ABSTRACT
Objective: Both antipsychotic and antidepressant medications have been associated with weight gain and hyperglycemia. Our previously published retrospective cohort study suggests that GLP-1 (glucagon-like peptide-1) analogs may be superior to alternative regimens for both glycemic and weight control in patients on antipsychotic plus/minus antidepressant medications. In the current study, we asked whether GLP-1 analogs or SGLT-2 (sodium-glucose-transporter-2) inhibitors would be similarly beneficial in patients on antidepressant medications alone.
Methods: In this retrospective cohort study, we included all patients with type 2 diabetes on antidepressant medications referred to our endocrine clinics between January 1, 2016, and January 1, 2017. Overall, 61 patients were started on a GLP-1 analog, 9 patients were started on an SGLT-2 inhibitor, and 134 were on alternative regimens (controls).
Results: The groups did not differ in age, sex, ethnicity, and glycosylated hemoglobin (HbA1c) levels, although body mass index levels were higher in patients started on a GLP-1 analog (P < .0001). After 12 months, patients on GLP-1 analogs lost 4 kg, patients on SGLT-2 inhibitors lost 2.4 kg, and controls gained 0.8 kg (P < .001 for controls versus GLP-1 analog group). Subanalyses revealed that GLP-1 analog–related weight loss was most notable in women and patients on selective serotonin reuptake inhibitors. On the other hand, all serotonin-norepinephrine reuptake inhibitor users lost weight over time, independent of the antidiabetic regimen applied. In contrast to the above noted differences in weight control, HbA1c reductions were comparable and somewhat diminished in all patients on antidepressant medications (−0.3% to 0.6%).
Conclusions: In this retrospective cohort study, we confirm superiority of GLP-1 analogs in mediating weight loss in patients on psychotropic and, more specifically, antidepressant medications. We also note overall blunted glycemic improvements in patients on antidepressant medications, a finding that was independent of the treatment strategy used. It could be a result of mental distress or suboptimal self-care and clearly requires further attention by future, prospective studies.
Prim Care Companion CNS Disord 2021;23(5):20m02868
To cite: Gonzalez CL, Azim S, Miedlich SU. GLP-1 analogs are superior in mediating weight loss but not glycemic control in diabetic patients on antidepressant medications: a retrospective cohort study. Prim Care Companion CNS Disord. 2021;23(5):20m02868.
To share: https://doi.org/10.4088/PCC.20m02868
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Medicine, Yale New Haven Hospital, New Haven, Connecticut
bUnity Diabetes and Endocrine Center, Rochester Regional Health, Rochester, New York
cDivision of Endocrinology, Diabetes and Metabolism, Department of Medicine, University of Rochester School of Medicine and Dentistry, Rochester, New York
*Corresponding author: Susanne U. Miedlich, MD, Division of Endocrinology, Diabetes and Metabolism, Department of Medicine, University of Rochester School of Medicine and Dentistry, Box 693, 601 Elmwood Ave, Rochester, NY 14642 ([email protected]).
Between 1999 and 2016, the adult US population has seen an increase in diabetes prevalence from 9.5% to 13.0%.1 Specifically, 34.1 million people aged 18 years or older have a known diagnosis of diabetes; an additional 7.3 million people are estimated to be undiagnosed.1 The increase is, at least in part, linked to the obesity epidemic; 85.2% of Americans with a diagnosis of type 2 diabetes (T2D) are overweight or obese.2 Notably, the rates of both obesity and T2D are especially high in patients with serious and/or persistent mental illness including major depressive disorder and/or schizophrenia compared to the general population.3,4
Psychotropic medications are at least in part to blame. Multiple studies, both in rodents as well as humans, have demonstrated that weight gain and hyperglycemia ensue from second-generation antipsychotic medication (APM) use. Olanzapine and clozapine in particular cause hyperphagia, weight gain, increased adiposity, hyperglycemia, and insulin resistance.5–9 Antidepressant medications (ADMs) can also lead to weight gain and potentially T2D, though the evidence is not as consistent across different ADMs as compared to second-generation APMs. Specifically, amitriptyline, paroxetine, and mirtazapine have been associated with weight gain.10 As a class, ADMs are associated with a 20% increased risk of T2D (corrected for depression and body mass index [BMI]11). The metabolic side effects of ADMs require attention, considering that up to 13% of Americans are using ADMs, with one-quarter of them having done so for ≥ 10 years.13 Mental illness, obesity, metabolic syndrome, and diabetes are all associated with an increased risk of cardiovascular disease and mortality. For example, cardiovascular events account for 68% of all deaths in diabetic patients.2
Currently available diabetes medications differ in their impact on weight and cardiovascular outcomes. For example, both sulfonylureas and insulin promote weight gain,13,14 and even though they are touted for reducing the risk of myocardial infarctions, it may take 20 years to experience those benefits.15 Newer diabetes medications on the other hand, for example GLP-1 (glucagon-like peptide-1) analogs or SGLT-2 (sodium-glucose-transporter-2) inhibitors, promote not only diabetes and weight control but also reduce adverse cardiovascular and renal outcomes within the span of only a few years.16–19
Studies addressing the efficacy of the above medications in vulnerable populations such as people with mental illness and diabetes are basically nonexistent. There are a few studies suggesting some degree of antidepressant action of liraglutide specifically, but those studies are small and do not address antihyperglycemic or weight loss effects.20 To our knowledge, there are no studies detailing glycemic and weight control related to GLP-1 analogs or SGLT-2 inhibitors in patients on ADM alone. However, GLP-1 analogs have been shown to mediate weight loss and improve glycemia overall in small prospective studies of mostly prediabetic patients on APM.21 In an earlier retrospective cohort study,22 we showed that obese, diabetic patients on APM may particularly benefit from GLP-1 analogs in terms of weight control. Furthermore, we noted that patients on both APM and ADM had blunted glycosylated hemoglobin (HbA1c) reductions compared to patients on APM alone, but not if they were treated with GLP-1 analogs. In other words, GLP-1 analogs may be superior for both weight and glycemic control in patients on psychotropic medications and perhaps specifically in patients on ADMs. In the current study, we therefore asked if patients on ADM alone would similarly benefit from GLP-1 analogs, and perhaps also from SGLT-2 inhibitors, in terms of weight and diabetes control. Thus, we performed a retrospective data analysis of diabetic patients on ADM only seen in outpatient endocrine clinics of the University of Rochester Medical Center (URMC).
METHODS
Design and Participants
A retrospective chart review was conducted of electronic medical records between January 1, 2016, and January 1, 2017. Patients included were at least 18 years old, had a diagnosis of T2D, were on an ADM, and had been seen in any of the outpatient endocrine clinics at URMC during the study time frame. The study protocol was reviewed and approved by the URMC Research Subjects Review Board (study #RSRB00003306).
Exposures
Potential patients were identified through the URMC Clinical Analytics (eRecord Reporting) team using the search parameters age: >18 years, ICD-10 codes: E11, and medication: any ADM. Patients fulfilling the above criteria who were continuously treated with an ADM for at least 1 year and had at least 1 follow-up visit at 1 of the URMC endocrine clinics were included in the final data set.
Data were obtained through review of electronic medical records and included age, sex, ethnicity/race, height, weight, BMI, smoking status, HbA1c, systolic and diastolic blood pressure, and medications. Specific medications recorded included antidepressant, antidiabetic (see below), and antihypertensive medications. Subjects were then divided into 3 groups based on antidiabetic medication use: control patients (no use of GLP-1 analogs or SGLT-2 inhibitors), patients on GLP-1 analogs, and patients on SGLT-2 inhibitors. Data for weight and HbA1c were recorded at 0, 3, 6, and 12 months following initial medication changes after referral to our endocrine clinics (controls) or after start of either GLP-1 analog or SGLT-2 inhibitor. Data for systolic and diastolic blood pressure were recorded at time points 0 and 12 months. When appointments did not fall exactly at 3, 6, or 12 months, data were applied to the closest time point.
Outcomes and Measures
Differences in HbA1c and weight were the main recorded outcomes in all 3 groups. Weight and HbA1c changes were calculated for each patient by subtracting the weight (kg) or HbA1c (%) at time 0 from each of the following time points: 3 months, 6 months, and 12 months after the initial visit (controls) or start of either GLP-1 analog or SGLT-2 inhibitor. Systolic and diastolic blood pressure values at 0 and 12 months for each group were also compared by similarly calculating changes of systolic and diastolic blood pressure values: systolic/diastolic blood pressure at baseline was subtracted from systolic/diastolic blood pressure after 12 months.
Statistical Analysis
SPSS version 24 was used for descriptive analyses and statistical comparisons between groups. Kolmogorov-Smirnov tests were used to determine if the continuous data were normally distributed (P > .1). Based on Kolmogorov-Smirnov testing, it was determined that the majority of the data were not normally distributed. Thus, nonparametric tests (Kruskal-Wallis, Mann-Whitney U, Wilcoxon signed rank tests) were used to compare continuous variables. Accordingly, medians and interquartile ranges (IQRs) are reported for descriptive analyses. For comparisons of categorical variables, χ2 tests were applied. A P value < .05 was considered statistically significant.
RESULTS
The retrospective analysis included 204 patients based on continuous use of an ADM over the course of 1 year after referral to a URMC endocrine clinic with at least 1 follow-up visit. Sixty-one patients were started on a GLP-1 analog (liraglutide for all cases), and 9 cases had an SGLT-2 inhibitor added to their regimen: 6 were started on canagliflozin, 2 on empagliflozin, and 1 on dapagliflozin. Of the 134 patients on alternative diabetes medications (control group), 104 were prescribed insulin (77.6%) and 80 (59.5%) had insulin added or doses adjusted over the course of 1 year. Thirty-five patients (26%) had metformin added or dose increased, and 21 (16%) had a DPP-4 (dipeptidylpeptidase) inhibitor added to their regimen. Of note, all patients started on a GLP-1 analog and 5 patients started on an SGLT-2 inhibitor were also on insulin.
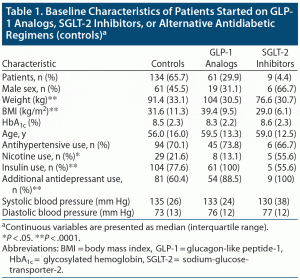
Patients in the GLP-1 analog group had a significantly higher weight and BMI at baseline (P < .0001) compared to the control group and the SGLT-2 inhibitor group (Table 1). There were also differences between the number of active smokers in each group: there were more active smokers among SGLT-2 inhibitor users compared to the control and GLP-1 analog groups (see Table 1). Additionally, there were significant differences in the use of insulin and additional ADMs between the 3 groups (see Table 1). There were no differences in sex, ethnicity/race distribution, age, systolic blood pressure, diastolic blood pressure, or HbA1c between the different treatment groups of patients at the beginning of the observation period (see Table 1).
In regard to the ADM used, the majority of all patients were on selective serotonin reuptake inhibitors (SSRIs, 49.5%). In the control group, 64 patients (47.8%) were on SSRIs, while 21 patients were on serotonin-norepinephrine reuptake inhibitors (SNRIs, 34.4%). In the GLP-1 analog group, 34 patients (55.7%) were on SSRIs, while 15 (25%) were on SNRIs. Four patients in the SGLT-2 inhibitor group were on SSRIs, and 4 patients were on SNRIs. Other ADMs, used much less frequently in all patients, included tricyclics (7.9%), bupropion (9.3%), trazodone (7.5%), mirtazapine (3.7%), and doxepin (0.9%).
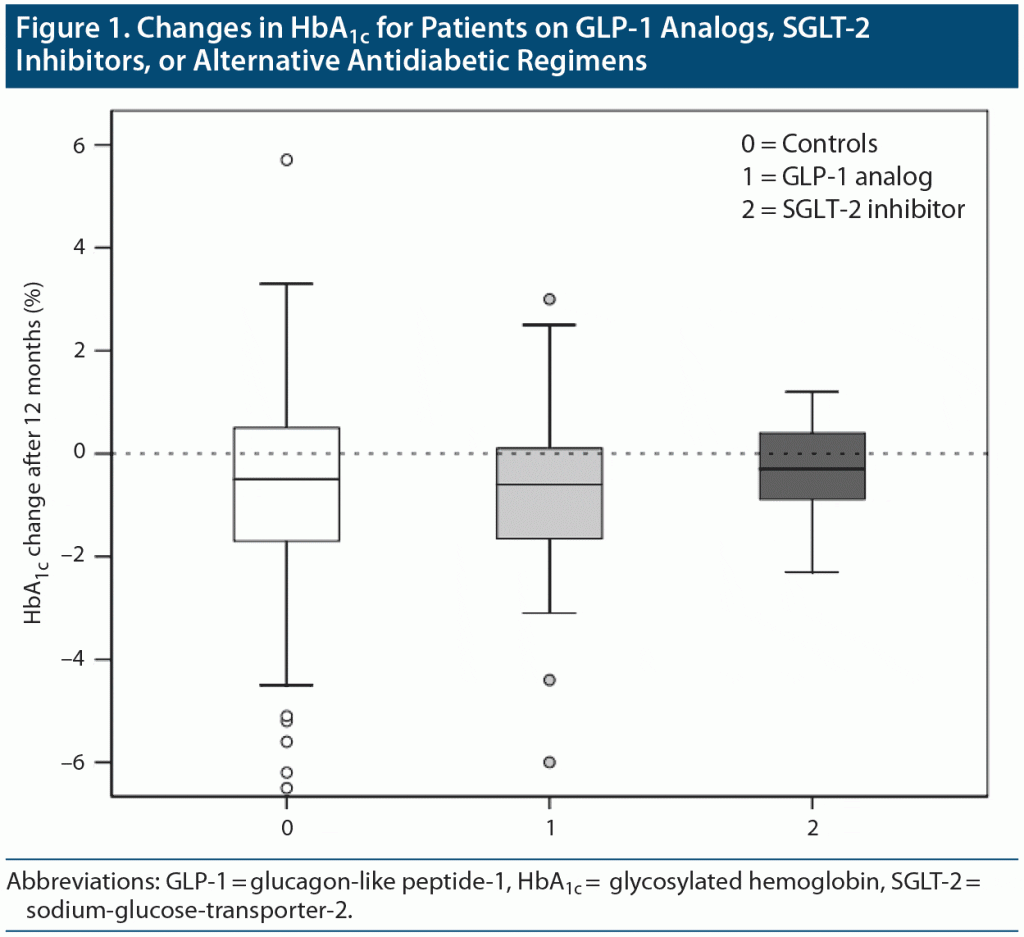
Over the course of 12 months, median (IQR) HbA1c levels improved similarly in all 3 treatment groups (Figure 1). In the control group, HbA1c levels at 3, 6, and 12 months decreased by 0.45% (1.9), 0.6% (2.3), and 0.5% (2.2), respectively. In the GLP-1 analog group, HbA1c reductions were 0.95% (1.98), 0.55% (1.65), and 0.6% (1.8) after 3, 6, and 12 months, respectively. The SGLT-2 inhibitor group had an HbA1c increase of 0.1% (1.4) after 3 months; reductions of 0.29% (2) and 0.3% (1.6) were observed after 6 and 12 months, respectively.
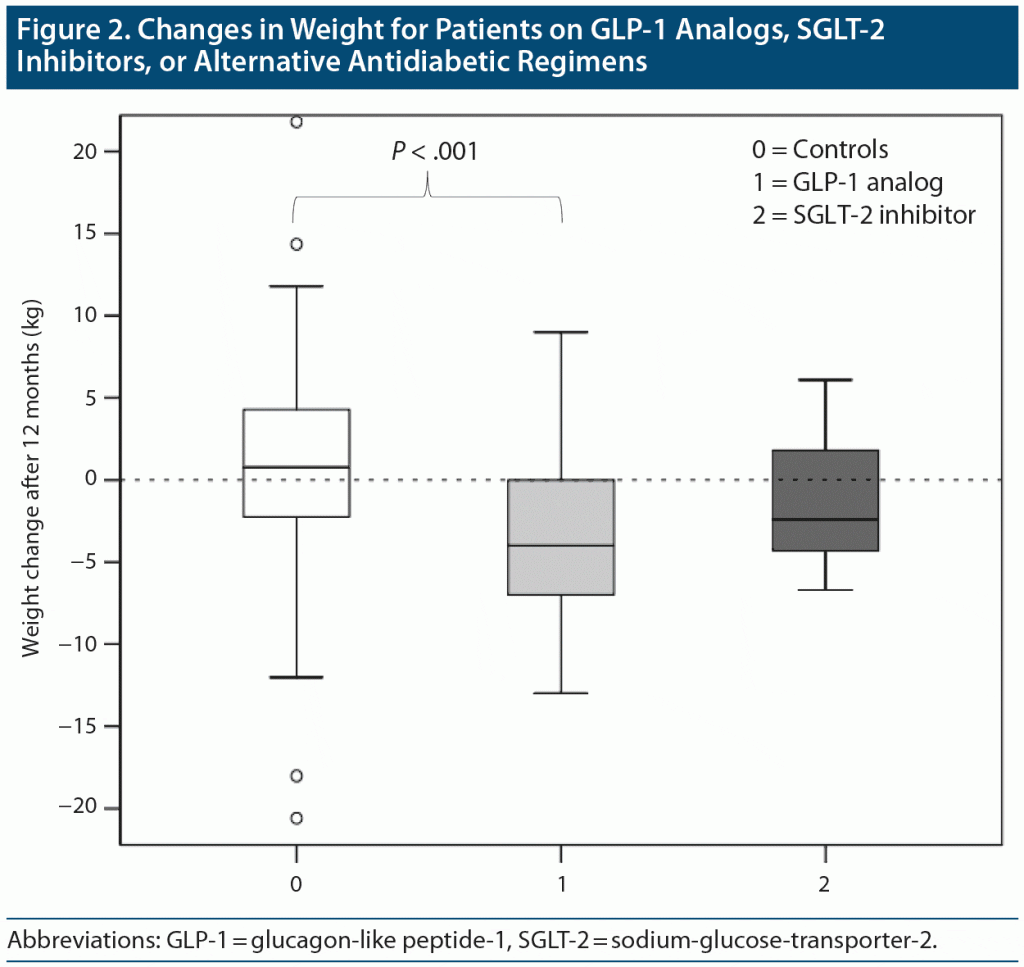
There were significant differences between the 3 treatment groups when comparing the median (IQR) weight changes over time: patients on a GLP-1 analog lost significantly more weight at all time points (P < .001) compared to patients in the control group (shown for 12-month time points in Figure 2). After 3, 6, and 12 months, the patients on GLP-1 analogs lost 2 kg (5), 2 kg (5), and 4 kg (7), respectively. In contrast, the weight of the control group patients was basically unchanged after 3 and 6 months, but then increased at 12 months: −0.08 kg (3.34), −0.01 kg (4.59), and +0.78 kg (6.55), respectively. Patients on SGLT-2 inhibitors also lost more weight than the control group, though the difference was not statistically significant for this comparison, which is likely owed to the small number of patients in this group (n = 9; P = .4, .06, and .17 at 3, 6, and 12 months, respectively; median [IQR]: −0.69 kg [4.96], −2.16 kg [4.93], and −2.4 kg [7.1] after 3, 6, and 12 months, respectively).
Comparing male and female patients separately revealed no significant sex-specific differences in glycemic or weight changes in all or between the 3 treatment groups. Notably though, women on the GLP-1 analog lost more weight (median [IQR]) than men at all time points (women: −2 kg [6], −2 kg [7], and −4.5 kg [7.5] and men: −1.5 kg [3.75], −1 kg [5.5], and −3 kg [8.5] at 3, 6, and 12 months, respectively), and the weight change was significantly different from control patients at all time points in women, while only reaching significance at 12 months for men (P < .01 at all time points in women, P = .01 at 12 months only in men).
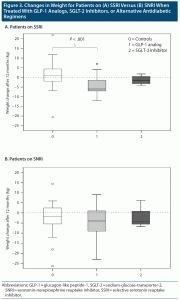
We then turned our attention to glycemic and weight changes for patients on SSRIs versus SNRIs. Glycemic improvements were again similar for all treatment groups, independent of whether the patients were on SSRIs or SNRIs (data not shown). Differences were noted for weight changes over time (P = .005, .02, and .11 at 3, 6 and 12 months, respectively). Specifically, all patients on SNRIs lost weight over time, particularly during the first 6 months of the observation period. Of note, the baseline weights of SNRI users were not different from SSRI users and thus are unlikely to account for the difference. The above changes (median [IQR]) were particularly evident in the control group: patients on SSRIs gained weight ( + 0.2 kg [3.29], + 0.03 kg [4.3], and + 0.9 kg [6.6] at 3, 6, and 12 months, respectively), while patients on SNRIs lost weight (−0.6 kg [3.92], −2.58 kg [6.85], and −1.87 kg [9.04] at 3, 6 and 12 months, respectively, P = .013–.069), reaching significance at 3 and 6 months. With that said, SSRI users lost weight when a GLP-1 analog was added, whereas patients on SSRIs in the control group gained weight, as stated previously. On the other hand, for SNRI users, the weight trends were not different between the 3 treatment groups, meaning patients in all groups lost weight over time (Figure 3).
We also explored whether treatment outcomes differed between users of only 1 as compared to more than 1 ADM. As a whole, patients on more than 1 ADM had better weight and HbA1c control during the 12-month observation compared to patients on only 1 ADM (P < .05). This trend was most apparent in the control group.
There were not enough smokers in each treatment group to compare HbA1c and weight changes between smokers and nonsmokers. Lastly, no statistically significant changes in systolic and diastolic blood pressure changes were seen between the 3 treatment groups over time. There were also no differences in blood pressure changes over time in male versus female patients or SSRI versus SNRI users.
DISCUSSION
Antidepressant medications have become 1 of the top 3 most commonly prescribed therapeutic drug classes in the United States over the last 40 years.12 SSRIs and SNRIs are not just prescribed for depression but also for anxiety, posttraumatic stress, obsessive-compulsive, eating, and somatoform disorders as well as chronic neuropathic pain (SNRIs for the latter indication). Unfortunately, many ADMs are associated with a number of adverse side effects, ranging from sexual dysfunction and nausea to constipation and drowsiness, but also weight changes and possibly hyperglycemia.11,23,24 SSRIs in particular are often associated with weight gain.23 In contrast, SNRIs are more likely to result in weight loss.25 While high affinity antagonism of central histamine receptors may contribute to weight gain seen with SSRIs,26,27 noradrenergic stimulation (SNRIs) could be mediating weight loss, but may also lead to hyperglycemia.28 In a retrospective cohort study,1 we previously showed that GLP-1 analogs can mediate impressive weight reductions (7 kg) in diabetic patients on APMs. Furthermore, in patients on both APMs and ADMs, GLP-1 analogs were superior to alternative regimens in terms of both weight and glycemic control. Thus, we had asked ourselves whether patients on ADM alone would similarly benefit from GLP-1 analogs and perhaps also from SGLT-2 inhibitors. Note that both agents have been ascribed to reducing not only weight and HbA1c levels, but also adverse cardiovascular events.16–19,29 Specifically, in several randomized controlled trials, therapy with the GLP-1 analog liraglutide (prescribed in all patients in this study) resulted in 1.3–3 kg weight loss and HbA1c reductions from 1% to 1.4%.17,30 Similarly, the SGLT-2 inhibitor canagliflozin (most often used in this analysis) led to weight loss between 1.7 kg and 3 kg and HbA1c reductions from 0.6% to 1%.18,30,31 Here, we confirm that both GLP-1 analogs and SGLT-2 inhibitors mediate weight loss, while alternative regimens result in weight gain in patients on ADM. However, the difference was only significant for patients on the GLP-1 analog and not for patients on SGLT-2 inhibitors. The latter finding is likely owed to the small number (n = 9) or the lower baseline weight of patients started on an SGLT-2 inhibitor. Overall, the weight loss observed here in patients on a GLP-1 analog was larger than in the aforementioned studies17,30 (4 kg versus 1.3–3 kg). Furthermore, women and patients on SSRIs had larger weight reductions following 1-year therapy with liraglutide, 4.5 kg (women) and 6 kg (patients on SSRI), which may point to sex-specific effects of GLP-1 analogs in the setting of ADM use. Of note, sex-specific effects were not noted in previous studies with liraglutide.17 The above result may also point to a specific advantage of GLP-1 analog therapy in patients on SSRIs, at least for weight control. In contrast, choosing a GLP-1 analog in an overweight or obese patient on an SNRI may not be superior to alternative antidiabetic regimens, as all patients on SNRIs lost weight over time.
Interestingly, the glycemic improvements observed in this study with either treatment strategy were notably blunted. We described similarly blunted HbA1c reductions (0.8% after 12 months) in patients on APM and ADM in our previous work.22 However, in the previous study,22 mean HbA1c reductions were significantly larger when GLP-1 analogs were used in patients on APM and ADM: 1.4% (GLP-1 analogs) versus 0.5% (controls) after 12 months. Somewhat surprisingly, a superior glycemic effect of GLP-1 analogs over alternative regimens was not noted in this analysis: HbA1c reductions were similar in all treatment groups, ranging from 0.3%–0.6% after 12 months. However, we should note that higher baseline HbA1c (> 9.5%) levels in our previous study could have amplified differences between treatment groups. It is also possible that adherence to diet, lifestyle, or medication administration may have been suboptimal in our patients on ADM alone. That said, if medication nonadherence was a major factor for the blunted glycemic outcomes, the question remains why GLP-1 analog therapy did mediate significant weight loss, especially in women and SSRI users.
One could also speculate that increased stress hormone activity (ie, catecholamines, cortisol) in the setting of anxiety or depression could have contributed to persistent hyperglycemia. As an example, the net effect of either catecholamines or cortisol is hyperglycemia.32,33 Both cortisol and catecholamine levels have been shown to be elevated under conditions of mental distress.34 Thus, one might argue that a blunted glycemic response could be a reflection of continued mental distress. Unfortunately, medication, diet and lifestyle adherence, and depression scores are not consistently documented in our clinic settings. Thus, none of the above hypotheses could be tested in this retrospective study, which is a major limitation of this study. Independent of the cause, we believe that failure to control hyperglycemia, despite best efforts in medication management, diabetes control, and diet and lifestyle education, should alert physicians to inquire about the patient’s mental health and trigger notification of the existing or referral to a mental health team as indicated.
In summary, in this retrospective cohort study we extend the findings of our previous work, confirming that in a real-world setting of an outpatient academic endocrine practice, GLP-1 analogs may be particularly well suited in mediating weight loss in obese, diabetic patients on psychotropic medications and specifically in patients on second-generation APMs and/or ADMs, particularly patients on SSRIs. With that in mind, are there risks to GLP-1 analog therapy? During the main prospective, placebo-controlled trial of liraglutide,18 gastrointestinal side effects, gallbladder disease, and injection site reactions were reported, though these rarely resulted in permanent discontinuation of the drug (less than 4%). Unfortunately, based on the retrospective nature of our study and lack of consistent data relating to medication adherence or adverse effects, we are not able to report whether the above reported adverse effects played a role in shaping any of the outcomes reported here.
A different picture emerges from the glycemic trends in patients on psychotropic medications from our cohort studies: all patients on ADM in this and our previous study22 had somewhat blunted HbA1c reductions over a 12-month period. However, while our previous study suggested that patients on both APM and ADM may specifically benefit from GLP-1 analogs in terms of both weight and glycemic control, the latter finding cannot be confirmed in this study of patients on ADM alone. In fact, all patients, independent of the treatment strategy, had less than expected HbA1c reductions. Based on the nature of both of our studies (retrospective chart reviews), the reasons for the differences in glycemic versus weight control in patients on psychotropic medications remain largely unknown at this time and clearly need further investigation by controlled, prospective trials.
Submitted: November 15, 2020; accepted February 3, 2021.
Published online: September 9, 2021.
Potential conflicts of interest: None.
Funding/support: None.
Previous presentation: These data were presented in part as a poster at the Annual Meeting of the Endocrine Society; March 23–26, 2019; New Orleans, LA.
Acknowledgements: The authors thank Steven Lamberti, MD, for his critical advice and review of the manuscript. Dr Lamberti has no conflicts of interest related to the subject of this article.
Clinical Points
- Weight gain and increased risk of hyperglycemia have been associated with the use of antidepressant medications.
- Compared to alternative antidiabetic regimens, GLP-1 analogs are particularly helpful in mediating weight loss in obese, diabetic patients on antidepressants, particularly in patients on selective serotonin reuptake inhibitors.
- Improvements in glycemic control are blunted overall in our diabetic patients on antidepressants; this should alert clinicians to address the patient’s mental health.
References (34)

- National Diabetes Statistics Report 2020 - Estimates of Diabetes and Its Burden in the United States. CDC website. https://www.cdc.gov/diabetes/data/statistics-report/index.html. 2020.
- Bhupathiraju SN, Hu FB. Epidemiology of obesity and diabetes and their cardiovascular complications. Circ Res. 2016;118(11):1723–1735. PubMed CrossRef
- Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry. 1999;156(11):1686–1696. PubMed
- Goff DC, Sullivan LM, McEvoy JP, et al. A comparison of ten-year cardiac risk estimates in schizophrenia patients from the CATIE study and matched controls. Schizophr Res. 2005;80(1):45–53. PubMed CrossRef
- Hegedűs C, Kovács D, Kiss R, et al. Effect of long-term olanzapine treatment on meal-induced insulin sensitization and on gastrointestinal peptides in female Sprague-Dawley rats. J Psychopharmacol. 2015;29(12):1271–1279. PubMed CrossRef
- Smith GC, Chaussade C, Vickers M, et al. Atypical antipsychotic drugs induce derangements in glucose homeostasis by acutely increasing glucagon secretion and hepatic glucose output in the rat. Diabetologia. 2008;51(12):2309–2317. PubMed CrossRef
- Smith GC, Vickers MH, Cognard E, et al. Clozapine and quetiapine acutely reduce glucagon-like peptide-1 production and increase glucagon release in obese rats: implications for glucose metabolism and food choice behaviour. Schizophr Res. 2009;115(1):30–40. PubMed CrossRef
- van der Zwaal EM, Janhunen SK, la Fleur SE, et al. Modelling olanzapine-induced weight gain in rats. Int J Neuropsychopharmacol. 2014;17(1):169–186. PubMed CrossRef
- Vidarsdottir S, de Leeuw van Weenen JE, Frölich M, et al. Effects of olanzapine and haloperidol on the metabolic status of healthy men. J Clin Endocrinol Metab. 2010;95(1):118–125. PubMed CrossRef
- Serretti A, Mandelli L. Antidepressants and body weight: a comprehensive review and meta-analysis. J Clin Psychiatry. 2010;71(10):1259–1272. PubMed CrossRef
- Salvi V, Grua I, Cerveri G, et al. The risk of new-onset diabetes in antidepressant users: a systematic review and meta-analysis. PLoS One. 2017;12(7):e0182088. PubMed CrossRef
- Pratt LA, Brody DJ, Gu Q. Antidepressant use among persons aged 12 and over: United States, 2011–2014. NCHS Data Brief. 2017;(283):1–8. PubMed
- Gerstein HC, Bosch J, Dagenais GR, et al; ORIGIN Trial Investigators. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367(4):319–328. PubMed CrossRef
- Kahn SE, Haffner SM, Heise MA, et al; ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006;355(23):2427–2443. PubMed CrossRef
- Holman RR, Paul SK, Bethel MA, et al. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(15):1577–1589. PubMed CrossRef
- Marso SP, Bain SC, Consoli A, et al; SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. PubMed CrossRef
- Marso SP, Daniels GH, Brown-Frandsen K, et al; LEADER Steering Committee; LEADER Trial Investigators. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322. PubMed CrossRef
- Neal B, Perkovic V, Matthews DR. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(21):2099. PubMed
- Wiviott SD, Raz I, Bonaca MP, et al; DECLARE–TIMI 58 Investigators. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357. PubMed CrossRef
- Woo YS, Lim HK, Wang SM, et al. Clinical evidence of antidepressant effects of insulin and anti-hyperglycemic agents and implications for the pathophysiology of depression:a literature review. Int J Mol Sci. 2020;21(18):6969. PubMed CrossRef
- Siskind D, Hahn M, Correll CU, et al. Glucagon-like peptide-1 receptor agonists for antipsychotic-associated cardio-metabolic risk factors: a systematic review and individual participant data meta-analysis. Diabetes Obes Metab. 2019;21(2):293–302. PubMed CrossRef
- Perlis LT, Lamberti JS, Miedlich SU. Glucagon-like peptide analogs are superior for diabetes and weight control in patients on antipsychotic medications: a retrospective cohort study. Prim Care Companion CNS Disord. 2020;22(1):19m02504. PubMed CrossRef
- Cascade E, Kalali AH, Kennedy SH. Real-world data on SSRI antidepressant side effects. Psychiatry (Edgmont). 2009;6(2):16–18. PubMed
- Garfield LD, Dixon D, Nowotny P, et al. Common selective serotonin reuptake inhibitor side effects in older adults associated with genetic polymorphisms in the serotonin transporter and receptors: data from a randomized controlled trial. Am J Geriatr Psychiatry. 2014;22(10):971–979. PubMed CrossRef
- Hainer V, Kabrnova K, Aldhoon B, et al. Serotonin and norepinephrine reuptake inhibition and eating behavior. Ann N Y Acad Sci. 2006;1083(1):252–269. PubMed CrossRef
- Salvi V, Barone-Adesi F, D’Ambrosio V, et al. High H1-affinity antidepressants and risk of metabolic syndrome in bipolar disorder. Psychopharmacology (Berl). 2016;233(1):49–56. PubMed CrossRef
- Salvi V, Mencacci C, Barone-Adesi F. H1-histamine receptor affinity predicts weight gain with antidepressants. Eur Neuropsychopharmacol. 2016;26(10):1673–1677. PubMed CrossRef
- Hennings JM, Schaaf L, Fulda S. Glucose metabolism and antidepressant medication. Curr Pharm Des. 2012;18(36):5900–5919. PubMed CrossRef
- Zinman B, Wanner C, Lachin JM, et al; EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128. PubMed CrossRef
- Lorenzi M, Ploug UJ, Langer J, et al. Liraglutide versus SGLT-2 inhibitors in people with type 2 diabetes: a network meta-analysis. Diabetes Ther. 2017;8(1):85–99. PubMed CrossRef
- Ali AM, Martinez R, Al-Jobori H, et al. Combination therapy with canagliflozin plus liraglutide exerts additive effect on weight loss, but not on HbA1c, in patients with type 2 diabetes. Diabetes Care. 2020;43(6):1234–1241. PubMed CrossRef
- Mesmar B, Poola-Kella S, Malek R. The physiology behind diabetes mellitus in patients with pheochromocytoma: a review of the literature. Endocr Pract. 2017;23(8):999–1005. PubMed CrossRef
- Kuo T, McQueen A, Chen TC, et al. Regulation of glucose homeostasis by glucocorticoids. Adv Exp Med Biol. 2015;872:99–126. PubMed CrossRef
- Joseph JJ, Golden SH. Cortisol dysregulation: the bidirectional link between stress, depression, and type 2 diabetes mellitus. Ann N Y Acad Sci. 2017;1391(1):20–34. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite