Objective: The current meta-analysis synthesizes previous findings on the effect of gabapentin on alcohol withdrawal and craving.
Data Sources: Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology, a search for relevant English-language literature published between January 1999 and February 2019 was conducted using PubMed and Google Scholar with the keywords alcohol use disorder, alcohol dependence, alcohol withdrawals, alcohol craving, “gabapentin in alcohol use, consumption,” and “gabapentin in alcohol withdrawals.”
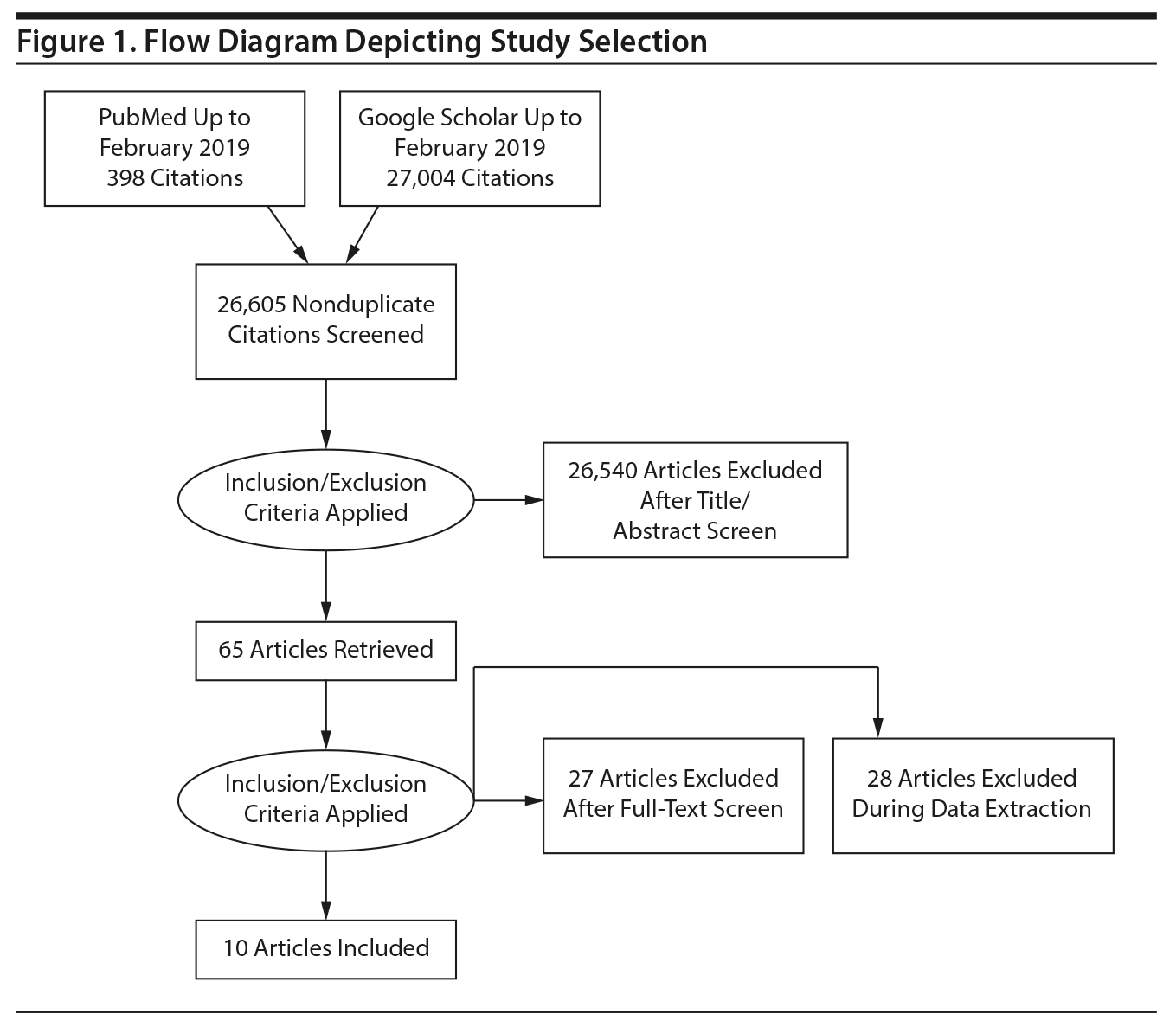
Study Selection and Data Extraction: Studies were included wherein gabapentin was used as an adjunctive or primary treatment of alcohol dependence/withdrawal. Studies included participants diagnosed with alcohol use disorder using DSM-IV, DSM-IV-TR, DSM-5, or the International Classification of Diseases, Tenth Revision (ICD-10). The search, as well as data extraction, was carried out by 3 blinded authors to preserve precision, using a template in Microsoft Excel to extract the needed data. Following the review of the initial 65 returns, 2 authors independently judged each trial by applying the inclusionary and exclusionary criteria, and any remaining disagreements were resolved by involving a third independent author. A total of 10 studies met the inclusion criteria and were selected for analysis. Subjects in these 10 studies were pooled using standard techniques of meta-analysis.
Data Synthesis: Three sets of meta-analyses examined outcomes from (1) single-group pretest-posttest changes, (2) posttest differences between independent groups, and (3) differences in pretest-posttest change scores between independent groups. Statistically significant effect sizes were found for craving (P < .01) and withdrawal (P < .01, P < .001) in the meta-analysis of single-group pretest-posttest outcome changes and were associated with a high level of heterogeneity. In contrast, the meta-analyses of posttest differences between independent groups—that of differences in pretest-posttest change scores between independent groups—did not yield significant effect sizes.
Conclusions: Our analysis of pooled data provides evidence that the use of gabapentin to manage alcohol withdrawal symptomatology and related cravings is at least moderately effective. However, given the limited number of available well-designed studies, these findings require further support through more rigorously designed studies.
Objective: The current meta-analysis synthesizes previous findings on the effect of gabapentin on alcohol withdrawal and craving.
Data Sources: Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology, a search for relevant English-language literature published between January 1999 and February 2019 was conducted using PubMed and Google Scholar with the keywords alcohol use disorder, alcohol dependence, alcohol withdrawals, alcohol craving, “gabapentin in alcohol use, consumption,” and “gabapentin in alcohol withdrawals.”
Study Selection and Data Extraction: Studies were included wherein gabapentin was used as an adjunctive or primary treatment of alcohol dependence/withdrawal. Studies included participants diagnosed with alcohol use disorder using DSM-IV, DSM-IV–TR, DSM-5, or the International Classification of Diseases, Tenth Revision (ICD-10). The search, as well as data extraction, was carried out by 3 blinded authors to preserve precision, using a template in Microsoft Excel to extract the needed data. Following the review of the initial 65 returns, 2 authors independently judged each trial by applying the inclusionary and exclusionary criteria, and any remaining disagreements were resolved by involving a third independent author. A total of 10 studies met the inclusion criteria and were selected for analysis. Subjects in these 10 studies were pooled using standard techniques of meta-analysis.
Data Synthesis: Three sets of meta-analyses examined outcomes from (1) single-group pretest-posttest changes, (2) posttest differences between independent groups, and (3) differences in pretest-posttest change scores between independent groups. Statistically significant effect sizes were found for craving (P < .01) and withdrawal (P < .01, P < .001) in the meta-analysis of single-group pretest-posttest outcome changes and were associated with a high level of heterogeneity. In contrast, the meta-analyses of posttest differences between independent groups—that of differences in pretest-posttest change scores between independent groups—did not yield significant effect sizes.
Conclusions: Our analysis of pooled data provides evidence that the use of gabapentin to manage alcohol withdrawal symptomatology and related cravings is at least moderately effective. However, given the limited number of available well-designed studies, these findings require further support through more rigorously designed studies.
Prim Care Companion CNS Disord 2019;21(4):19r02465
To cite: Ahmed S, Stanciu CN, Kotapati PV, et al. Effectiveness of gabapentin in reducing cravings and withdrawal in alcohol use disorder: a meta-analytic review. Prim Care Companion CNS Disord. 2019;21(4):19r02465.
To share: https://doi.org/10.4088/PCC.19r02465
© Copyright 2019 Physicians Postgraduate Press, Inc.
aBoston University Medical Center, Boston University School of Medicine, Boston, Massachusetts
bDepartment of Psychiatry, Geisel School of Medicine at Dartmouth, Dartmouth-Hitchcock Medical Center, Lebanon, New Hampshire
cAddiction Services, New Hampshire Hospital, Concord, New Hampshire
dDepartment of Psychiatry, Manhattan Psychiatric Center, New York, New York
eLiaquat College of Medicine and Dentistry (Dar-ul Sehat Hospital), Karachi, Pakistan
fDepartment of Psychiatry, St Elizabeth’s Medical Center, Brighton, Massachusetts
gDepartment of Psychiatry, University of Texas Rio Grande Valley, Brownsville, Texas
hNew York Institute of Technology (NYIT) College of Osteopathic Medicine, Old Westbury, New York
iDepartment of Psychiatry, Texas Tech University Health Science-Permian Basin, Odessa, Texas
jDepartment of Psychiatry, Nassau University Medical Center, East Meadow, New York
*Corresponding author: Siddhi Bhivandkar, MD, St Elizabeth’s Medical Center, 736 Cambridge St, Brighton, MA 02135 ([email protected]).
Alcohol withdrawal occurs as a result of cessation of or reduction in alcohol use, particularly after a period of heavy and prolonged drinking. The diagnosis requires the presence of ≥ 2 of a set of 8 criteria: autonomic hyperactivity (eg, sweating or pulse rate > 100 beats per minute); increased hand tremor; insomnia; nausea or vomiting; transient visual, tactile, or auditory hallucinations or illusions; psychomotor agitation; anxiety; or generalized tonic-clonic seizures. The symptoms develop within several hours to a few days after this cessation or reduction in alcohol use and can be potentially life-threatening.1 The “protracted abstinence” phase of withdrawal causes negative affective changes that are associated with increased cravings and relapse risk.2
At present, benzodiazepines (BZDs) serve as a standard of care in the treatment of alcohol withdrawal and mediate primary γ-aminobutyric acid (GABA) effects of alcohol by targeting the GABA-A receptors. While most patients with alcohol withdrawal respond to standard treatment that includes benzodiazepines, there is a subgroup of this patient population in whom deficiency of endogenous GABA or acquired conformational changes in GABA-A receptor may resist therapy (resistant alcohol withdrawal).3 These patients require large doses of BZD (ie, ≥ 50 mg of intravenous diazepam in the first hour of treatment) and additional sedatives adjunctive to BZDs such as phenobarbital, dexmedetomidine, or propofol and some may undergo complicated hospitalizations.4-6
Although BZDs are the standard treatment for managing withdrawal, BZDs are approved for a diverse set of clinical indications and are increasingly prescribed. In 2012, for every 100 Americans, 37.6 BZD prescriptions were written by prescribers. The rise in overuse, misuse, and addiction increased overdose deaths involving BZDs from 1,135 in 1999 to 8,791 in 2015.7
The results from the National Epidemiologic Survey on Alcohol and Related Conditions report8 estimated the US prevalence of DSM-5 12-month alcohol use disorder among adults aged 18 years and older was 13.9%, while the lifetime estimate was 29.1%. Evidence suggests that the US Food and Drug Administration (FDA)-approved relapse preventive medications are currently underused in the management of alcohol use disorder.9 In the context of such a high prevalence of alcohol use disorder, this underutilization by prescribing health care professionals raises concern.
Gabapentin, or 1-(aminomethyl)cyclohexane acetic acid, is a nonbenzodiazepine anticonvulsant with high affinity for voltage-gated calcium channels. Its mechanism of action is believed to be blocking of a specific α-2d subunit of the voltage-gated calcium channel at selective presynaptic sites, thereby indirectly modulating GABA neurotransmission via a downstream cascade of resulting events.10,11 Gabapentin has also been reported to enhance GABA activity by possibly stimulating GABA synthesis directly.12 Although its FDA indication is for treatment of epileptic seizures and neuropathic pain, gabapentin has been increasingly used off-label in recent years. A rising number of reports10,11 support its use for alcohol dependence as well as alcohol withdrawal symptomatology. Possible mechanisms include normalization of stress-induced GABA activation in the amygdala, which is associated with alcohol dependence. During withdrawal, it also potentially reduces withdrawal excitability in the hippocampus.13 Studies11 have also reported that gabapentin reduces alcohol craving as well as sleep and mood disturbances in alcohol-dependent individuals. Continued use of gabapentin has also been associated with reduced relapse rates and reduced risk of return to heavy drinking.12 Furthermore, gabapentin has been reported to be non-habit forming and to have low abuse potential when compared to BZDs.
Despite the numerous studies involving off-label use of gabapentin for alcohol use disorder, there have been no meta-analyses conducted to assess its efficacy in reducing craving and withdrawal symptoms. Multiple studies14 suggest gabapentin has some efficacy in reducing alcohol dependence, withdrawal, and craving, but current evidence is complicated by the challenges of the variable dosing of gabapentin between trials, the heterogeneity of diagnoses, and absence of clear and comprehensive primary study outcomes. Craving, as a criterion in the diagnosis of alcohol use disorder, was added in the DSM-5 and was unlikely to be considered as a primary outcome in pre-DSM-5 studies. Additionally, most studies were small or conducted in limited treatment settings. With this in mind, we decided to perform a meta-analysis of all available studies to assess the efficacy of gabapentin for craving and withdrawal symptoms. Our findings from these analyses are hypothesized to complement conclusions already drawn from systematic reviews.12,14,15
METHODS
Data Sources and Searches
Using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) methodology,16 a search for relevant English-language literature published between January 1999 and February 2019 was conducted using PubMed and Google Scholar (Figure 1) with the following keywords: alcohol use disorder, alcohol dependence, alcohol withdrawals, alcohol craving, “gabapentin in alcohol use, consumption,” and “gabapentin in alcohol withdrawals.”
Inclusion Criteria
- We included studies from the past 20 years until the final search was conducted in February 2019.
- Studies had to be published in the English language.
- Gabapentin was used as an adjunctive or primary treatment of alcohol dependence/withdrawal. Thus, studies could have included other medications or psychotherapy in addition to gabapentin.
- Studies had to use a measure of either craving or withdrawal symptoms as one of the outcome measures, since these were the most frequently used scales across the studies we examined.
- Studies had to include participants diagnosed with an alcohol use disorder using the DSM-IV, DSM-IV-TR, DSM-5, or International Classification of Diseases, Tenth Revision (ICD-10).
- Eligible study designs included single-group pretest-posttest, independent-groups posttest, or independent-groups pretest-posttest designs. For the latter 2 designs, the design-specific effect sizes were calculated only if the comparison group received a placebo, rather than an alternative medication (since comparisons to alternative medications are beyond the scope of the current study). However, effect size calculations for studies that used an alternative medication for the comparison group were conducted using only the gabapentin group data and only for single-group pretest-posttest effect size calculations.
Exclusion Criteria
- Studies published in any language other than English.
- Gabapentin was used solely for illnesses other than alcohol dependence, such as seizure disorder, mood disorders, neuropathic pain, or any other neuropsychiatric illness.
- Case reports, case series, and those studies in which there were no descriptive statistics or any statistics that could be converted to an effect size.
- Review articles, commentaries, or opinion pieces.
- Unpublished studies such as conference posters or presentations.
Data Extraction, Synthesis, and Analysis
The search was carried out by 2 independent authors (S.A. and S.B.). These researchers independently performed screening of titles and abstracts for initial exclusion based on publication type. Where required, the full texts were immediately screened to ascertain inclusion or exclusion. Following the initial exclusion screening, the researchers further evaluated the full-text articles for inclusion based on the 6 inclusion criteria. At each step, their results were compared, and any discrepancies were resolved through discussion. Any remaining disagreements were resolved by involving a third independent author (C.N.S.).
Means and standard deviations (SDs) of the withdrawal symptoms and craving measures were extracted from the studies by 2 independent volunteer researchers (S.A. and S.B.). For studies with more than 1 follow-up time point,10,17-20 only data from the earliest measure were extracted to minimize loss of participants. In addition, there were 2 studies that divided their gabapentin treatment participants into subgroups of different treatment dosages and reported their results across these subgroups.10,11 For these studies, the sample sizes, means, and SDs from these subgroups were combined, using the formula suggested in the Cochrane Handbook for Systematic Reviews of Interventions,21 such that only 1 sample size and 1 set of means and SDs from all participants regardless of subtypes were obtained. If the means and SDs were not reported in a numeric format but were instead presented in graphs, these means and SDs were extracted from the graphs using a web-based application designed to facilitate data extraction from graphs.22

- While benzodiazepines are considered the standard of care for alcohol withdrawal, gabapentin is a valuable alternative that can also help with cravings and abstinence long term.
- Larger, double-blinded studies are needed to more reliably demonstrate gabapentin’s efficacy in the management of alcohol withdrawal and cravings.
Design-specific standardized mean difference (SMD) scores were calculated for each study using 1 (or more when a study provided data that could be used in more than 1 of the meta-analyses conducted) of 3 formulas (Supplementary Appendix 1). In all cases of effect size calculations, the direction was coded so that negative effect sizes reflect favorable outcomes for the gabapentin group. Calculation of effect sizes was generally straightforward (some exceptions are discussed in Supplementary Appendix 1). Given the variability in types of trial designs, we aggregated only design-specific effect sizes across studies. Publication bias was assessed by funnel plots. In addition, a trim and fill analysis23 was conducted to assess the degree to which publication bias may have influenced the meta-analytic results. Finally, leave-one-out analyses were carried out for each of the 3 meta-analyses to assess the replicability and robustness of the results. Supplementary Appendix 1 provides a detailed description of these analyses.
Description of Studies
Information, where available, regarding the participants (age, sex, sample type, and clinical diagnoses) and the intervention design, dosage, and intervention interval for each study was obtained. Ten studies10,11,17-20,24-27 were deemed eligible for inclusion. These studies utilized a variety of designs, the most common (6 studies) being that of a single-group pretest-posttest design. In all but 1 study,24 participants were explicitly reported to have met DSM-IV or ICD-10 diagnoses of alcohol dependence/withdrawal. This study24 was included in the meta-analysis since it reported data on alcohol withdrawal from a US Veterans Affairs medical center between November 2001 and April 2002. Since the DSM-IV was published in 1994 and was widely used at the time of this study, we can infer that patients met DSM-IV criteria for alcohol withdrawal. In addition, the leave-one-out data analyses specifically address this issue of whether including this study impacted the meta-analytic results.
RESULTS
Treatment Outcomes
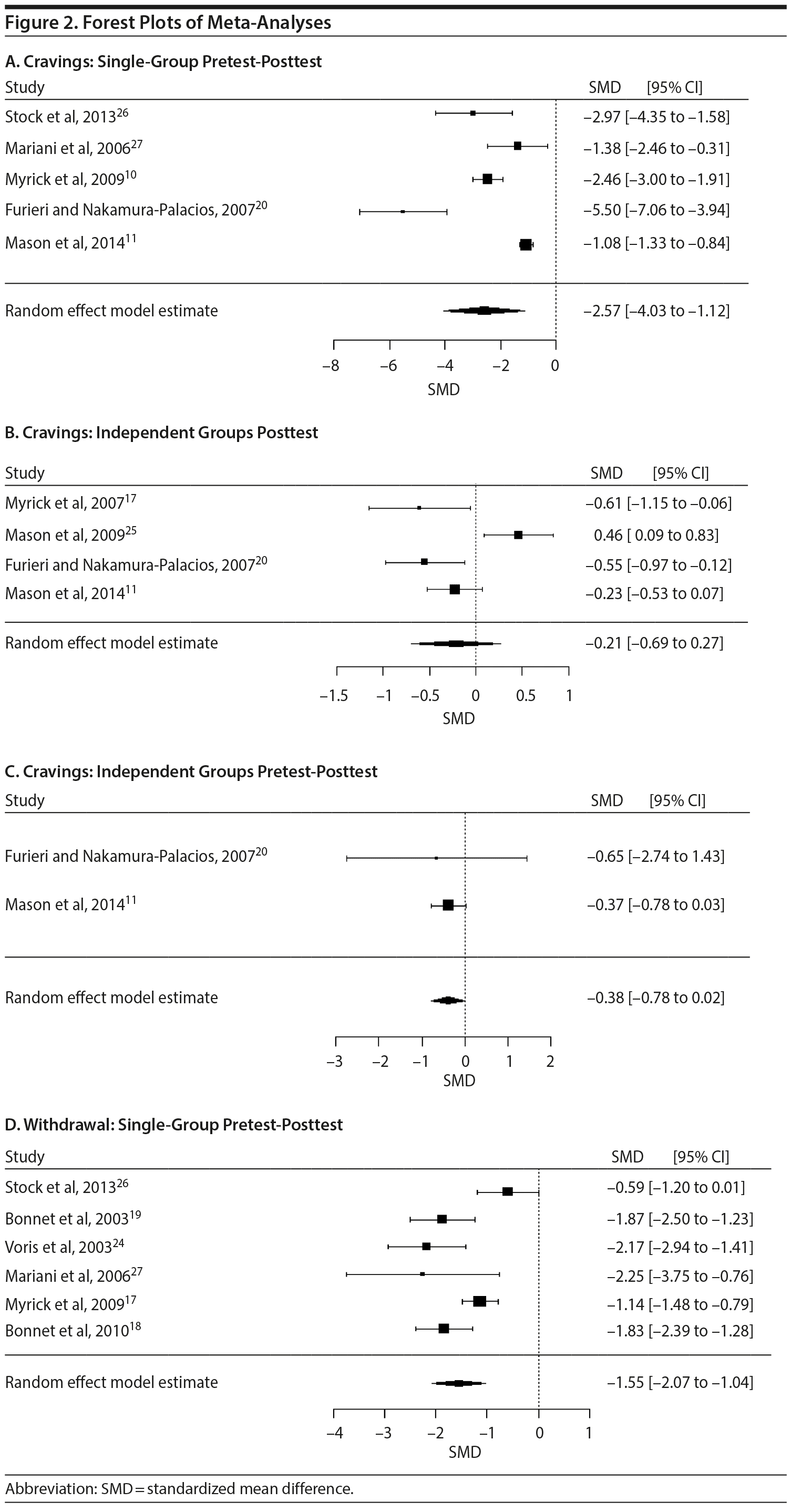
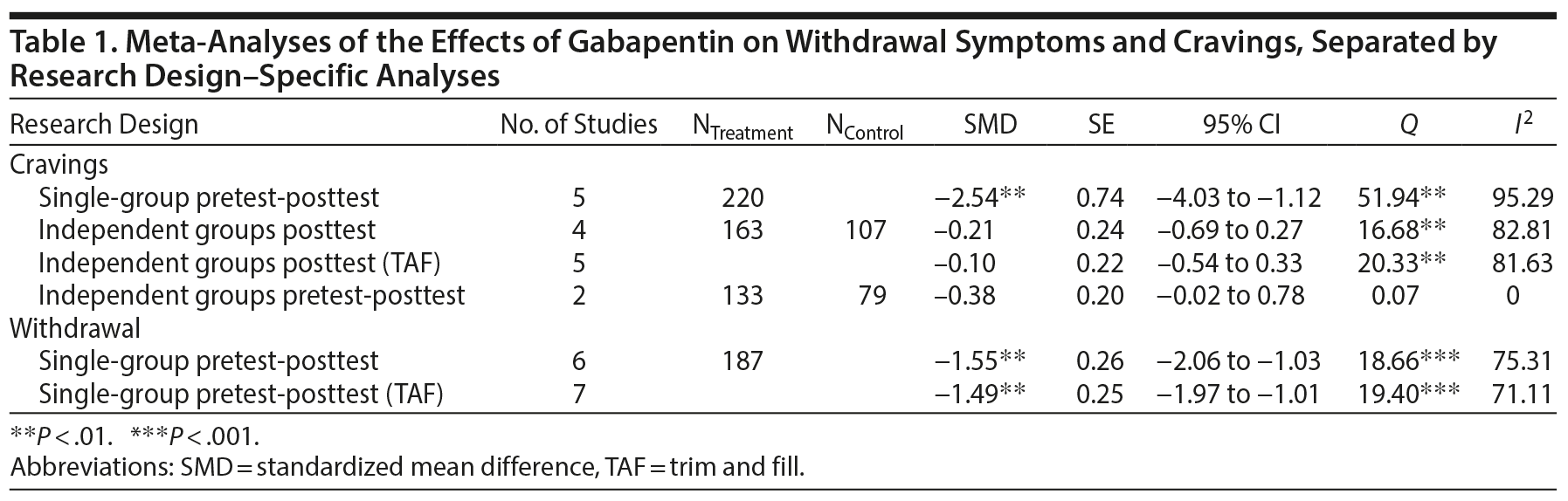
The meta-analyses of the single-group pretest-posttest results yielded significant SMDs for both cravings and withdrawal. However, the meta-analyses of the independent groups’ posttest and independent groups’ pretest-posttest outcomes on cravings did not result in any significant SMDs—studies that included withdrawal as an outcome variable had only a single-group pretest-posttest design, and no studies on withdrawal had either of the other 2 research design types. Substantial heterogeneity for all of the meta-analyses was demonstrated by the significant Q statistics and high I2 values. Forest plots for all meta-analyses are shown in Figure 2. The results for each of the meta-analyses, together with the trim and fill-adjusted results, are presented in Table 1.
Sensitivity Analysis
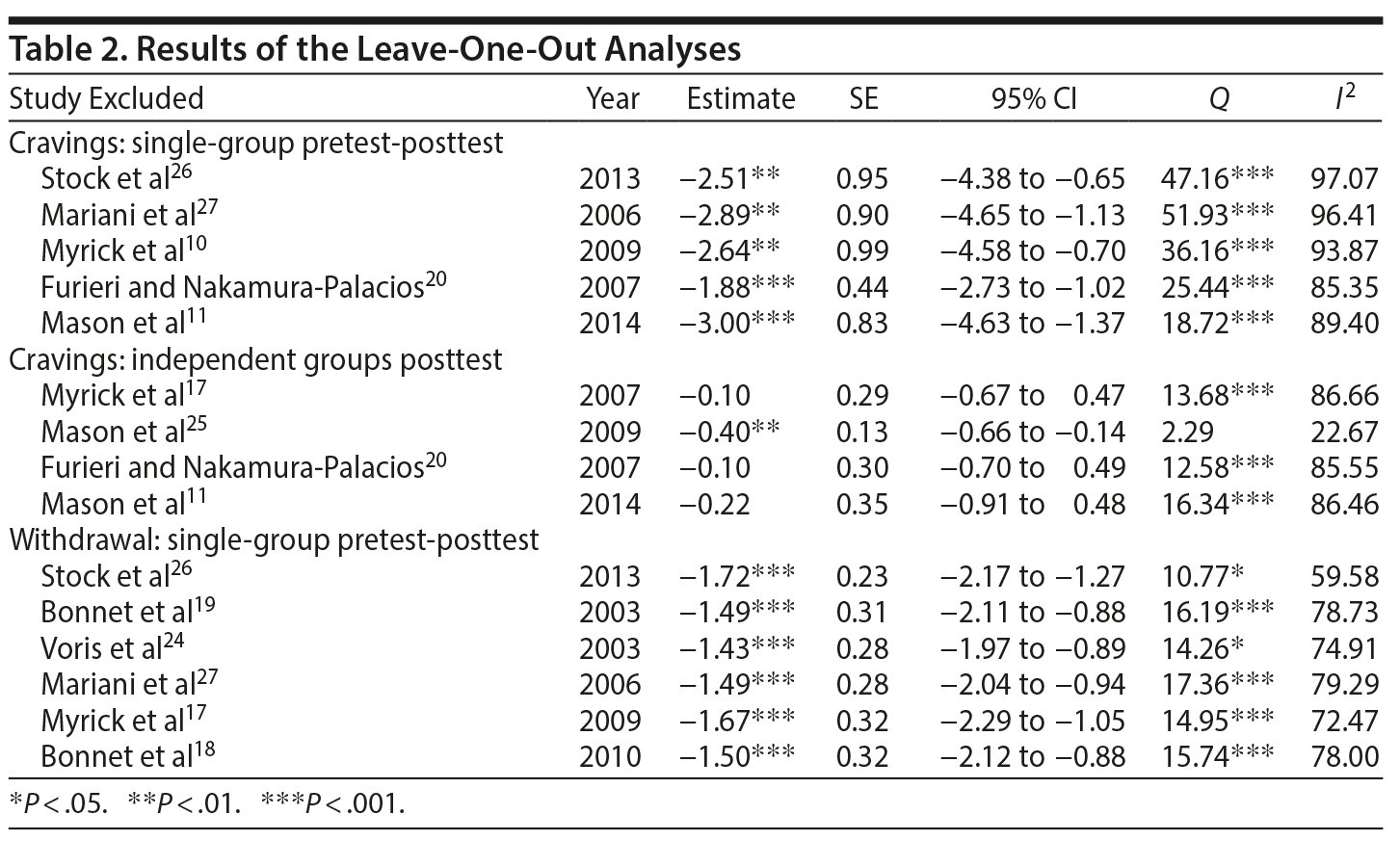
Results of the leave-one-out analyses generally suggested reliable and robust results for the single-group pretest-posttest meta-analyses for both cravings and withdrawal symptoms. No one study affected the overall estimates by more than 15.35%. As for heterogeneity, the exclusion of Lembke et al7 (this study was not included with the final 10 studies) impacted the Q statistic, which was no longer statistically significant (P = .09). Apart from this study, the exclusion of any other study did not significantly affect the heterogeneity results; the Q statistics remained significant (P ≤ .03).
The leave-one-out analyses revealed less reliable results for the independent groups’ posttest meta-analysis of cravings. First, the SMD for the independent group’s posttest cravings became significant with the exclusion of Mason et al.25 Next, the exclusion of Myrick et al17 and Furieri and Nakamura-Palacios20 each resulted in a 52.38% decrease in the SMD. Furthermore, the exclusion of Mason et al25 resulted in a 90.48% increase in the SMD and rendered the Q statistic nonsignificant (P = .32)
Leave-one-out analyses were not carried out for the independent groups’ meta-analyses since there were only 2 studies. The results of the leave-one-out analyses are presented in Table 2.
Trim and Fill Analyses
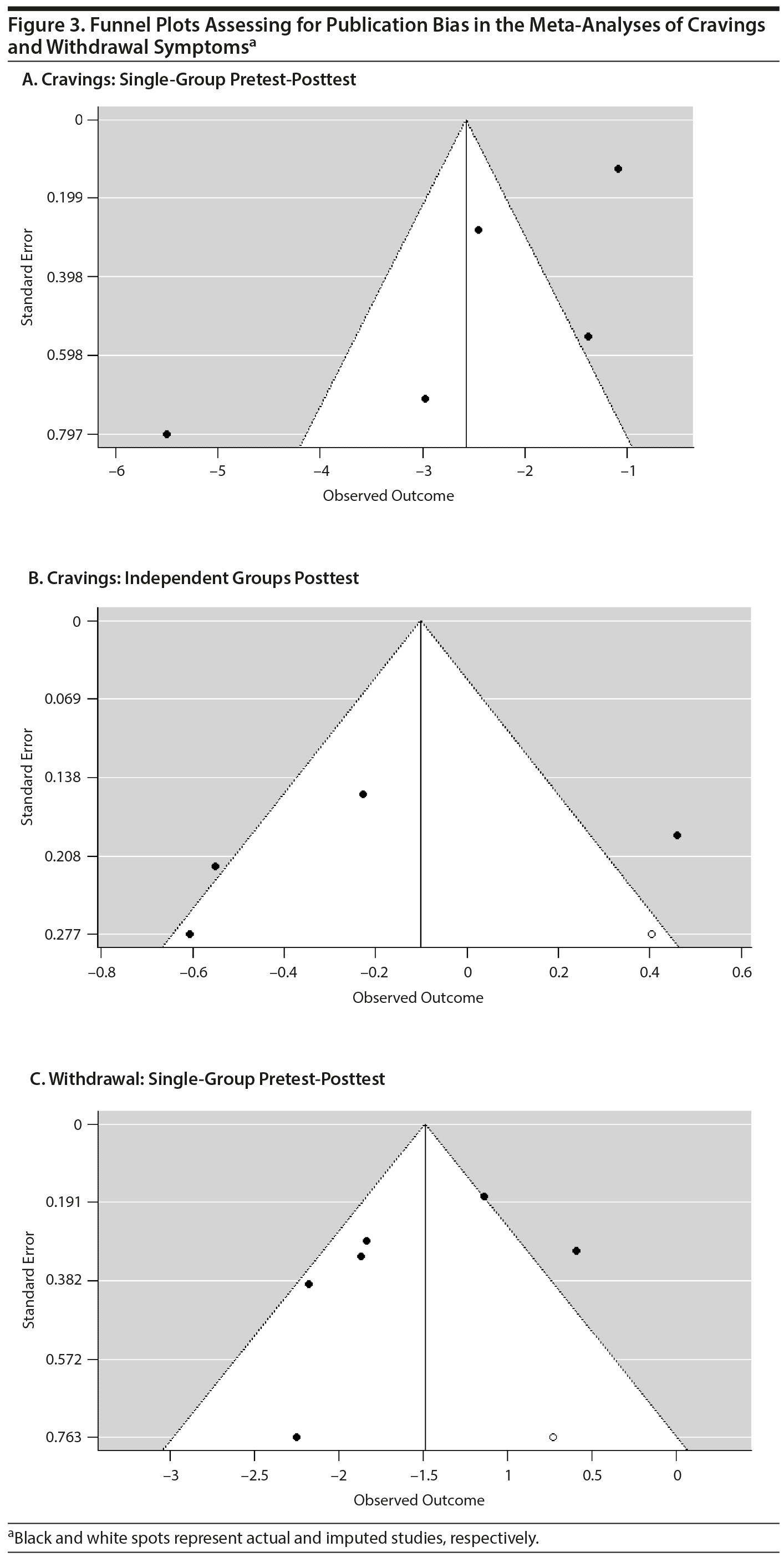
The funnel plots for the single-group pretest-posttest meta-analyses for cravings and withdrawal, as well as the funnel plot for the independent groups’ posttest analyses for cravings, are shown in Figure 2. The funnel plot for the independent groups’ pretest-posttest meta-analysis is not shown since there are too few studies for it to be meaningful. Using the trim and fill analysis, 2 studies were imputed in the meta-analysis of the single-group pretest-posttest results for cravings, 1 study was imputed in the meta-analysis of the independent groups’ posttest results for cravings, and 1 study was imputed in the meta-analysis of the single-group pretest-posttest results for withdrawal symptoms. If the asymmetry is due to publication bias, the results suggest that the true effect is slightly overestimated (Figure 3). Trim and fill analysis was not conducted for the independent groups’ pretest-posttest meta-analysis of cravings because there were only 2 studies. The results of the trim and fill-adjusted results, together with the rest of the results of the meta-analyses, are presented in Table 1.
DISCUSSION
The significant pooled estimates obtained for the treatment outcomes in the single-group pretest-posttest meta-analytic results provide support for the use of gabapentin in treating alcohol craving and withdrawal. It has been suggested that it may be as effective as BZDs in treating the symptoms of alcohol withdrawal.10,11,28-31 Patients might resume drinking if withdrawal symptoms are not adequately managed or if the medication given does not suppress the craving for alcohol or aggravates the craving. Some studies suggest that BZD use may increase craving and early relapse to alcohol use,17 whereas other studies28,32,33 have shown that gabapentin use did not trigger alcohol craving.
As described previously, barriers to the effective use of the FDA-approved agents in the management of alcohol use disorder include medical and psychiatric comorbidities, poor medication adherence, and problems with tolerability. As the body of research on alternatives to these agents continues to grow, we sought to improve the understanding of gabapentin’s strategic role in the management of alcohol dependence and withdrawal. Gabapentin is not hepatically metabolized,14 making it preferable to current FDA-approved agents, especially in a population with a high predisposition to hepatic insufficiency. Unlike acamprosate, gabapentin can also be used in patients with renal function < 20 mg/dL. Gabapentin is a promising agent in alcohol use disorder treatment because it is generally well tolerated, has a favorable safety profile with few side effects, does not interact with other medications, and improves sleep, mood, and anxiety.11,34,35 Unlike for carbamazepine and valproic acid, regular blood draws to determine gabapentin blood levels are not required. Furthermore, gabapentin has been shown to have some positive impact in the management of anxiety disorders and insomnia, which are frequent risks factors for alcohol use relapse.12,14
There is a general debate in clinical settings about the appropriate or recommended dose of gabapentin for alcohol dependence and withdrawal symptoms. Numerous clinical trials10,11,17-20,24-27 have suggested several dosing regimens of gabapentin for the treatment of alcohol withdrawal, which range from 300 mg twice daily to 600 mg 3 times a day. There is no consensus, to our knowledge, on the recommended or optimum dose of gabapentin in alcohol use disorder.
By examining the studies included in this review, it is difficult to determine an optimum dose of gabapentin in patients with alcohol dependence and withdrawal symptoms. In this context, we suggest the randomized controlled trial by Mason and colleagues11 as a model study for appropriate dosing while treating these patients. Mason et al11 randomized patients to 3 groups to receive placebo, gabapentin 300 mg thrice daily (900 mg), or gabapentin 600 mg thrice daily (1,800 mg). The results indicated that gabapentin had a significant linear dose effect on complete abstinence rate and no days of heavy drinking. In the 1,800-mg group, the rate of sustained 12 weeks of abstinence was significant with concomitant manual-guided counseling. Also, linear dose effects related to relapse-related symptoms such as cravings, mood, and sleep were statistically significant in the 1,800-mg group compared with other groups.11 Additional studies replicating this outcome would further establish gabapentin’s role in the treatment of alcohol use disorder.
There is a growing concern that gabapentin is being misused. The misuse has increased rapidly in recent years, especially in patients with substance use disorder. Gabapentin is noted, especially in those using opioids, to produce a rapid euphoric effect or “high” and reduce withdrawals.36 Patients with opioid use disorder misuse gabapentinoids to potentiate the effects of opioids in some cases, and patients also abuse it with prescription opioids such as methadone or buprenorphine/naloxone. The combined use of gabapentin and opioids also increases the risk of respiratory depression and deaths in this patient population.37 Although, gabapentin has a beneficial role in some substance use disorders such as alcohol use disorder, clinicians should adopt a cautious approach when considering prescribing gabapentin to patients with known prior history of drug dependence. Clinicians should educate their patients about the benefits and risks of gabapentin and its fatal interactions with other opioids (prescription opioids or illicit nonprescribed opioids).
Larger-sampled, double-blinded studies are required to more reliably demonstrate gabapentin’s efficacy in the treatment of alcohol withdrawal and craving. The unique advantages of gabapentin offer an opportunity for the development of a fairly reasonable alcohol detoxification program. For instance, gabapentin could be used initially as a detoxification agent and subsequently used as a relapse prevention agent with a lower and more tolerable dose for several months of maintenance treatment. Because protracted alcohol withdrawal symptoms of insomnia, anxiety, dysphoria, and alcohol craving can complicate the immediate period of recovery after detoxification, short-term maintenance treatment with gabapentin may relieve these symptoms and reduce relapse rates. Given gabapentin’s favorable safety profile, tolerability, and comparatively lower abuse potential than BZDs, it is an ideal candidate to study as a relapse prevention agent.
Limitations
One key limitation of this meta-analysis is that not all included studies were randomized controlled trials. In addition, there were several studies for which we were able to extract only pretest-posttest data, and these studies could, therefore, be included in only the single-group pretest-posttest meta-analyses. This meta-analysis does not, by definition of the eligibility criteria, compare the relative effectiveness of gabapentin with any alternative treatment.
In spite of the finding of significant single-group pretest-posttest effect sizes for both craving and withdrawal, there was a high degree of heterogeneity in both meta-analyses, as demonstrated by significant Q statistics and high I2 values. In the absence of a comprehensive qualitative analysis of studies in the meta-analyses, possible explanations for this degree of heterogeneity include varying severities of alcohol use disorder in the various study participants, the presence of psychiatric comorbidities in studied participants, variations in gabapentin dosage across included studies, and that follow-up time points were not homogenous across the studies.
Another limitation to the present study is the small number of individual primary studies included in the meta-analyses, a factor that also contributes to the significant Q statistics and high I2 values. The trim and fill analyses also point to some potential for publication bias in our findings, suggesting the possibility of a slight overestimate of the effect sizes in the meta-analyses. Given these limitations, one should exercise caution in generalizing the results of this study.
Submitted: March 31, 2019; accepted June 6, 2019.
Published online: August 22, 2019.
Potential conflicts of interest: None.
Funding/support: None.
Disclaimer: The findings of this meta-analysis are provided for educational purposes. These findings suggest conclusions based on analytical review of published data that should be interpreted cautiously in practical settings.
Ethics statement: The current meta-analysis identified published material and synthesized existing evidence that met prespecified eligibility criteria to answer specific questions regarding efficacy of gabapentin in alcohol withdrawal and cravings. The study was conducted entirely based on analytical interpretation of published papers. The authors did not require any consent from any human subjects.
Supplementary material: Supplementary Appendix 1 See accompanying pages.
REFERENCES
1.American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders. Fifth Edition. Washington, DC: American Psychiatric Association; 2013.
2.Heilig M, Egli M, Crabbe JC, et al. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15(2):169-184. PubMed CrossRef
3.Hack JB, Hoffmann RS, Nelson LS. Resistant alcohol withdrawal: does an unexpectedly large sedative requirement identify these patients early? J Med Toxicol. 2006;2(2):55-60. PubMed CrossRef
4.Gold JA, Rimal B, Nolan A, et al. A strategy of escalating doses of benzodiazepines and phenobarbital administration reduces the need for mechanical ventilation in delirium tremens. Crit Care Med. 2007;35(3):724-730. PubMed CrossRef
5.Mueller SW, Preslaski CR, Kiser TH, et al. A randomized, double-blind, placebo-controlled dose range study of dexmedetomidine as adjunctive therapy for alcohol withdrawal. Crit Care Med. 2014;42(5):1131-1139. PubMed CrossRef
6.Wong A, Benedict NJ, Lohr BR, et al. Management of benzodiazepine-resistant alcohol withdrawal across a healthcare system: Benzodiazepine dose-escalation with or without propofol. Drug Alcohol Depend. 2015;154:296-299. PubMed CrossRef
7.Lembke A, Papac J, Humphreys K. Our other prescription drug problem. N Engl J Med. 2018;378(8):693-695. PubMed CrossRef
8.Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 alcohol use disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72(8):757-766. PubMed CrossRef
9.Enoch M-A. Genetic influences on response to alcohol and response to pharmacotherapies for alcoholism. Pharmacol Biochem Behav. 2014;123:17-24. PubMed CrossRef
10.Myrick H, Malcolm R, Randall PK, et al. A double-blind trial of gabapentin versus lorazepam in the treatment of alcohol withdrawal. Alcohol Clin Exp Res. 2009;33(9):1582-1588. PubMed CrossRef
11.Mason BJ, Quello S, Goodell V, et al. Gabapentin treatment for alcohol dependence: a randomized clinical trial. JAMA Intern Med. 2014;174(1):70-77. PubMed CrossRef
12.Leung JG, Hall-Flavin D, Nelson S, et al. The role of gabapentin in the management of alcohol withdrawal and dependence. Ann Pharmacother. 2015;49(8):897-906. PubMed CrossRef
13.Clemens KJ, Vendruscolo LF. Anxious to drink: gabapentin normalizes GABAergic transmission in the central amygdala and reduces symptoms of ethanol dependence. J Neurosci. 2008;28(37):9087-9089. PubMed CrossRef
14.Berlin RK, Butler PM, Perloff MD. Gabapentin therapy in psychiatric disorders: a systematic review. Prim Care Companion CNS Disord. 2015;17(5):15r01821. PubMed
15.Swift RM, Aston ER. Pharmacotherapy for alcohol use disorder: current and emerging therapies. Harv Rev Psychiatry. 2015;23(2):122-133. PubMed CrossRef
16.Moher D, Liberati A, Tetzlaff J, et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. PubMed CrossRef
17.Myrick H, Anton R, Voronin K, et al. A double-blind evaluation of gabapentin on alcohol effects and drinking in a clinical laboratory paradigm. Alcohol Clin Exp Res. 2007;31(2):221-227. PubMed CrossRef
18.Bonnet U, Hamzavi-Abedi R, Specka M, et al. An open trial of gabapentin in acute alcohol withdrawal using an oral loading protocol. Alcohol Alcohol. 2010;45(2):143-145. PubMed CrossRef
19.Bonnet U, Banger M, Leweke FM, et al. Treatment of acute alcohol withdrawal with gabapentin: results from a controlled two-center trial. J Clin Psychopharmacol. 2003;23(5):514-519. PubMed CrossRef
20.Furieri FA, Nakamura-Palacios EM. Gabapentin reduces alcohol consumption and craving: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry. 2007;68(11):1691-1700. PubMed CrossRef
21.Higgins JP, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.0.0 [updated September 2008]. London, UK: The Cochrane Collaboration. 2008.
22.Rohatgi A. WebPlotDigitizer [computer program]. Automeris website. https://automeris.io/WebPlotDigitizer/. 2011. Accessed July 22, 2019.
23.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56(2):455-463. PubMed CrossRef
24.Voris J, Smith NL, Rao SM, et al. Gabapentin for the treatment of ethanol withdrawal. Subst Abus. 2003;24(2):129-132. PubMed CrossRef
25.Mason BJ, Light JM, Williams LD, et al. Proof-of-concept human laboratory study for protracted abstinence in alcohol dependence: effects of gabapentin. Addict Biol. 2009;14(1):73-83. PubMed CrossRef
26.Stock CJ, Carpenter L, Ying J, et al. Gabapentin versus chlordiazepoxide for outpatient alcohol detoxification treatment. Ann Pharmacother. 2013;47(7-8):961-969. PubMed CrossRef
27.Mariani JJ, Rosenthal RN, Tross S, et al. A randomized, open-label, controlled trial of gabapentin and phenobarbital in the treatment of alcohol withdrawal. Am J Addict. 2006;15(1):76-84. PubMed CrossRef
28.Leggio L, Kenna GA, Swift RM. New developments for the pharmacological treatment of alcohol withdrawal syndrome: a focus on non-benzodiazepine GABAergic medications. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(5):1106-1117. PubMed CrossRef
29.Wojnar M, Bizoń Z, Wasilewski D. Assessment of the role of kindling in the pathogenesis of alcohol withdrawal seizures and delirium tremens. Alcohol Clin Exp Res. 1999;23(2):204-208. PubMed CrossRef
30.Gonzalez LP, Veatch LM, Ticku MK, et al. Alcohol withdrawal kindling: mechanisms and implications for treatment. Alcohol Clin Exp Res. 2001;25(5 suppl ISBRA):197S-201S. PubMed CrossRef
31.Modesto-Lowe V, Huard J, Conrad C. Alcohol withdrawal kindling: is there a role for anticonvulsants? Psychiatry (Edgmont). 2005;2(5):25-31. PubMed
32.Poulos CX, Zack M. Low-dose diazepam primes motivation for alcohol and alcohol-related semantic networks in problem drinkers. Behav Pharmacol. 2004;15(7):503-512. PubMed CrossRef
33.Roberto M, Gilpin NW, O’ Dell LE, et al. Cellular and behavioral interactions of gabapentin with alcohol dependence. J Neurosci. 2008;28(22):5762-5771. PubMed CrossRef
34.Malcolm R, Myrick LH, Veatch LM, et al. Self-reported sleep, sleepiness, and repeated alcohol withdrawals: a randomized, double blind, controlled comparison of lorazepam vs gabapentin. J Clin Sleep Med. 2007;3(1):24-32. PubMed
35.Karam-Hage M, Brower KJ. Gabapentin treatment for insomnia associated with alcohol dependence. Am J Psychiatry. 2000;157(1):151. PubMed CrossRef
36.Evoy KE, Morrison MD, Saklad SR. Abuse and misuse of pregabalin and gabapentin. Drugs. 2017;77(4):403-426. PubMed CrossRef
37.Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in Appalachian Kentucky. Am J Psychiatry. 2015;172(5):487-488. PubMed CrossRef
Please sign in or purchase this PDF for $40.00.
Save
Cite