ABSTRACT
Objective: To examine the impact of antipsychotic dose adjustments (mainly reduction) on the efficacy and tolerability of antipsychotic medications (APMs) to facilitate hospital discharge in long-term hospitalized forensic patients with treatment-refractory psychosis.
Methods: This was a retrospective review of the medical charts of 22 patients with psychosis who were discharged from January 2020 to August 2020 from a long-term state psychiatric facility after restoration of their competency to stand trial. Due to the lack of specific guidelines, the high-dose therapy was defined as a dose ≥ 50% above the average package insert dose. The primary outcome was discharge time after the antipsychotic dosing adjustments.
Results: Sixty-eight percent of subjects, who were hospitalized for a mean ± SD total of 11.6 ± 5.3 months, were discharged after 2.3 ± 0.78 months of 44.4% antipsychotic dose reduction. Two patients, who were hospitalized for 14.5 ± 6.7 months, were discharged after 4 months of optimizing their subtherapeutic doses. Five patients, who were already receiving effective dosages, were discharged after a total hospital duration of 6.8 ± 2.17 months.
Conclusions: The results from this study extend the finding of beneficial effects of antipsychotic dose reduction from prior reports to the forensic population.
Prim Care Companion CNS Disord 2022;24(6):21m03214
To cite: Shad MU. High dose therapy in treatment-refractory psychosis: a retrospective study. Prim Care Companion CNS Disord. 2022;24(6):21m03214.
To share: https://doi.org/10.4088/PCC.21m03214
© 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, University of Nevada Las Vegas, Las Vegas, Nevada
bDepartment of Psychiatry, Touro University Nevada, Las Vegas, Nevada
cPsychiatry Residency Training Program, Valley Health System, Las Vegas, Nevada
*Corresponding author: Mujeeb U. Shad, MD, MSCS, DFAPA, Napa State Hospital, 2100 Napa Vallejo Hwy, Napa, CA 94558 ([email protected]).
High-dose antipsychotic medications (APMs) and polypharmacy (mega polypharmacy) frequently occur, particularly in the treatment-refractory schizophrenia population. Despite the lack of managed care pressures to quickly discharge patients from long-term state psychiatric hospitals, antipsychotic doses rapidly escalate beyond the conventional dosing range without waiting for the delayed antipsychotic response. This rapid dose escalation deprives patients of the chance to respond to the lowest effective dose, which is generally the most tolerable. Often, higher antipsychotic doses are justifiably used to manage behavioral aggression observed during acute psychosis rather than to prevent relapse (ie, maintenance doses) or treat early psychosis.1 However, the concern is not the high antipsychotic doses used during acute psychosis, but the repeated dose escalations after each behavioral disruption, often with no poststabilization dose adjustments. Although some suggest that the continuation of high-dose therapy effectively prevents relapse,2 there is no formal evidence to support this point of view, except data from a few case reports and case series, which need to be interpreted cautiously due to publication bias. In contrast, the evidence against high-dose therapy started decades ago3 and has only grown with time.4–11
Lack of evidence-based guidelines for effective maintenance doses as opposed to dosing recommendations for acute psychosis12,13 may be one reason for the continuation of high-dose therapy, especially in the treatment-refractory population. An initial nonresponse and urgency to treat also encourage high-dose treatment, creating a potential bias in perception of outcome. Some patients may also exaggerate or falsely report improvement to please their providers. Opposing guidelines have added further complexity with regard to defining effective maintenance doses. For example, the American Psychiatric Association guidelines recommend using the lowest effective doses for maintenance treatment.14
In contrast, the Expert Consensus Guidelines suggest continued use of antipsychotic doses effective during acute psychosis.2 It is worth noting that even the US Food and Drug Administration (FDA)–approved dosages, determined in a near-perfect patient population in preclinical trials, may not be helpful in real-world patients who often have comorbidities. The postmarketing deviations from FDA-approved dosages can often be explained based on genetically mediated interindividual variability in plasma levels or drug interactions.15 In addition, excessive sedation with high-dose therapy may also be misperceived as an improvement in psychosis. However, the high-dose therapy may also result in some not so benign adverse effects, ranging from extrapyramidal symptoms (EPS)16 to potentially fatal cardiac arrhythmias17 and neuroleptic malignant syndrome.18
In contrast, lower doses have been associated with significantly lower risk for some of these adverse effects.6,19–21 In addition, high-dose therapy with APMs with anticholinergic properties are associated with dryness of the mouth (resulting in partial or complete loss of taste), retention of urine, blurring of vision, constipation, loss of sweating, tachycardia, and a further worsening of preexisting cognitive dysfunction. Many APMs can also cause postural hypotension and dose-dependent weight gain, increasing the risk for metabolic syndrome in a patient population with a significant preexisting risk for medical comorbidities, most notably diabetes and hypertension.22
Mechanistically, continued high-dose therapy exposes patients to higher dopamine-2 (D2) receptor blockade than that required to control psychosis (ie, 60%–80%).23–25 Any D2 receptor blockade ≥ 80% is associated with adverse effects, especially EPS and hyperprolactinemia. High antipsychotic doses also promote medication nonadherence due to adverse effects, which makes it difficult to maintain stability after hospital discharge and to reintegrate these patients into the community, resulting in so-called “revolving door syndrome,” wherein patients are admitted repeatedly with significant health costs. A retrospective review26 showed that high-dose antipsychotic treatment to manage psychosis at hospital discharge might increase the risk for readmission in patients with borderline personality disorder. Another study27 reported an increased readmission rate within 6 months of hospital discharge for patients on antipsychotic polypharmacy.
These are reasons to prioritize an adequate trial with conventional antipsychotic doses, which can be facilitated by monitoring EPS or plasma drug and prolactin levels. Although prior studies have documented the positive effects of dose reduction in schizophrenia patients, there is little evidence to support lower doses in the forensic population residing in long-term psychiatric facilities. This retrospective study demonstrates how informed dosing adjustments can enhance antipsychotic effectiveness to facilitate competency restoration and hospital discharge of forensic subjects from a long-term state psychiatric facility.
METHODS
A review was conducted of medical charts of 22 inpatients at or after their discharge (January 2020 to August 2020) from a long-term state psychiatric hospital back to the court system to stand trial. This was a retrospective review of the medical charts of discharged patients, which makes it difficult to obtain informed consents from the study subjects. In addition, these patients were in the hospital for restoration of competency, reducing the validity of informed consents. Since the findings from this study are of high clinical relevance in an understudied population, it was important to communicate the study results to the clinicians working in a forensic inpatient setting. Every effort was made to keep the personal health information of these patients confidential.
Study Subjects
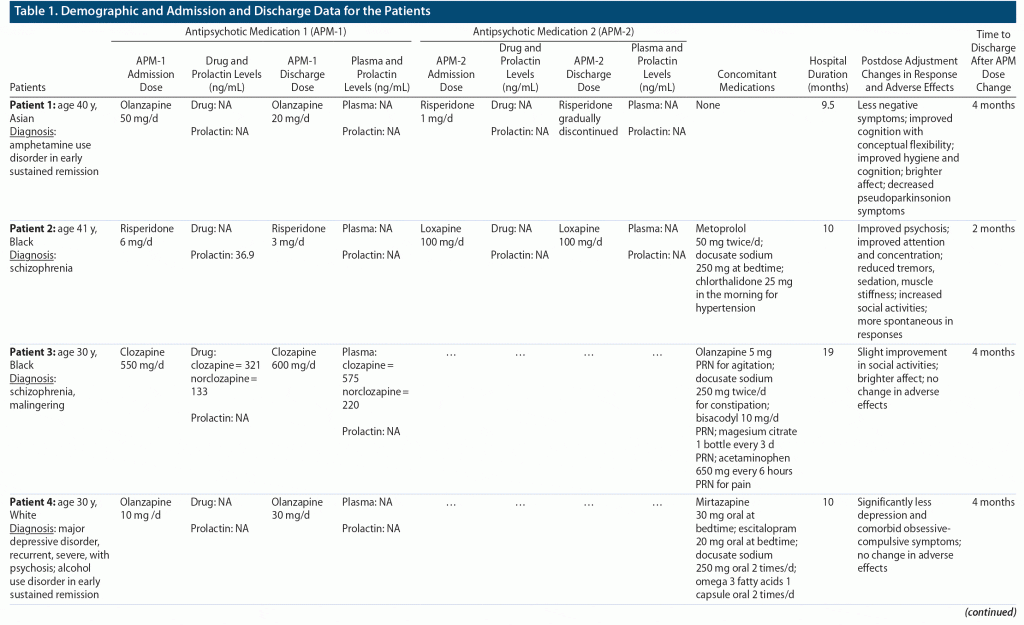
All patients selected for this study were males who responded to their antipsychotic treatment, facilitating competency restoration to stand trial as determined by the state hospital’s forensic staff. The study subjects were managed in a single inpatient unit and were discharged back to the state court system to stand trial by a staff psychiatrist during 7 months of inpatient management. The psychiatric diagnoses of these patients were established by a live interview with the treatment team and review of the medical records by certified psychiatrists after the hospital admission using DSM-5 criteria. However, regardless of their diagnosis, all patients were treated with 1 or more APMs for their psychosis as the primary presenting symptom. The antipsychotic-induced adverse effects were physically monitored for extrapyramidal symptoms, and drug and prolactin levels were monitored where indicated and consented by the patients. The patients’ demographic data, diagnoses, APM(s), change in doses, hospital duration, and time to discharge after dose adjustments are presented in Table 1.
Study Data and Procedures
All study data were derived from the discharged patients’ electronic medical records by the author to examine the quality of clinical documentation. The missing data are reported in Table 1. The primary outcome assessed was the time to discharge after dose adjustments to the antipsychotic medications. Since there are no clear guidelines to differentiate between conventional and high antipsychotic doses and to account for high variability in dose equivalency to occupy 60% to 80% D2 receptor blockade, the high-dose therapy was defined as a dose ≥ 50% above average package insert dose. Any dose roughly ≥ 50% of the maximum package insert dose was labeled as a high-dose therapy. A subtherapeutic dose was defined as less than the lowest effective dose in the package insert, except for risperidone due to postmarketing effectiveness of lower doses. High antipsychotic dosages were not adjusted if the plasma levels were within the laboratory reference range. The doses of 4 patients who were on long-acting injectables (LAIs) were also adjusted based on their oral dose equivalency and prolactin and antipsychotic plasma levels. Clinically, effective dose was described as the dose that produced an antipsychotic response (≥ 20% decrease in total Positive and Negative Syndrome Scale28 scores) without significant adverse effects, such as EPS and hyperprolactinemia.
RESULTS
As shown in Table 1, all 22 patients were adult males with a mean ± SD age of 34.04 ± 5.49 years. Eight patients were White, 6 were Hispanic, 5 were Black, and 3 were Asian. Unspecified schizophrenia was the primary diagnosis in 8 patients, schizophrenia in 7, and schizoaffective disorder, bipolar type in 2. One patient had a primary diagnosis of major depressive disorder with psychosis and another of bipolar affective disorder with psychosis. Although only 2 patients had a primary diagnosis of amphetamine and opioid use disorder, substance use disorder was the most common comorbid diagnosis in these cases, including amphetamine use disorder in 4 patients, alcohol use in 7, cannabis in 3, cocaine in 2, and opioid use in 2. Malingering was the primary diagnosis in one and a comorbid diagnosis in another of the reviewed cases. One of the cases also had a comorbid diagnosis of antisocial personality disorder.
A total of 22 patients, hospitalized for a mean ± SD of 10.77 ± 5.08 months, were discharged from a state psychiatric facility managed by a single staff psychiatrist over 7 months. Seventeen (77%) of 22 patients, hospitalized for 11.94 ± 5.3 months, were discharged after 2.5 ± 0.93 months of dose adjustments or reduced polypharmacy. Fifteen (68%) of these patients, hospitalized for a total of 11.6 ± 5.3 months, were discharged after 2.3 ± 0.78 months of their dose reduction or reduced polypharmacy. Two patients, hospitalized for 14.5 ± 6.7 months, were discharged after 4 months of optimizing their subtherapeutic dosages (Table 1). No dose adjustments were made for the rest of the patients (23%), as they were already taking effective doses and were discharged after being hospitalized for 6.8 ± 2.17 months. The 15 patients in the dose reduction group had an average of 44.4% reduction in their first APM. Nine of these patients had antipsychotic polypharmacy, of which 4 had their second APM discontinued, and one had dose reductions in both the first and second APM (Table 1). Only 4 of 22 patients were on LAIs. Olanzapine was the most frequently prescribed orally administered high-dose APM in this group (ie, 12 of 18 patients). Still, 1 of these patients had a subtherapeutic dose, and 2 did not need any dosing changes, thus leaving 9 patients with a mean dose of 51.7 mg/d reduced to 27.8 mg/d. In contrast, risperidone was reduced by ≥ 50% (10 mg/d to 4.5 mg/d) in 2 patients.
DISCUSSION
This is the first retrospective study of forensic patients hospitalized in a long-term psychiatric facility, to my knowledge, to report the positive effects of antipsychotic dose reduction on treatment effectiveness, competency restoration, and hospital discharge with no symptom relapse. The utility of dose adjustments is supported by a longer average hospital stay in patients before dose reduction (9.3 months) compared to after dose reduction (2.3 months). However, the shortest hospitalization was reported in 2 patients who were already on effective doses (ie, < 7 months), and the longest hospital duration was observed in patients who required optimization of their subtherapeutic doses (ie, 14.5 months). These results suggest that response to increasing a subtherapeutic dose takes longer than a dose reduction. This could be attributable to a decrease in adverse effects that precedes loss of, or decrease in, efficacy following a dose reduction. However, relapse after discharge cannot be completely ruled out, despite continued stability for a 10-week hospitalization after a slow and gradual dose reduction being associated with a relatively low risk for relapse.10,11
Of note, a total hospitalization of > 11 months in this study is longer than the 6 months required to restore competency in 75% of a similar patient population in earlier studies.29,30 Interestingly the average dose reduction of 44.4% in this study is close to a 42% dose reduction in a randomized, rater-blinded trial with no relapse.10 Similarly, 50% dose reduction in 2 randomized controlled trials was not associated with any worsening in psychosis but instead resulted in an improvement in negative and cognitive symptoms.31,32 These findings are further supported by a significant functional improvement in > 50% of the study subjects after about 60% reduction in the antipsychotic dose in another study.11 Along the same lines, a comprehensive Cochrane review4 found no clear advantages of increasing the dose over continuing the previous dose.
Although high-dose therapy is justifiable to manage acute psychosis and associated behaviors, the high doses are often continued as maintenance doses beyond patients’ stabilization. The rationale provided is the biological differences between treatment-refractory patients and the general patient population, justifying the continuation of high-dose therapy to avoid aggression and violence. However, one can also make a counterargument against high-dose therapy, as it carries an increased risk for adverse effects in biologically vulnerable patients with a high prevalence of medical, substance use, and psychiatric comorbidities. More importantly, there are no formal data to support high-dose therapy except for a few case reports and case series, which may overrepresent the evidence due to publication bias. There is only 1 study33 with an LAI that used high enough doses to be relevant to this discussion. Although there were no significant differences in efficacy between the top 3 LAI doses, the adverse effects were numerically higher with the highest dose of 200 mg/month than with the lower dosages. In contrast, data from most studies18,33–38 with l LAI reported effective maintenance dosages that are more like conventional than high dosages.
Executive cognition is one of the critical elements required for competency restoration. Since dopamine is one of the most important neurotransmitters for executive cognitive function,39 the continuation of high-dose therapy with excessive dopamine blockade can further compromise preexisting executive dysfunction, delaying competency restoration. This view is supported by improved performance on several cognitive measures without relapse after reduction in maintenance doses or even complete discontinuation of APMs.32,40 In another study,41 patients found to have a short-term increase in relapse rates after the dose reduction were reported to have a higher rate of symptomatic and functional remission than those who were continued on their previous doses over a 7-year follow-up.42 In addition, improved performance was reported on measures of psychosocial adjustment in patients receiving the low dose compared to those receiving standard doses.43 Moreover, even discontinuation of APMs was associated with more extended recovery periods, with no relapse in up to one-third of the schizophrenia patients who stopped taking their APM.44 For these reasons, the goal of antipsychotic dose adjustments in this study was to achieve an effective dose, defined as providing the best compromise between efficacy and tolerability, and allowing enough dopamine function for the forensic rehabilitation to succeed.
However, extreme caution is required to discontinue an APM or to lower its dose below the standard doses due to increased risk for relapse43 until biomarkers are developed to identify patients who will do well on reduced doses or even after discontinued medication(s).32,40,44 Until then, high-dose therapy should only be employed after ruling out the confounding effects of environmental stress, pharmacogenetic variance, seasonal changes in symptoms, or medication nonadherence on patients’ psychopathology. Also, a physical examination for EPS and laboratory investigations for prolactin and drug levels can help determine effective dosages. Uninformed antipsychotic polypharmacy can be wholly ineffective or intolerable and should be avoided or replaced by logical antipsychotic combinations. In this study, 4 patients in the dose reduction group were successfully stabilized by reducing their first APM dose and discontinuing their second APM. However, the key to a successful dose reduction/discontinuation is to avoid abrupt stoppage and follow a gradual dose reduction while monitoring psychotic relapse and discontinuation symptoms, such as rebound psychosis and akathisia.8
Still, there will be a minority of patients that will require and respond to high-dose therapy with no plausible genetic or clinical explanations for it. However, high-dose treatment should only be employed after failing an adequate trial with the conventional doses of APMs. Finally, LAIs are more effective in preventing relapse than oral APMs due to improved medication adherence, and LAIs should be a priority, especially in patients with a known history of medication nonadherence.
Limitations
The findings of this study should be interpreted with caution, as they are based on retrospective chart review without a priori hypotheses. Another limitation is the naturalistic setting of the study data, which limits providing control for various confounding factors that may affect the study results. In addition, lack of clear definition of effective maintenance doses makes it difficult to define various dosing levels and accurately interpret findings.
CONCLUSION
This study is the first to extend findings from earlier studies to a forensic population showing the effectiveness of antipsychotic dose reduction in facilitating competency restoration, hospital discharge, and community reintegration in a long-term hospitalized forensic patient population. The reduced dosages also offered a significantly better adverse effect profile, which is of high clinical significance in a patient population with multiple preexisting cognitive deficits and a high prevalence of medical, substance use, and psychiatric comorbidities.
Submitted: December 9, 2021; accepted April 11, 2022.
Published online: November 24, 2022.
Relevant financial relationships: None.
Funding/support: None.
Acknowledgments: It is essential to recognize the significant role of nonpsychopharmacologic interventions in patients’ stabilization and hospital discharge. The nurses’ and supporting staff’s dedication to working with one of the most challenging patient populations and a significant contribution from the psychologists, social workers, and rehabilitation and other therapists all contributed to the positive outcomes in these patients. In addition, efficient management of frequent medical comorbidities by the internal medicine team also contributed to the overall psychiatric stability of these patients.
ORCID: Mujeeb U. Shad: https://orcid.org/0000-0002-5136-9452
Clinical Points
- There is growing evidence against high-dose therapy in patients with psychosis.
- Beneficial effects of antipsychotic dose reduction may be extended to the forensic population.
- Clinicians should cautiously consider antipsychotic dose reduction to facilitate hospital discharge in the long-term hospitalized forensic population.
References (45)

- Haddad PM, Correll CU. The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses. Ther Adv Psychopharmacol. 2018;8(11):303–318. PubMed CrossRef
- Kane JM, Leucht S, Carpenter D, et al; Expert Consensus Panel for Optimizing Pharmacologic Treatment of Psychotic Disorders. The expert consensus guideline series. optimizing pharmacologic treatment of psychotic disorders. introduction: methods, commentary, and summary. J Clin Psychiatry. 2003;64(suppl 12):5–19. PubMed
- Baldessarini RJ. A summary of current knowledge of tardive dyskinesia. Encephale. 1988;14(Spec No):263–268. PubMed
- Samara MT, Klupp E, Helfer B, et al. Increasing antipsychotic dose for nonresponse in schizophrenia. Cochrane Database Syst Rev. 2018;5(5):CD011883. PubMed CrossRef
- Samara MT, Klupp E, Helfer B, et al. Increasing antipsychotic dose versus switching antipsychotic for non response in schizophrenia. Cochrane Database Syst Rev. 2018;5(5):CD011884. PubMed CrossRef
- Uchida H, Suzuki T, Takeuchi H, et al. Low dose vs standard dose of antipsychotics for relapse prevention in schizophrenia: meta-analysis. Schizophr Bull. 2011;37(4):788–799. PubMed CrossRef
- Patel MX, Matonhodze J, Baig MK, et al. Naturalistic outcomes of community treatment orders: antipsychotic long-acting injections versus oral medication. J Psychopharmacol. 2013;27(7):629–637. PubMed CrossRef
- Leucht S, Tardy M, Komossa K, et al. Antipsychotic drugs versus placebo for relapse prevention in schizophrenia: a systematic review and meta-analysis. Lancet. 2012;379(9831):2063–2071. PubMed CrossRef
- Leucht S, Samara M, Heres S, et al. Dose equivalents for second-generation antipsychotics: the minimum effective dose method. Schizophr Bull. 2014;40(2):314–326. PubMed CrossRef
- Huhn M, Leucht C, Rothe P, et al. Reducing antipsychotic drugs in stable patients with chronic schizophrenia or schizoaffective disorder: a randomized controlled pilot trial. Eur Arch Psychiatry Clin Neurosci. 2020;271(2):293–302. PubMed
- Suzuki T, Uchida H, Tanaka KF, et al. Reducing the dose of antipsychotic medications for those who had been treated with high-dose antipsychotic polypharmacy: an open study of dose reduction for chronic schizophrenia. Int Clin Psychopharmacol. 2003;18(6):323–329. PubMedvv
- Goff DC, Posever T, Herz L, et al. An exploratory haloperidol-controlled dose-finding study of ziprasidone in hospitalized patients with schizophrenia or schizoaffective disorder. J Clin Psychopharmacol. 1998;18(4):296–304. PubMed CrossRef
- Beasley CM, Dellva MA, Tamura RN, et al. Randomised double-blind comparison of the incidence of tardive dyskinesia in patients with schizophrenia during long-term treatment with olanzapine or haloperidol. Br J Psychiatry. 1999;174(1):23–30. PubMed CrossRef
- Keepers GA, Fochtmann LJ, Anzia JM, et al; (Systematic Review). The American Psychiatric Association Practice Guideline for the treatment of patients with schizophrenia. Focus Am Psychiatr Publ. 2020;18(4):493–497. PubMed
- Hiemke C, Baumann P, Bergemann N, et al. AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update 2011. Pharmacopsychiatry. 2011;44(6):195–235. CrossRef
- Lemmens P, Brecher M, Van Baelen B. A combined analysis of double-blind studies with risperidone vs placebo and other antipsychotic agents: factors associated with extrapyramidal symptoms. Acta Psychiatr Scand. 1999;99(3):160–170. PubMed CrossRef
- Ray WA, Chung CP, Murray KT, et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med. 2009;360(3):225–235. PubMed CrossRef
- Jeste DV, Caligiuri MP, Paulsen JS, et al. Risk of tardive dyskinesia in older patients: a prospective longitudinal study of 266 outpatients. Arch Gen Psychiatry. 1995;52(9):756–765. PubMed CrossRef
- Kane JM, Rifkin A, Woerner M, et al. Low-dose neuroleptic treatment of outpatient schizophrenics, I: preliminary results for relapse rates. Arch Gen Psychiatry. 1983;40(8):893–896. PubMed CrossRef
- Barbui C, Esposito E, Cipriani A. Selective serotonin reuptake inhibitors and risk of suicide: a systematic review of observational studies. CMAJ. 2009;180(3):291–297. PubMed CrossRef
- Barbui C, Saraceno B. Low-dose neuroleptic therapy and extrapyramidal side effects in schizophrenia: an effect size analysis. Eur Psychiatry. 1996;11(8):412–415. PubMed CrossRef
- De Hert M, Mittoux A, He Y, et al. Metabolic parameters in the short- and long-term treatment of schizophrenia with sertindole or risperidone. Eur Arch Psychiatry Clin Neurosci. 2011;261(4):231–239. PubMed CrossRef
- Seeman P, Kapur S. Schizophrenia: more dopamine, more D2 receptors. Proc Natl Acad Sci U S A. 2000;97(14):7673–7675. PubMed CrossRef
- Nyberg S, Nordström AL, Halldin C, et al. Positron emission tomography studies on D2 dopamine receptor occupancy and plasma antipsychotic drug levels in man. Int Clin Psychopharmacol. 1995;10(suppl 3):81–85. PubMed
- Uchida H, Takeuchi H, Graff-Guerrero A, et al. Dopamine D2 receptor occupancy and clinical effects: a systematic review and pooled analysis. J Clin Psychopharmacol. 2011;31(4):497–502. PubMed CrossRef
- Yamada Y, Yokoi Y, Narita Z, et al. High-dose antipsychotic drug use as a predictor for readmission of inpatients with borderline personality disorder: a retrospective chart review in a Japanese psychiatric hospital. Neuropsychopharmacol Rep. 2020;40(4):365–370. PubMed CrossRef
- Kadra G, Stewart R, Shetty H, et al. Antipsychotic polypharmacy prescribing and risk of hospital readmission. Psychopharmacology (Berl). 2018;235(1):281–289. PubMed CrossRef
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. PubMed CrossRef
- Morris DR, Parker GF. Jackson’s Indiana: state hospital competence restoration in Indiana. J Am Acad Psychiatry Law. 2008;36(4):522–534. PubMed
- Nicholson RA, McNulty JL. Outcome of hospitalization for defendants found incompetent to stand trial. Behav Sci Law. 1992;10(3):371–383. PubMed CrossRef
- Takeuchi H, Suzuki T, Remington G, et al. Effects of risperidone and olanzapine dose reduction on cognitive function in stable patients with schizophrenia: an open-label, randomized, controlled, pilot study. Schizophr Bull. 2013;39(5):993–998. PubMed CrossRef
- Zhou Y, Li G, Li D, et al. Dose reduction of risperidone and olanzapine can improve cognitive function and negative symptoms in stable schizophrenic patients: a single-blinded, 52-week, randomized controlled study. J Psychopharmacol. 2018;32(5):524–532. PubMed CrossRef
- Kane JM, Davis JM, Schooler N, et al. A multidose study of haloperidol decanoate in the maintenance treatment of schizophrenia. Am J Psychiatry. 2002;159(4):554–560. PubMed CrossRef
- Kane JM, Rifkin A, Woerner M, et al. High-dose versus low-dose strategies in the treatment of schizophrenia. Psychopharmacol Bull. 1985;21(3):533–537. PubMed
- Marder SR, Van Putten T, Mintz J, et al. Low- and conventional-dose maintenance therapy with fluphenazine decanoate. two-year outcome. Arch Gen Psychiatry. 1987;44(6):518–521. PubMed CrossRef
- Marder SR, Van Putten T, Mintz J, et al. Costs and benefits of two doses of fluphenazine. Arch Gen Psychiatry. 1984;41(11):1025–1029. PubMed CrossRef
- Hogarty GE, McEvoy JP, Munetz M, et al. Dose of fluphenazine, familial expressed emotion, and outcome in schizophrenia: results of a two-year controlled study. Arch Gen Psychiatry. 1988;45(9):797–805. PubMed CrossRef
- Schooler NR, Keith SJ, Severe JB, et al. Relapse and rehospitalization during maintenance treatment of schizophrenia: the effects of dose reduction and family treatment. Arch Gen Psychiatry. 1997;54(5):453–463. PubMed CrossRef
- Weintraub D, Chahine LM, Hawkins KA, et al; PARS Investigators. Cognition and the course of prodromal Parkinson’s disease. Mov Disord. 2017;32(11):1640–1645. PubMed CrossRef
- Faber G, Smid HG, Van Gool AR, et al. The effects of guided discontinuation of antipsychotics on neurocognition in first onset psychosis. Eur Psychiatry. 2012;27(4):275–280. PubMed CrossRef
- Wunderink L, Nienhuis FJ, Sytema S, et al. Guided discontinuation versus maintenance treatment in remitted first-episode psychosis: relapse rates and functional outcome. J Clin Psychiatry. 2007;68(5):654–661. PubMed CrossRef
- Wunderink L, Nieboer RM, Wiersma D, et al. Recovery in remitted first-episode psychosis at 7 years of follow-up of an early dose reduction/discontinuation or maintenance treatment strategy: long-term follow-up of a 2-year randomized clinical trial. JAMA Psychiatry. 2013;70(9):913–920. PubMed CrossRef
- Kreisman D, Blumenthal R, Borenstein M, et al. Family attitudes and patient social adjustment in a longitudinal study of outpatient schizophrenics receiving low-dose neuroleptics: the family’s view. Psychiatry. 1988;51(1):3–13. PubMed CrossRef
- Harrow M, Jobe TH, Faull RN. Do all schizophrenia patients need antipsychotic treatment continuously throughout their lifetime? a 20-year longitudinal study. Psychol Med. 2012;42(10):2145–2155. PubMed CrossRef
- Tani H, Takasu S, Uchida H, et al. Factors associated with successful antipsychotic dose reduction in schizophrenia: a systematic review of prospective clinical trials and meta-analysis of randomized controlled trials. Neuropsychopharmacology. 2020;45(5):887–901. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite