and BD. Identification of common factors that are important to patient-consumers in making treatment decisions may serve to improve the quality of informed consent discussions through anticipating and directly addressing these factors rather than solely focusing on discussing efficacy and safety data. An understanding of patient prioritized factors may also serve to further inform the development of patient-centered outcomes in future clinical studies.
METHODS
The current study was conducted by the Depression and Bipolar Support Alliance (DBSA). The DBSA is an education, advocacy, and support organization for individuals living with mood disorders governed, staffed, and run primarily by people with lived experience of depression and bipolar disorder. The DBSA’s outreach activities include periodic constituent surveys regarding affected individuals’ and family members’ views on clinical, research, and policy issues. These surveys are available on the DBSA website (DBSAlliance.org). All surveys are anonymous; respondents are not asked to provide any identifying information.

- Side effects, doctor recommendations, cost, and how quickly the treatment will begin to work are the most important factors for patients when making treatment decisions.
- Cost and impact on pregnancy/lactation are more likely to affect treatment decisions for patients who are not currently taking medications, whereas doctor recommendations are less likely to play an influential role in this group.
- Patients currently taking medications are more likely to perceive their current treatment plan as effective compared to patients not taking medications.
For the current study, the DBSA conducted an online survey from February 2016 to April 2016 asking participants about their current treatments, perceived effectiveness, factors influencing treatment decisions, and reasons for wanting to change treatments. This article summarizes results of the subset of questions pertaining to factors influencing treatment decisions. Individuals were invited to complete the survey through DBSA’s online monthly newsletter, chapter network, and social media pages. The survey link was also shared with several other mental health organizations to be distributed to their members.
All respondents were advised regarding the goals and content of the survey, including intent to publish survey results online and in scientific journals. Because all responses were anonymous and no protected health information was collected, written informed consent was not required. The local research ethics board at University Health Network in Toronto, Ontario, Canada, verified that approval was not required for the current study (under section 2.2.b). Of note, demographic information was not collected to keep the current survey brief, maximize respondent rates, and minimize discomfort from respondents disclosing any potentially identifying information. Frequency of survey responses, cross-tabulation, and χ2 tests were completed using IBM SPSS Statistics Software, version 25.
As part of a secondary analysis, participants with bipolar and unipolar depression were combined, and response rates for the group of participants currently taking medications were compared with the group not currently taking any medications. After pooling frequencies of responses, response rates for the 2 groups (ie, those currently using medications versus those not currently using medications) were compared using a 2-tailed χ2 test to determine if there was a statistically significant difference between groups. Similarly, responses for factors influencing treatment decisions were cross-tabulated with number of medication trials and evaluated for statistically significant differences using linear-by-linear, 2-tailed χ2 tests to compare response rates.
RESULTS
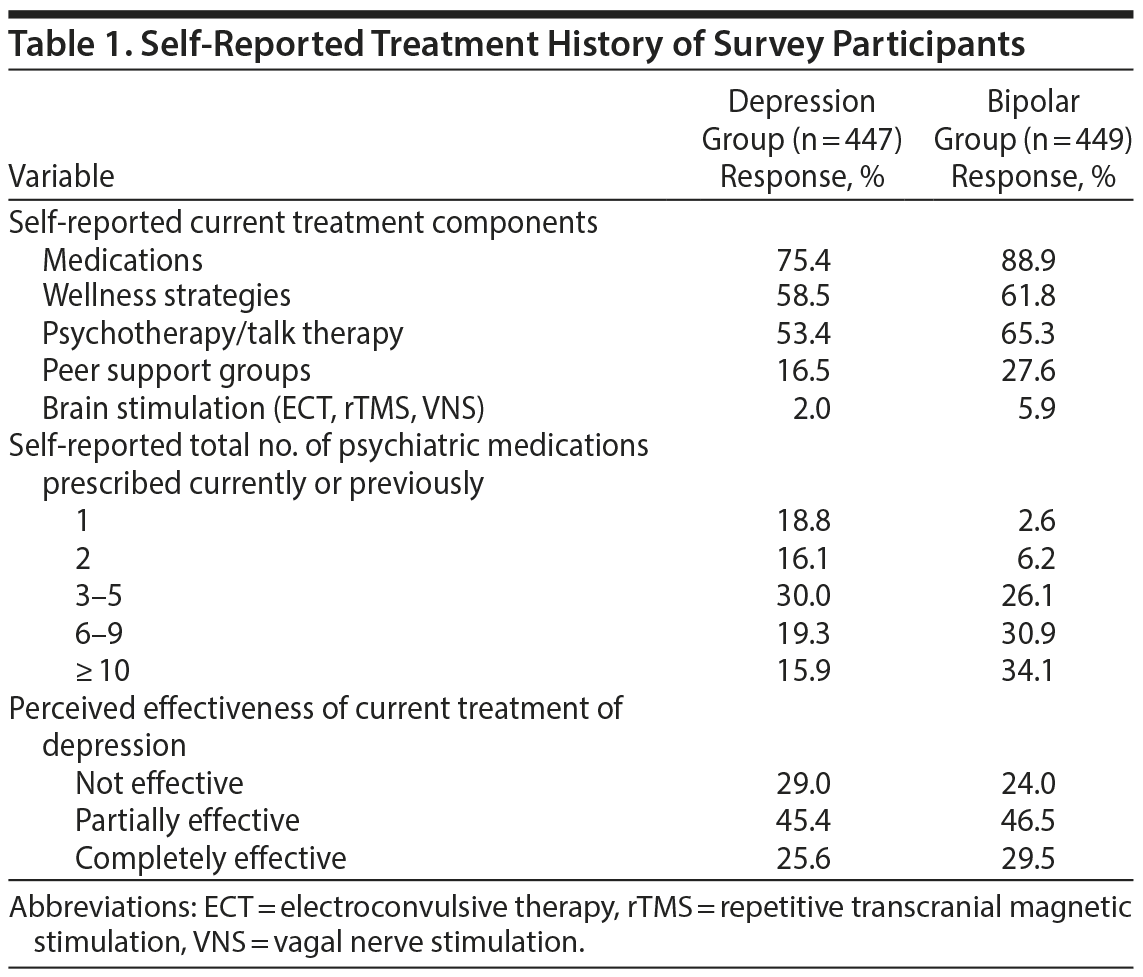
Characteristics and Treatment History of Survey Participants
In total, 896 respondents completed the survey. Based on self-report, 49.9% had unipolar depression (“depression group,” MDD, n = 447) and 50.1% had BD (“bipolar group,” n = 449). No demographic or identifying data were obtained in the survey to further characterize this sample. As shown in Table 1, the majority of participants were receiving medications as part of their current treatment plan. The majority of respondents taking medications had numerous medication trials, with 65% of the depression group and 91% of the bipolar group reporting ≥ 3 medication trials. In both groups, the minority of respondents felt their current treatment plan was completely effective, with only 26% of the depression group and 30% of the bipolar group reporting that their current treatment was completely effective.
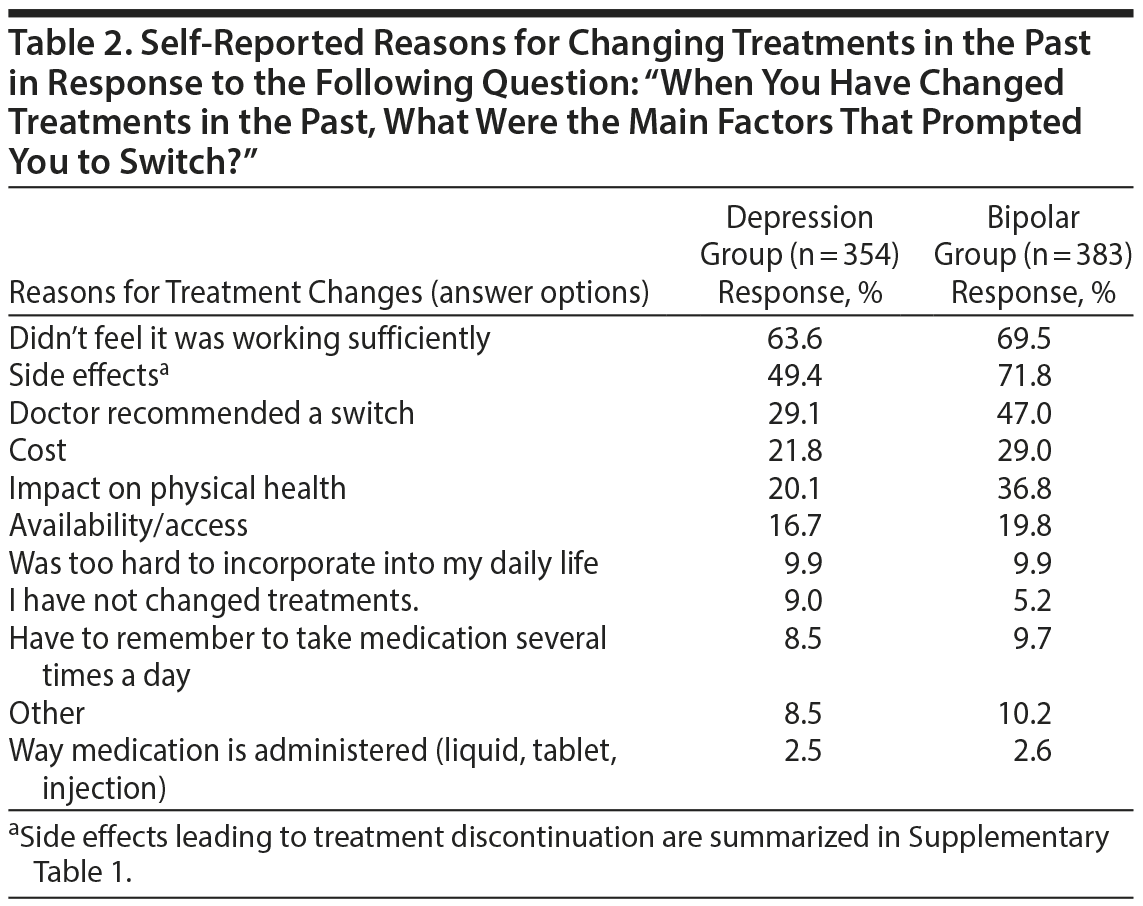
Reasons for Changing Treatments
In the depression group, the most commonly reported reason for changing treatment was an inadequate response (“didn’ t feel it was working sufficiently”) (Table 2). Conversely, side effects was the most commonly reported reason for changing treatment in the bipolar group, followed closely by having an inadequate response. Specific side effects were identified as more commonly leading to treatment discontinuation, as summarized in Supplementary Table 1. Weight gain was the side effect that most often led respondents to discontinue a specific medication in both groups. In addition to side effects and inadequate effectiveness, doctor recommendations and cost were other commonly reported factors leading to a change in treatment. Interestingly, doctor recommendations played a larger role in the bipolar group, with 47% of participants reporting it as an important factor versus only 29% in the depression group.
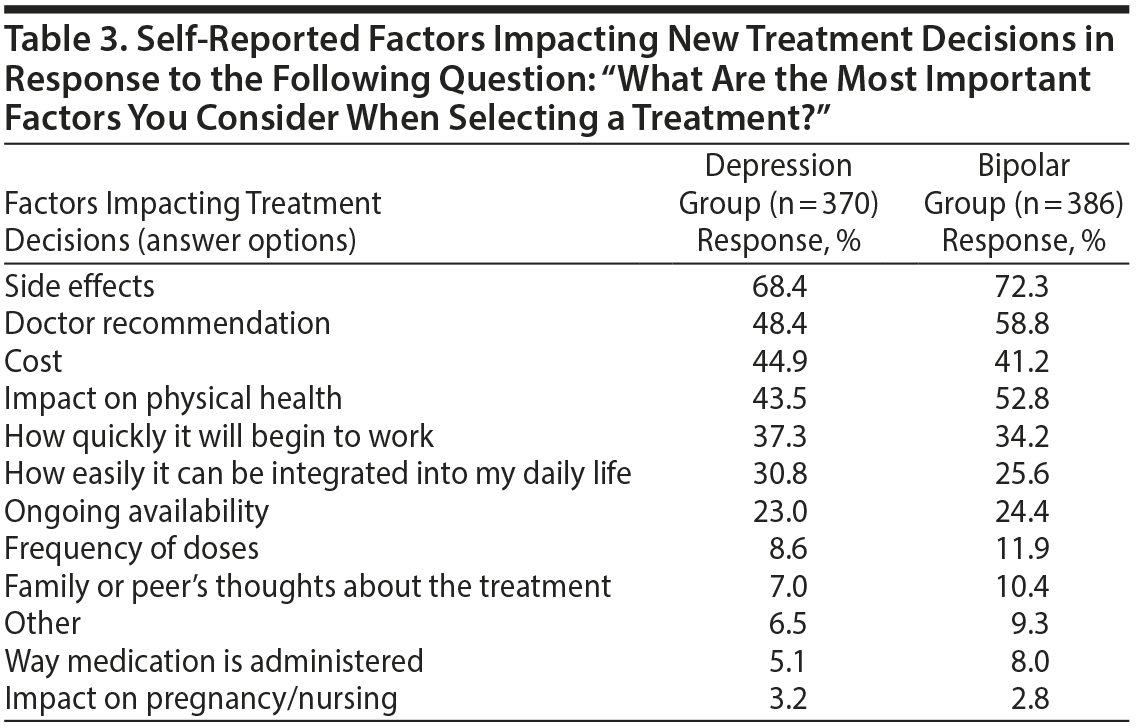
Common Factors Impacting Treatment Decisions
In both bipolar and unipolar depression, side effects was the most commonly reported factor impacting new treatment decisions, followed by doctor recommendations, cost, and impact on physical health (Table 3). “How quickly it will begin to work” was also reported as an important factor by over one-third of respondents in both groups. Frequency of dosing, route of administration, and impact on pregnancy/nursing were reported as important factors for only a small minority of respondents (< 10%).
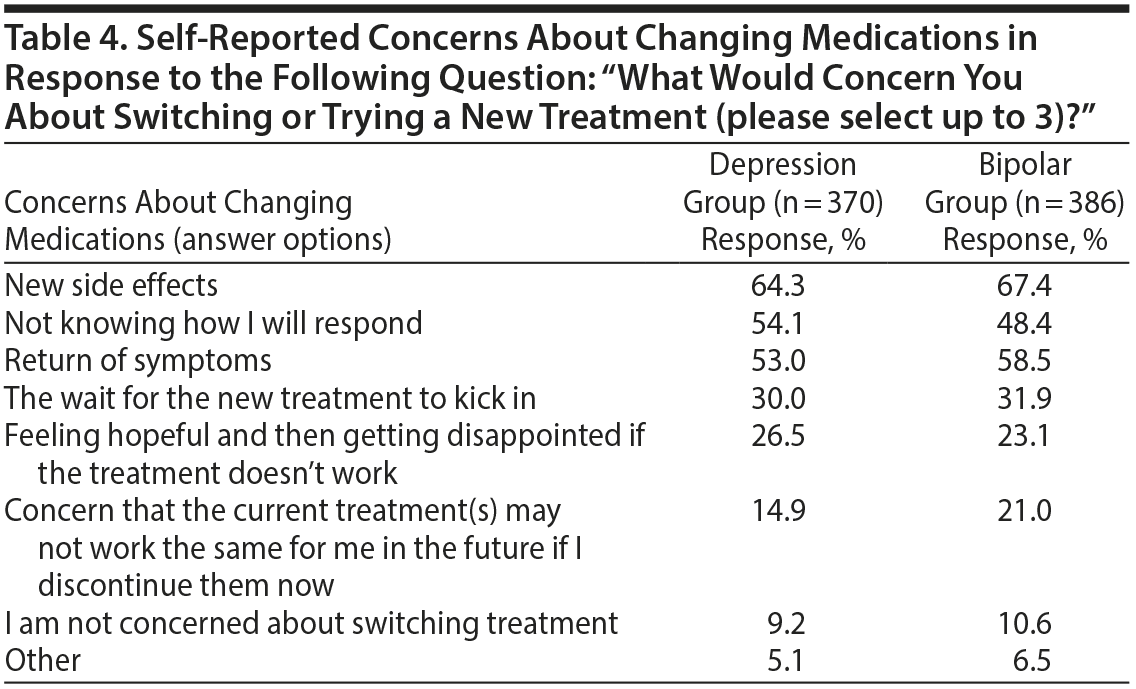
Concerns About Changing Medications
When respondents were asked about concerns with regard to changing medications, the emergence of new side effects was the most common concern in both groups (Table 4). “Not knowing how I will respond” and return of symptoms were also reported frequently. “The wait for the new treatment to kick in” and “feeling hopeful and then getting disappointed if the treatment doesn’ t work” were also common concerns reported in approximately one-quarter of respondents. Only a minority of respondents (approximately 10%) were not concerned about switching treatments.
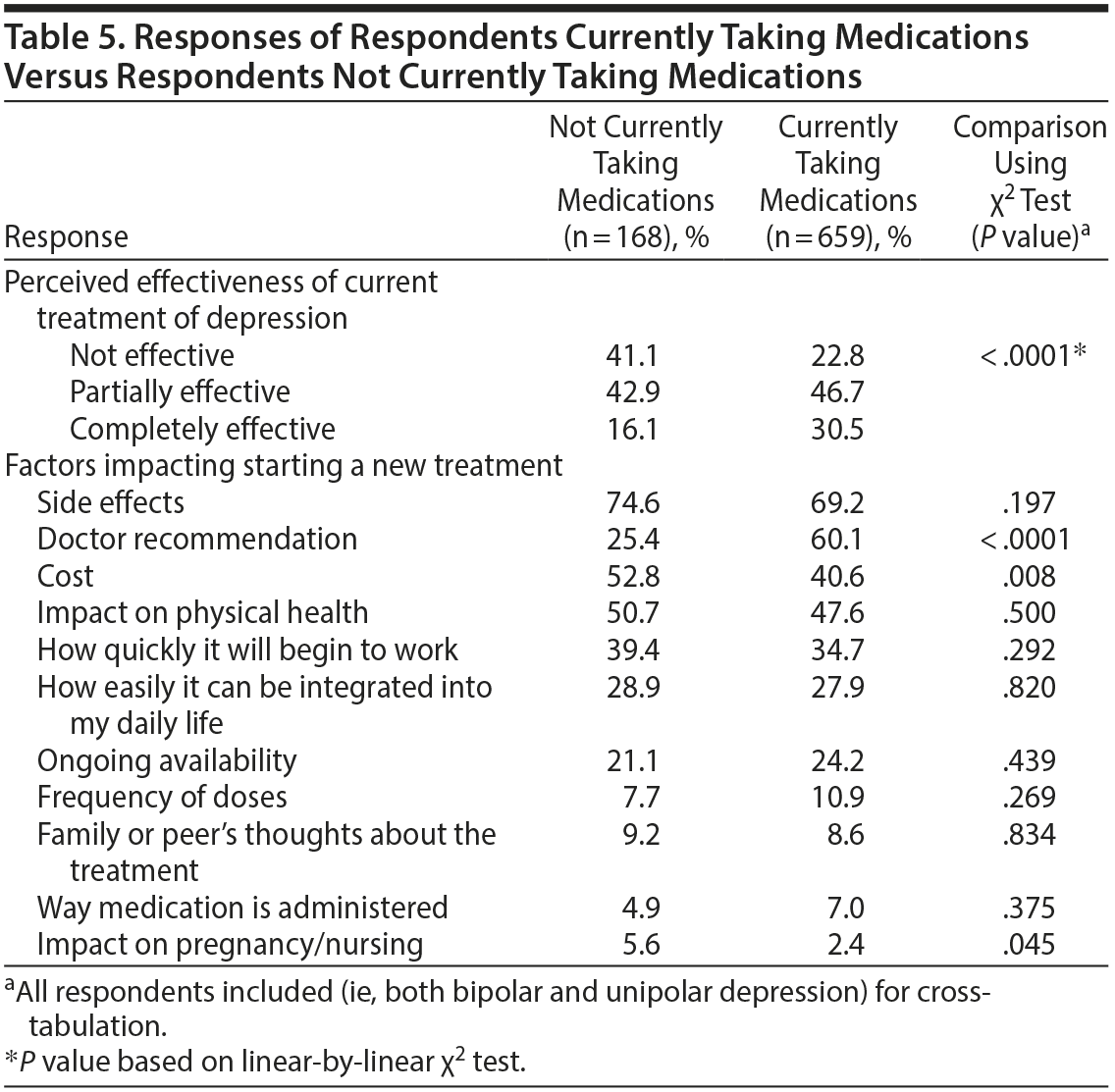
Comparison of Respondents Based On Medication Use History
Response rates were also cross-tabulated comparing participants reporting current medication use versus participants reporting not currently using any medications (Table 5). Participants currently taking medications were twice as likely to perceive their current treatment plan as completely effective compared to those not currently taking medications (30.5% vs 16.1%, respectively, P < .0001). Three statistically significant differences in factors impacting treatment decisions emerged when comparing these 2 groups. Doctor recommendations were more likely to be influential for those taking medications (60.1% vs 25.4%, P < .0001). Conversely, cost (40.6% versus 52.8%, P = .008) and impact on pregnancy/nursing (2.4% vs 5.6%, P = .045) were more likely to impact treatment decision in participants not currently taking medications.
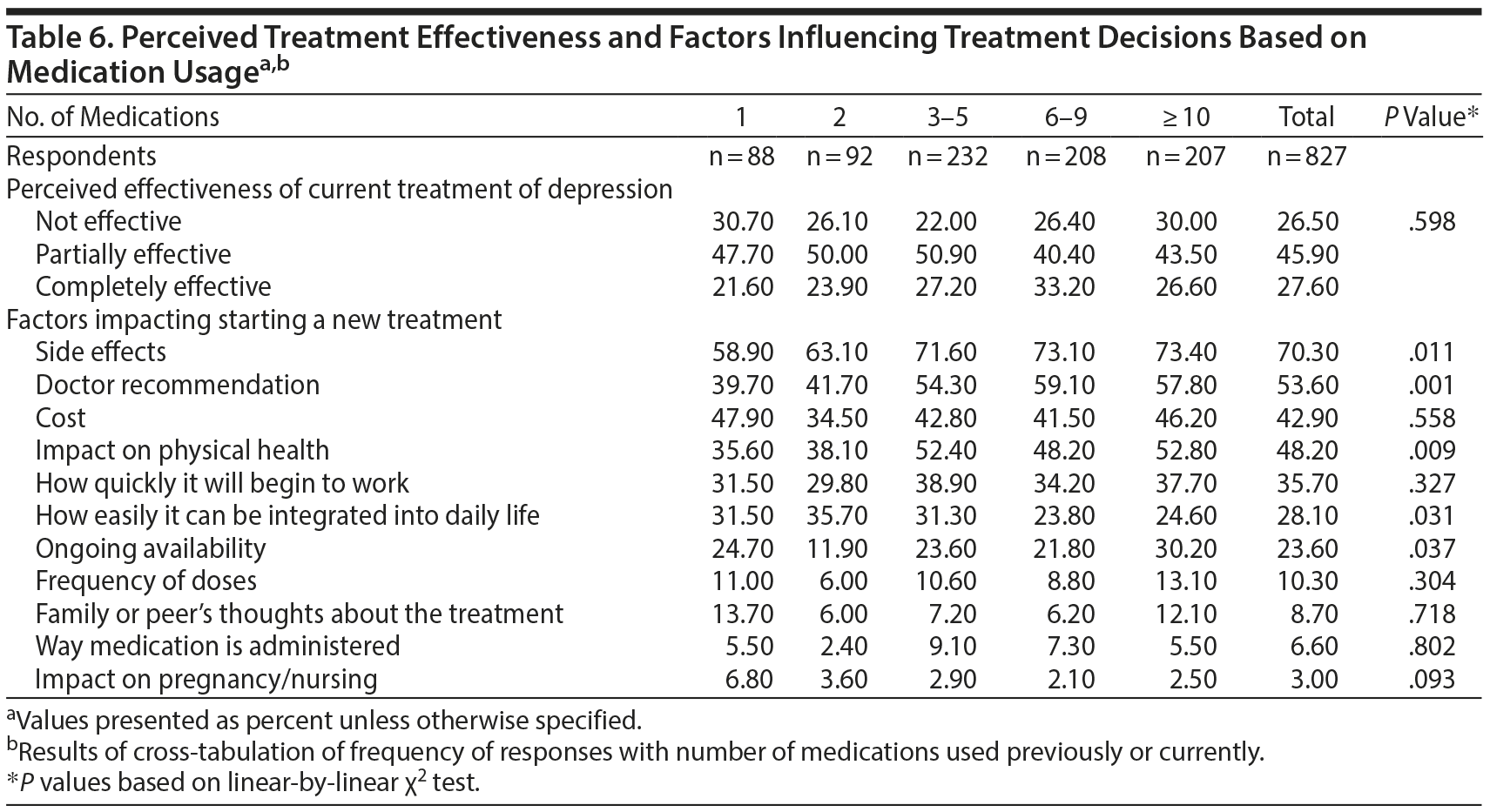
Responses were also compared based on the number of medications used for depression previously or currently (Table 6). No statistically significant differences in perceived effectiveness were observed (P = .598). However, factors that impacted starting a new treatment varied based on medication history: side effects (P = .011), doctor recommendations (P = .001), and impact on physical health (P = .009) were more frequently influential in respondents with greater number of medication trials. Conversely, how easily the medication would integrate into daily life was more frequently reported as influential in respondents with fewer medication trials (P = .031). Ongoing availability was a factor most commonly reported in participants with ≥ 10 medication trials (P = .037).
DISCUSSION
The majority of respondents reported receiving some form of pharmacotherapy as part of their current treatment plan. Further, most respondents reported a history of several trials of different medications, with only a minority of respondents endorsing complete effectiveness of the current treatment plan. Given the number of previous medication trials, the majority of respondents most likely had significant experience in making treatment decisions and numerous previous discussions with care providers regarding starting and stopping various psychiatric medications. As such, the current sample is most likely suitable for answering questions regarding factors influencing treatment decisions.
Side effects, doctor recommendations, cost, and rapidity of antidepressant effectiveness were found to be particularly important factors in making treatment decisions. Side effects and ineffectiveness were the most common reasons for discontinuing a medication and were also the primary concerns when making treatment changes. Weight gain and impact on physical health were identified as important factors, further emphasizing the importance of prioritizing weight-neutral treatments, as there is great variability in the cardiometabolic effects of different psychiatric medications.13 The importance of “how quickly it will begin to work” is also of interest, given the potential new class of rapid-acting antidepressants, such as ketamine, currently being investigated.14
In making treatment decisions, some of these factors (eg, efficacy and tolerability) are currently usually considered by prescribers and are discussed with patients as part of the standard of care during the informed consent process.9,10 In addition to these standard and expected factors, other potentially unexpected factors, such as impact on physical health and time to onset of antidepressant effects, were also reported as important in making treatment decisions. Therefore, these additional factors should be considered while engaging in treatment decision discussions, as the identified factors are likely to impact the shared decision-making process.
Intriguingly, doctor recommendations were more likely to be influential for participants currently taking medications, whereas cost and impact on pregnancy/lactation were more likely to influence treatment decisions for participants not taking medications. Moreover, current medication usage significantly increased the proportion of individuals perceiving their current treatment plan as completely effective (Table 5). As such, our results suggest that the inability to pay for medications, concerns about the effect of medications on pregnancy/lactation, and valuing doctor recommendations less are associated with a decreased likelihood of taking medications, which is associated with decreased subjective treatment effectiveness. Therefore, exploring these 3 factors with patients who are refusing potentially effective treatments may be beneficial to improve treatment outcomes. Notably, however, the significant impact of doctor recommendations is subject to facilitating paternalistic practices rather than patient-centered care.15
The current study had a number of important limitations to acknowledge. First, all results were entirely based on self-report and not verified via any objective measures. The use of self-report is both a strength and weakness of the current study. As the DBSA was most interested in the patient-consumer perspective (rather than the clinician’s perspective), the use of self-report was helpful. However, in the absence of collateral information, the accuracy of some of the data may be called into question (eg, diagnosis was not confirmed, medication trials unverified). The lack of demographic information is also a limitation, making it difficult to understand what population the current results are generalizable to. However, results of previous DBSA surveys suggest that survey respondents are diverse with regard to age, sex, ethnicity, and socioeconomic status.16 Finally, the current survey did not capture the perspective of substitute decision makers, which may be important to consider as well.
In summary, numerous factors emerged that are commonly considered by patients when selecting, changing, or discontinuing pharmacologic treatments for BD and MDD. Some of the common influential factors are already routinely considered by prescribers, such as evaluating comparative efficacy and side effects of treatment options. However, numerous other factors emerged that were important to respondents that are not routinely discussed with patient-consumers, such as inter-medication differences in the lag time to have antidepressant effects. The results from the current survey add to the growing literature on understanding what factors are most important to patients when selecting treatments for depression.17 Considering these important consumer perspectives in future clinical studies and direct clinical care may serve to improve consumer satisfaction and the therapeutic alliance, as consumers and providers collaboratively make important treatment decisions.
Submitted: June 13, 2018; accepted August 24, 2018.
Published online: November 1, 2018.
Author contributions: The Depression and Bipolar Support Alliance (Drs Simon, Sachs, and McIntyre; Mss Deetz, DePeralta, and Dean; and Mr Doederlein) conducted the online survey and tabulated frequencies of responses. Dr Rosenblat wrote the initial draft of the manuscript. Drs Simon, Sachs, and McIntyre; Mss Deetz, DePeralta, and Dean; and Mr Doederlein provided edits and revisions. All authors read and approved the final submission.
Potential conflicts of interest: Dr Simon is an employee of Kaiser Permanente and has received research grant support from Novartis for research regarding suicide risk in psoriasis. Dr Sachs is an employee of Bracket and Collaborative Care Initiative and has received support from Supernus for investigator-initiated research. Dr McIntyre has served on the advisory boards and received speaker fees and grant support from Lundbeck, Pfizer, AstraZeneca, Eli-Lilly, JanssenOrtho, Purdue, Johnson & Johnson, Moksha8, Sunovion, Mitsubishi, Takeda, Forest, Otsuka, Bristol-Myers Squibb, and Shire. Dr Rosenblat; Mss Deetz, DePeralta, and Dean; and Mr Doederlein have no conflicts of interest to declare.
Funding/support: The study was conducted with support from Alkermes.
Role of the sponsor: Alkermes reviewed the publication to ensure none of Alkermes’ confidential information was disclosed.
Supplementary material: See accompanying pages.
REFERENCES
1. WHO. Depression Fact Sheet. 2017. WHO website. http://www.who.int/news-room/fact-sheets/detail/depression. Accessed September 20, 2018.
2. McIntyre RS. Florida Best Practice Psychotherapeutic Medication Guidelines for Adults, 2015. Florida Medicaid Drug Therapy Management Program for Behavioral Health website. http://www.medicaidmentalhealth.org/_assets/file/Guidelines/Web_2015-Psychotherapeutic%20Medication%20Guidelines%20for%20Adults_Final_Approved1.pdf. Accessed September 20, 2018.
3. Gaynes BN, Warden D, Trivedi MH, et al. What did STAR*D teach us? results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv. 2009;60(11):1439-1445. PubMed CrossRef
4. Grande I, Berk M, Birmaher B, et al. Bipolar disorder. Lancet. 2016;387(10027):1561-1572. PubMed CrossRef
5. Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357-1366. PubMed CrossRef
6. Miura T, Noma H, Furukawa TA, et al. Comparative efficacy and tolerability of pharmacological treatments in the maintenance treatment of bipolar disorder: a systematic review and network meta-analysis. Lancet Psychiatry. 2014;1(5):351-359. PubMed CrossRef
7. Whitney SN, McGuire AL, McCullough LB. A typology of shared decision making, informed consent, and simple consent. Ann Intern Med. 2004;140(1):54-59. PubMed CrossRef
8. Gopal AA, Cosgrove L, Shuv-Ami I, et al. Dynamic informed consent processes vital for treatment with antidepressants. Int J Law Psychiatry. 2012;35(5-6):392-397. PubMed CrossRef
9. Bauer M, Monz BU, Montejo AL, et al. Prescribing patterns of antidepressants in Europe: results from the Factors Influencing Depression Endpoints Research (FINDER) study. Eur Psychiatry. 2008;23(1):66-73. PubMed CrossRef
10. Zimmerman M, Posternak M, Friedman M, et al. Which factors influence psychiatrists’ selection of antidepressants? Am J Psychiatry. 2004;161(7):1285-1289. PubMed CrossRef
11. Trivedi MH, Daly EJ. Measurement-based care for refractory depression: a clinical decision support model for clinical research and practice. Drug Alcohol Depend. 2007;88(suppl 2):S61-S71. PubMed CrossRef
12. McHugh RK, Whitton SW, Peckham AD, et al. Patient preference for psychological vs pharmacologic treatment of psychiatric disorders: a meta-analytic review. J Clin Psychiatry. 2013;74(6):595-602. PubMed CrossRef
13. Rosenblat JD, McIntyre RS. Pharmacological approaches to minimizing cardiometabolic side effects of mood stabilizing medications. Curr Treat Options Psychiatry. 2017;4(4):319-332.
14. Caddy C, Amit BH, McCloud TL, et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev. 2015;CD011612(9):CD011612. PubMed CrossRef
15. Drake RE, Deegan PE. Shared decision making is an ethical imperative. Psychiatr Serv. 2009;60(8):1007. PubMed CrossRef
16. DBSA Consumer and Family Survey Center. Depression and Bipolar Support Alliance website. http://www.dbsalliance.org/site/PageServer?pagename=help_surveycenter. Accessed September 20, 2018.
17. Ahmed S, Berzon RA, Revicki DA, et al; International Society for Quality of Life Research. The use of patient-reported outcomes (PRO) within comparative effectiveness research: implications for clinical practice and health care policy. Med Care. 2012;50(12):1060-1070. PubMed CrossRef
Please sign in or purchase this PDF for $40.00.
Save
Cite