Donepezil is an acetylcholinesterase inhibitor1 with a half-life of 70 hours in the blood2 that shows antidementia effects when administered once daily.3,4 Dementia is primarily characterized by short-term memory impairment.3 Cases of overdose of medication due to cognitive decline have been reported.4 We present a case of donepezil overdose in a patient with Alzheimer’s disease.
Case Report
A 77-year-old woman with Alzheimer’s disease presented with a history of cellulitis, sciatic neuropathy, and cervical spondylosis. Regular medications included cilostazol and donepezil.
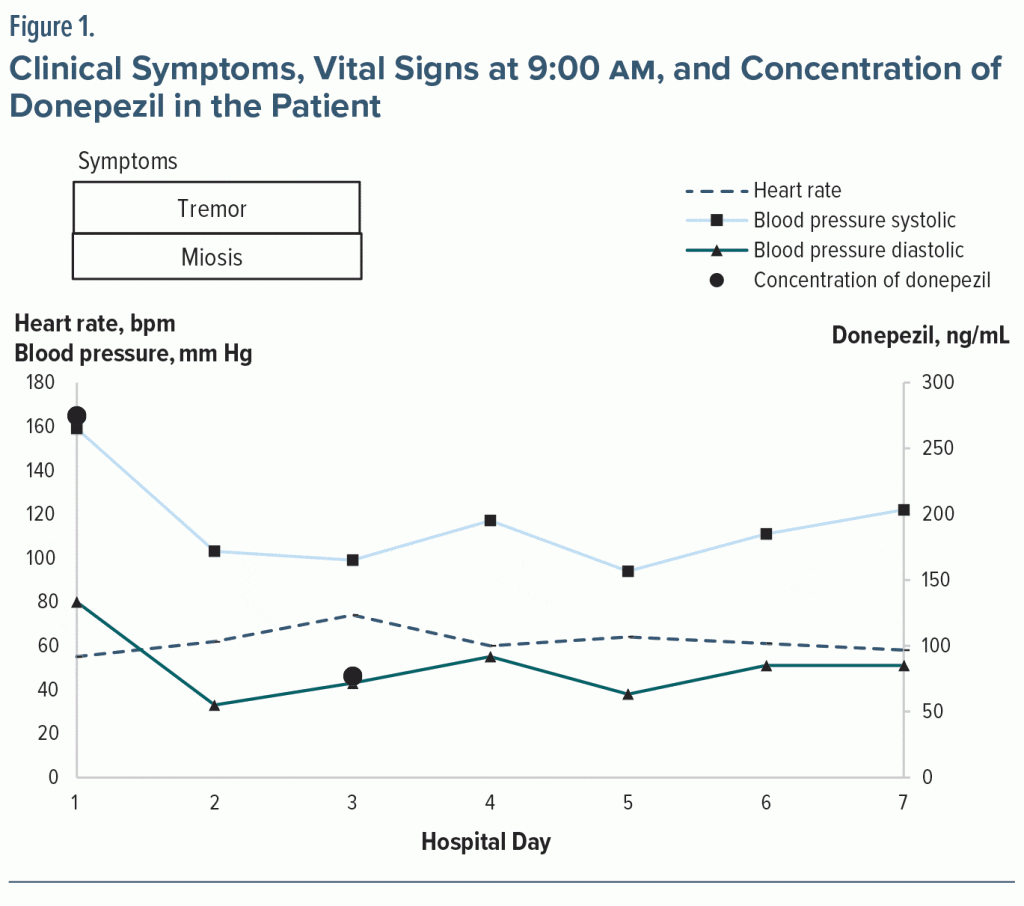
The patient accidentally overdosed at 11 pm with 15 tablets (150 mg) of donepezil by mistake, as out of 24 tablets, 9 were remaining after the dosage. At 8:20 am the next day, the patient fell and was taken to the hospital. On arrival, the clinical findings were as follows: temperature: 35.5°C (95.9°F), elevated blood pressure: 159/80 mm Hg, SpO2: 98%, heart rate: 67 bpm, elevated respiratory rate: 22 breaths/minute, and Glasgow Coma Scale: 4 (eyes), 4 (verbal), 6 (motor). No electrocardiographic changes were found. Pupil constriction of 1 mm × 1 mm and postural tremor of the upper extremities were observed. Blood test results at 12:30 pm (hospital day 1) revealed normal cholinesterase levels of 270 U/L but increased donepezil blood concentration of 272 ng/mL. The patient was admitted to the hospital, and infusion therapy of hypotonic fluid 1.5 L was administered. No oral medications were used.
On day 2, her heart rate was 45 bpm at 4:00 am. At 9:00 am, her heart rate was 62 bpm, and her blood pressure was 103/33 mm Hg (Figure 1). Mydriasis and tremor were observed; therefore, infusion therapy of hypotonic fluid 1 L was continued.
On day 3, her heart rate was 54 bpm, and her blood pressure was 139/75 mm Hg at 2:00 am. At 9:00 am, her heart rate was 74 bpm, and her blood pressure was 99/43 mm Hg. Mydriasis was observed, but the tremor decreased in frequency. At 1:45 pm, her donepezil blood concentration level was 77 ng/mL (normal cholinesterase levels).
On day 4, her pupils were 3 mm x 3 mm, but tremors were absent. On day 9, donepezil was resumed. On day 17, the patient was transferred to a hospital for rehabilitation due to decreased activity level.
Discussion
In this report, we present a case of donepezil overdose (150 mg). After initiating treatment, the blood concentration of donepezil decreased rapidly, but the clinical symptoms persisted. The half-life of donepezil is 67.3 hours2; however, our patient’s blood concentration rapidly decreased with treatment from 275 ng/mL to 77 ng/mL within 48 hours. One of the main reasons for this rapid decrease may be the effect of infusion therapy in diluting the blood with a 2.5-L transfusion and ensuring hepatic blood flow. Also, donepezil has high tissue transferability,2 so rapid tissue distribution may have influenced this decrease. Furthermore, donepezil has a high protein-binding rate (93%),5 hence it slows metabolization due to saturation of protein binding.6 High doses increased free donepezil levels, which would have caused rapid metabolism.
Even after a rapid decrease in donepezil blood concentration, the clinical symptoms (mydriasis and tremor) persisted. The effective blood concentration was 70 ng/mL,2,6 but the clinical symptoms persisted even at a concentration of 77 ng/mL. Persistence of symptoms may be due to high tissue translocation and active metabolites. Donepezil exhibits high tissue migration,6 and rapid tissue distribution may result in residual symptoms. 6-O-desmethyl donepezil (6-ODD) is an active metabolite,7 and at normal doses it is not problematic.8 However, our patient overdosed on 150 mg of donepezil. The half-life of 6-ODD is > 300 hours with a high muscle accumulation rate.9 This may be the reason for persistence of clinical symptoms.
In conclusion, despite the rapid decrease in blood concentration of donepezil upon treatment, the clinical symptoms persisted. Therefore, in donepezil overdose, it is necessary to observe not only blood levels but also clinical symptoms.
Article Information
Published Online: September 28, 2023. https://doi.org/10.4088/PCC.23cr03511
© 2023 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord 2023;25(5):23cr03511
Submitted: February 15, 2023; accepted April 7, 2023.
To Cite: Mori K, Hirose T, Matsumura T, et al. Follow-up of clinical symptoms and blood concentration in donepezil overdose. Prim Care Companion CNS Disord. 2023;25(5):23cr03511.
Author Affiliations: Gifu Pharmaceutical University, Gifu, Japan (Mori, Hirose, Yoshimura); Meijo University, Aichi, Nagoya, Japan (Matsumura).
Corresponding Author: Koki Mori, BPharm, Department of Pharmacy, Ogaki Municipal Hospital, 4-86 Minaminokawa-cho, Ogaki-shi, Gifu 503-8502, Japan ([email protected]).
Relevant Financial Relationships: None.
Funding/Support: None.
Patient Consent: Consent was received from the patient to publish the case report, and information (including dates) has been de-identified to protect anonymity.
ORCID: Koki Mori: https://orcid.org/0000-0001-8616-3422
References (9)

- Kosasa T, Kuriya Y, Yamanishi Y. Effect of donepezil hydrochloride (E2020) on extracellular acetylcholine concentration in the cerebral cortex of rats. Jpn J Pharmacol. 1999;81(2):216–222. PubMed CrossRef
- Rogers SL, Friedhoff LT. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following single oral doses. Br J Clin Pharmacol. 1998;46(suppl 1):1–6. PubMed CrossRef
- Wilson RS, Boyle PA, Yu L, et al. Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology. 2015;85(11):984–991. PubMed CrossRef
- Mitchell RJ, Harvey LA, Brodaty H, et al. Dementia and intentional and unintentional poisoning in older people: a 10-year review of hospitalization records in New South Wales, Australia. Int Psychogeriatr. 2015;27(11):1757–1768. PubMed CrossRef
- Noetzli M, Eap CB. Pharmacodynamic, pharmacokinetic and pharmacogenetic aspects of drugs used in the treatment of Alzheimer’s disease. Clin Pharmacokinet. 2013;52(4):225–241. PubMed CrossRef
- Tiseo PJ, Rogers SL, Friedhoff LT. Pharmacokinetic and pharmacodynamic profile of donepezil HCl following evening administration. Br J Clin Pharmacol. 1998;46(suppl 1):13–18. PubMed CrossRef
- Bures J, Tacheci I, Kvetina J, et al. The impact of dextran sodium sulfate-induced gastrointestinal injury on the pharmacokinetic parameters of donepezil and its active metabolite 6-o-desmethyldonepezil, and gastric myoelectric activity in experimental pigs. Molecules. 2021;26(8):2160. PubMed CrossRef
- Shintani EY, Uchida KM. Donepezil: an anticholinesterase inhibitor for Alzheimer’s disease. Am J Health Syst Pharm. 1997;54(24):2805–2810. PubMed CrossRef
- Patel BN, Sharma N, Sanyal M, et al. Quantitation of donepezil and its active metabolite 6-O-desmethyl donepezil in human plasma by a selective and sensitive liquid chromatography-tandem mass spectrometric method. Anal Chim Acta. 2008;629(1-2):145–157. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite