ABSTRACT
Objective: To evaluate whether a history of suicide attempt increases the odds of receiving clozapine treatment in veterans with schizophrenia or schizoaffective disorder.
Methods: Electronic health record data were obtained for veterans with schizophrenia or schizoaffective disorder treated at any US Veterans Affairs Medical Center between January 1, 2000, and January 31, 2021 (N = 134,692). Logistic regression (adjusted and unadjusted) was applied to estimate odds ratios (ORs) for clozapine treatment in suicide attempters relative to nonattempters.
Results: 3,407 patients had a documented history of suicide attempt, while 6,867 patients had received clozapine treatment. Also, 9.4% (n = 321) of suicide attempters versus 5.0% (n = 6546) of nonattempters had received clozapine treatment. The odds of being treated with clozapine was approximately 2-fold in patients with a history of suicide attempt in unadjusted (OR = 1.98, 95% CI, 1.76–2.22) and adjusted (OR = 1.91, 95% CI, 1.67–2.15) analyses.
Conclusions: Despite the higher odds of clozapine treatment in suicide attempters with schizophrenia or schizoaffective disorder, clozapine was underutilized in the current sample of veterans. Concerted efforts should be made to expand the use of clozapine in patients with schizophrenia or schizoaffective disorder, especially those with a history of suicide attempt.
Prim Care Companion CNS Disord 2022;24(6):21m03231
To cite: Jones GH, Mitchell BG, Bernard J, et al. History of suicide attempt and clozapine treatment in veterans with schizophrenia or schizoaffective disorder. Prim Care Companion CNS Disord. 2022;24(6):21m03231.
To share: https://doi.org/10.4088/PCC.21m03231
© 2022 Physicians Postgraduate Press, Inc.
aMental Health Care Line, Michael E. DeBakey Veterans Affairs Medical Center, Houston, Texas
bDepartment of Psychiatry, University of Texas Health Science Center at Houston, Houston, Texas
cMenninger Department of Psychiatry and Behavioral Sciences, Baylor College of Medicine, Houston, Texas
dCenter for Innovations in Quality, Effectiveness and Safety (IQuEST), Michael E. DeBakey VA Medical Center, Houston, Texas
*Corresponding author: Olaoluwa O. Okusaga, MD, MScPHR, Michael E. DeBakey Veterans Affairs Medical Center, 2002 Holcombe Blvd, 580/116 MHCL rTMS, Houston, Texas 77030 ([email protected]).
Suicide attempts are highly prevalent among veterans with schizophrenia1 and are correlated with death by suicide. Approximately 5%–13% of patients with schizophrenia or schizoaffective disorder commit suicide2,3—a self-inflicted mortality rate 4 to 13 times that of the general population.4 Currently, clozapine is the only pharmacologic intervention approved for reducing suicidal behavior in patients with schizophrenia or schizoaffective disorder. However, despite its proven efficacy, only 5% of patients with schizophrenia in the United States are treated with clozapine, compared to 20%–35% in other developed countries.5 Moreover, estimates for clozapine utilization among veterans are below the national average, ranging from 0% to 3% across regional networks.6–8 On average, veterans trial 7 antipsychotics before initiating clozapine,7 resulting in a significant delay in starting the recommended evidence-based intervention for treatment-resistant cases. Intensive monitoring requirements, potential for severe adverse drug reactions, cardiometabolic side effects, and concern for patient adherence are frequently cited as barriers to guideline observance.9
To gain a better understanding of the extent of clozapine prescribing, specifically for veterans with schizophrenia or schizoaffective disorder who have a history of a suicide attempt, we sought to determine whether the odds of receiving clozapine is higher in patients with a history of a suicide attempt relative to those without a history of a suicide attempt. Based on the US Food and Drug Administration (FDA) indication of clozapine, we hypothesized that a history of a suicide attempt would be positively associated with clozapine treatment. Knowing the extent to which clozapine is being prescribed in a high-risk population such as suicide attempters with schizophrenia or schizoaffective disorder is the first step prior to designing initiatives to increase utilization of this proven intervention by prescribers.
METHODS
We analyzed cross-sectional data on veterans with a diagnosis of schizophrenia or schizoaffective disorder who received treatment at any US Veterans Affairs Medical Center (VAMC) from January 1, 2000, to January 31, 2021. We performed the analyses on de-identified electronic health records obtained from the Corporate Data Warehouse (CDW) and the Veterans Affairs Informatics and Computing Interface (VINCI). CDW integrates information from several locations (including electronic medical records) throughout the Veteran’s Health Administration (VHA) and makes the database available via VINCI. Notably, care received by veterans outside of VHA facilities is not automatically captured by CDW/VINCI. The Institutional Review Board of Baylor College of Medicine and the Michael E. DeBakey VAMC Research and Development Committee provided approval for the study and granted a waiver of consent and HIPAA authorization to use de-identified protected health information from individuals involved in the study.
We identified patients via ICD-9 and ICD-10 codes corresponding to diagnoses of schizophrenia and schizoaffective disorder and documentation of suicide attempts. We also extracted the following variables: age (at last visit), sex, race, marital status, body mass index (BMI), and Charlson-Deyo Comorbidity Index (CDCI)10,11 score. The CDCI assigns an aggregate score (0–6) to patients based on their quantity and severity of comorbid chronic medical conditions. The CDCI is a validated age-independent predictor of various outcomes including mortality, disability, hospital readmissions, and length of stay.
We separated the entire sample into 2 groups based on whether patients had a documented history of suicide attempt. Demographic and clinical variables were compared between the 2 groups using t test or χ2 test as appropriate. All variables are reported as mean ± SD and n (%). Logistic regression was applied to estimate the odds of having a history of clozapine treatment based on existence of a prior suicide attempt (or lack thereof), with additional analysis adjusting for age, sex, race, BMI, marital status, and CDCI score. P values < .05 were considered statistically significant. All statistical analyses were performed with IBM SPSS, version 27.
RESULTS
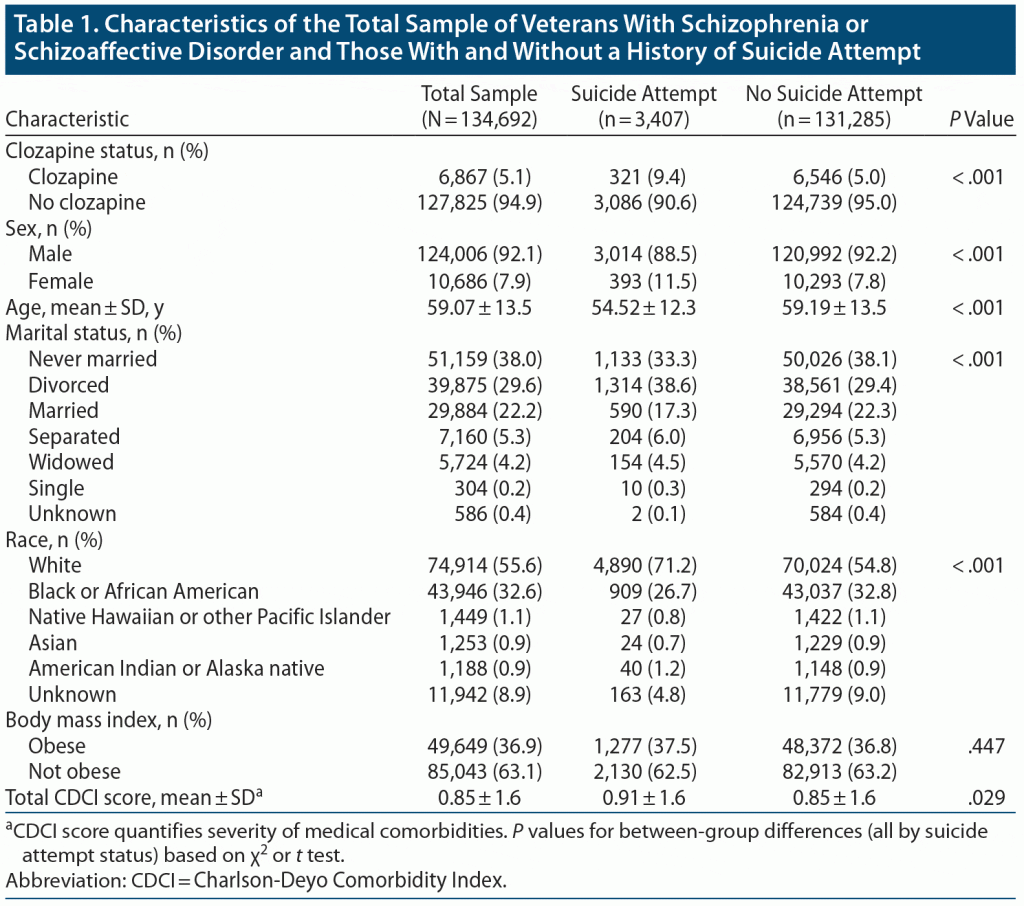
The sample (N = 134,692) was predominantly male (92%) and white (55%), with a mean age of 59 years. Approximately one-third (37%) were obese but with mild severity of medical comorbidities (CDCI = 0.85). Suicide attempters were almost 5 years younger on average, had a slight female and White predominance, and were more likely to have been divorced (Table 1).
The results showed that 9.4% (n = 321) of patients with a history of suicide attempt versus 5.0% (n = 6,546) of patients without a documented history of suicide attempt had received clozapine treatment. The odds of being treated with clozapine was approximately 2-fold higher in patients with a history of suicide attempt (OR = 1.98, 95% CI, 1.76–2.22), and this value remained relatively unchanged after controlling for age, sex, race, BMI, marital status, and comorbid medical burden (OR = 1.91, 95% CI, 1.67–2.15).
DISCUSSION
As hypothesized, among veterans with schizophrenia or schizoaffective disorder, prior suicide attempt (relative to no prior suicide attempt) was associated with almost twice the odds of being prescribed clozapine. Therefore, our results suggest adherence to treatment guidelines by prescribers in the VHA. In addition, the reported frequency of clozapine administration in this large cohort exceeds that of prior VA studies (0%–3%), but still falls short of expectations based on official recommendations and worldwide prescribing patterns.5,9
Several studies2,12–16 that employed different research designs have consistently demonstrated the efficacy of clozapine in reducing the risk of suicide in patients with schizophrenia or schizoaffective disorder. Indeed, in the United States, it has been estimated that one-third of suicides among patients with schizophrenia or schizoaffective disorder may be prevented if treated with clozapine.17 Although further validation is needed, evidence also suggests that recent suicidal ideation in veterans may confer a superior response to clozapine.18 In that regard, leveraging psychopharmacology (ie, clozapine) can work synergistically with other targeted approaches (eg, multidisciplinary interventions for select patients based on suicide risk stratification) to reduce suicidality. Moreover, clozapine’s antisuicidal effect remains consistent provided there is adherence to the medication and appears to be independent of its increased clinical monitoring requirements.2
Although risk of increasing side effects exists with expanding the use of clozapine, net benefits in suicide reduction and secondary enhancements (eg, potential for improved cognition, adherence, and quality of life) must be considered as well, particularly given that these are some of the most important predictors of functional outcomes in this population.19 Likewise, clozapine appears to have some advantages in patients with comorbid substance use disorder or affective components (ie, schizoaffective disorder and bipolar with psychotic features)—attributes that are likewise associated with increased risk of suicide.20,21
Contrary to the general population in which suicide risk increases into middle age, among veterans and patients with schizophrenia, young adults are at highest risk for completing suicide.22,23 Despite having greater tolerability for the medication, younger patients are much less likely to be started on clozapine compared to their middle-aged counterparts.24 Older patients in turn experience higher rates of postural hypotension, confusion, leukopenia, and agranulocytosis.24–26 In addition to these side effects, clozapine also carries black box warnings for myocarditis, cardiomyopathy, seizures (dose dependent), and increased mortality in elderly patients with dementia-related psychosis.27 While myocarditis risk is highest among patients aged 15–44 years, 90% of cases have been found to occur within the first 2 months following initiation.28 Likewise, rates of agranulocytosis decrease significantly after 18 weeks of treatment.29 Thus, increasing clozapine utilization to target suicidality in younger patients should also entail closer cardiac monitoring and gradual titrations to optimize tolerability.
Severe agranulocytosis has been observed to occur in 0.05%–0.86% of patients, which has necessitated frequent monitoring of neutrophil counts as part of the FDA’s Risk Evaluation and Mitigation Strategy for clozapine.30 Despite recent attempts to streamline surveillance, practitioners often cite this paradigm as a major barrier to initiation.9 However, evidence suggests that clinicians vastly overestimate the burden of routine monitoring, assuming that 52% of patients will be inconvenienced by testing compared to only 19% of patients themselves.31 Despite increased need for monitoring, it has been estimated that clozapine initiation would save approximately $22,444 per veteran per year, mostly in reduced hospitalizations.32 Furthermore, the risk of self-inflicted mortality in this population is over 10 times that of fatal agranulocytosis.33
While suicide is the leading cause of premature death in schizophrenia, cardiovascular disease represents the greatest proportion of overall mortality.4 Compared to other antipsychotics, clozapine is associated with the highest risk of cardiometabolic side effects.34 However conflicting results exist as to whether clozapine itself is associated with higher cardiovascular disease mortality compared to other antipsychotics.35,36 Some studies37,38 have suggested that the benefits from suicide prevention with clozapine may be mostly offset by increases in cardiometabolic mortality. However, a more recent systematic review39 does not support this hypothesis. Meta-analyses36,39–41 have consistently demonstrated lower disease-specific and all-cause mortality risk with long-term clozapine use compared to other antipsychotics or nontreatment. Moreover, viable yet underutilized strategies exist to mitigate metabolic side effects from these medications.42
Strengths and Limitations
This study collected data from a larger nationwide cohort of patients than has previously been assessed in this subject area. It also combines data from both inpatient and outpatient centers, more accurately reflecting real-world conditions. However, given the scope of sites included in this sample, it was not possible to stratify prescribing odds across individual centers. Future studies designed to uncover any possible regional barriers to prescription are highly warranted.
Further limitations of this study include the strong male predominance of our sample (which limits the generalizability of our findings) and the lack of information on duration of treatment, mean dosage, and adjunctive therapy. Concurrent use of lithium may be a significant confounder. It should be noted, however, that a recent randomized placebo-controlled trial using adjunctive lithium to prevent suicide-related events in veterans with mood disorders was halted for futility43—a finding that underscores the need to consider multimodal strategies for suicide prevention in the veteran population.
A final limitation inherent in electronic medical records is that suicide attempts could have been underreported, as some patients might not have reported their suicide attempt(s) either because they were not asked directly or due to feeling embarrassed. Finally, we do not have data on deaths from suicide.
CONCLUSION
Rather than interpreting cause and effect from our data, we hope to give a snapshot of the current situation. Increased likelihood of clozapine usage in suicidal patients in our study reflects the notion that many clinicians are attempting to combat this public health crisis through pharmacologic means, as we have also recommended. Though the higher rates of clozapine usage reported in our study are encouraging, they are still inadequate by evidence-based standards. Clozapine’s promise to ameliorate a substantial portion of the tragedy of suicide is belied by perceived risks and burdens from clinicians—often inappropriately so. Expanding the use of clozapine, particularly to younger patients, and initiating treatment earlier in the disease course represent viable strategic additions to multimodal suicide prevention. Special focus should be placed on enhancing strategies to reduce cardiometabolic burden, particularly given the ancillary benefits associated with prolonged use.
Submitted: December 27, 2021; accepted May 25, 2022.
Published online: November 1, 2022.
Relevant financial relationships: None.
Funding/support: None.
Clinical Points
- Clozapine, the only medication approved for suicidal behavior in patients with schizophrenia or schizoaffective disorder, is underutilized in the United States.
- Prior suicidal behavior nearly doubles the odds of receiving clozapine among veterans with schizophrenia or schizoaffective disorder, surpassing previous estimates in this population and nationwide averages.
- Perceived barriers and long-term risks with clozapine exist but may be overstated, necessitating a reappraisal by providers.
References (43)

- Harvey PD, Posner K, Rajeevan N, et al. Suicidal ideation and behavior in US veterans with schizophrenia or bipolar disorder. J Psychiatr Res. 2018;102:216–222. PubMed CrossRef
- Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82–91. PubMed CrossRef
- Hor K, Taylor M. Suicide and schizophrenia: a systematic review of rates and risk factors. J Psychopharmacol. 2010;24(suppl):81–90. PubMed CrossRef
- Olfson M, Gerhard T, Huang C, et al. Premature mortality among adults with schizophrenia in the United States. JAMA Psychiatry. 2015;72(12):1172–1181. PubMed CrossRef
- Fuller Torrey E, Knable MB, Quanbeck C, et al. Clozapine for treating schizophrenia: a comparison of the states. Treatment Advocacy Center website. Accessed August 23, 2021. https://www.treatmentadvocacycenter.org/storage/documents/clozapine-for-treating-schizophrenia.pdf
- Gören JL, Meterko M, Williams S, et al. Antipsychotic prescribing pathways, polypharmacy, and clozapine use in treatment of schizophrenia. Psychiatr Serv. 2013;64(6):527–533. PubMed CrossRef
- Zuschlag ZD, Fowler CA, Devendorf A, et al. Clozapine utilization at the United States Veterans Health Administration: a descriptive report of prescribing patterns and patient characteristics among Operation Enduring Freedom/Operation Iraqi Freedom Veterans. Int Clin Psychopharmacol. 2020;35(6):322–328. PubMed CrossRef
- Blow FC, McCarthy JF, Valenstein M, et al. Care for Veterans with Psychosis in the Veterans Health Administration, FY05, 7th Annual National Psychosis Registry Report. 2005. Accessed August 23, 2021. https://czresearch.com/dropbox/NPRReport04.pdf
- Kelly DL, Freudenreich O, Sayer MA, et al. Addressing barriers to clozapine underutilization: a national effort. Psychiatr Serv. 2018;69(2):224–227. PubMed CrossRef
- Kurichi JE, Stineman MG, Kwong PL, et al. Assessing and using comorbidity measures in elderly veterans with lower extremity amputations. Gerontology. 2007;53(5):255–259. PubMed CrossRef
- Papaleontiou M, Banerjee M, Reyes-Gastelum D, et al. Risk of osteoporosis and fractures in patients with thyroid cancer: a case-control study in US veterans. Oncologist. 2019;24(9):1166–1173. PubMed CrossRef
- Modestin J, Dal Pian D, Agarwalla P. Clozapine diminishes suicidal behavior: a retrospective evaluation of clinical records. J Clin Psychiatry. 2005;66(4):534–538. PubMed CrossRef
- Taipale H, Tanskanen A, Mehtälä J, et al. 20-year follow-up study of physical morbidity and mortality in relationship to antipsychotic treatment in a nationwide cohort of 62,250 patients with schizophrenia (FIN20). World Psychiatry. 2020;19(1):61–68. PubMed CrossRef
- Reid WH, Mason M, Hogan T. Suicide prevention effects associated with clozapine therapy in schizophrenia and schizoaffective disorder. Psychiatr Serv. 1998;49(8):1029–1033. PubMed CrossRef
- Ringbäck Weitoft G, Berglund M, Lindström EA, et al. Mortality, attempted suicide, rehospitalization and prescription refill for clozapine and other antipsychotics in Sweden: a register-based study. Pharmacoepidemiol Drug Saf. 2014;23(3):290–298. PubMed CrossRef
- Meltzer HY, Okayli G. Reduction of suicidality during clozapine treatment of neuroleptic-resistant schizophrenia: impact on risk-benefit assessment. Am J Psychiatry. 1995;152(2):183–190. PubMed CrossRef
- Meltzer HY. Suicide in schizophrenia, clozapine, and adoption of evidence-based medicine. J Clin Psychiatry. 2005;66(4):530–533. PubMed CrossRef
- Sajatovic M, Bingham CR, Garver D, et al. An assessment of clinical practice of clozapine therapy for veterans. Psychiatr Serv. 2000;51(5):669–671. PubMed CrossRef
- Olagunju AT, Clark SR, Baune BT. Clozapine and psychosocial function in schizophrenia: a systematic review and meta-analysis. CNS Drugs. 2018;32(11):1011–1023. PubMed CrossRef
- Ciapparelli A, Dell’Osso L, Pini S, et al. Clozapine for treatment-refractory schizophrenia, schizoaffective disorder, and psychotic bipolar disorder: a 24-month naturalistic study. J Clin Psychiatry. 2000;61(5):329–334. PubMed CrossRef
- Arranz B, Garriga M, García-Rizo C, et al. Clozapine use in patients with schizophrenia and a comorbid substance use disorder: a systematic review. Eur Neuropsychopharmacol. 2018;28(2):227–242. PubMed CrossRef
- Olfson M, Stroup TS, Huang C, et al. Suicide risk in Medicare patients with schizophrenia across the life span. JAMA Psychiatry. 2021;78(8):876–885. PubMed CrossRef
- United States Department of Veterans Affairs. National Veteran Suicide Prevention Annual Report. Published online 2019:32. US Department of Veterans Affairs website. Accessed August 23, 2021. https://www.mentalhealth.va.gov/docs/data-sheets/2019/2019_National_Veteran_Suicide_Prevention_Annual_Report_508.pdf
- Toyoda K, Hata T, Yamauchi S, et al. Clozapine is better tolerated in younger patients: risk factors for discontinuation from a nationwide database in Japan. Psychiatry Investig. 2021;18(2):101–109. PubMed CrossRef
- Munro J, O’Sullivan D, Andrews C, et al. Active monitoring of 12,760 clozapine recipients in the UK and Ireland: beyond pharmacovigilance. Br J Psychiatry. 1999;175(6):576–580. PubMed CrossRef
- Bishara D, Taylor D. Adverse effects of clozapine in older patients: epidemiology, prevention and management. Drugs Aging. 2014;31(1):11–20. PubMed CrossRef
- Clozaril Prescribing Information. Novartis Pharmaceuticals Corporation. Published online 2004. Accessed August 23, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/019758s054lbl.pdf
- Merrill DB, Ahmari SE, Bradford JME, et al. Myocarditis during clozapine treatment. Am J Psychiatry. 2006;163(2):204–208. PubMed CrossRef
- Rajagopal S. Clozapine, agranulocytosis, and benign ethnic neutropenia. Postgrad Med J. 2005;81(959):545–546. PubMed CrossRef
- Moody BL, Eatmon CV. Perceived barriers and facilitators of clozapine use: a national survey of Veterans Affairs prescribers. Fed Pract. 2019;36(suppl 6):S22–S27. PubMed
- Hodge K, Jespersen S. Side effects and treatment with clozapine: a comparison between the views of consumers and their clinicians. Int J Ment Health Nurs. 2008;17(1):2–8. PubMed CrossRef
- Gören JL, Rose AJ, Smith EG, et al. The business case for expanded clozapine utilization. 2016;67(11):1197–1205.
- Sinyor M, Remington G. Is psychiatry ignoring suicide? The case for clozapine. J Clin Psychopharmacol. 2012;32(3):307–308. PubMed CrossRef
- De Hert M, Detraux J, van Winkel R, et al. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2011;8(2):114–126. PubMed CrossRef
- Kelly DL, McMahon RP, Liu F, et al. Cardiovascular disease mortality in patients with chronic schizophrenia treated with clozapine: a retrospective cohort study. J Clin Psychiatry. 2010;71(3):304–311. PubMed CrossRef
- Tiihonen J, Lönnqvist J, Wahlbeck K, et al. 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet. 2009;374(9690):620–627. PubMed CrossRef
- Henderson DC, Nguyen DD, Copeland PM, et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry. 2005;66(9):1116–1121. PubMed CrossRef
- Fontaine KR, Heo M, Harrigan EP, et al. Estimating the consequences of antipsychotic-induced weight gain on health and mortality rate. Psychiatry Res. 2001;101(3):277–288. PubMed CrossRef
- Vermeulen JM, van Rooijen G, van de Kerkhof MPJ, et al. Clozapine and long-term mortality risk in patients with schizophrenia: a systematic review and meta-analysis of studies lasting 1.1–12.5 years. Schizophr Bull. 2019;45(2):315–329. PubMed CrossRef
- Walker AM, Lanza LL, Arellano F, et al. Mortality in current and former users of clozapine. Epidemiology. 1997;8(6):671–677. PubMed CrossRef
- Kiviniemi M, Suvisaari J, Koivumaa-Honkanen H, et al. Antipsychotics and mortality in first-onset schizophrenia: prospective Finnish register study with 5-year follow-up. Schizophr Res. 2013;150(1):274–280. PubMed CrossRef
- Marvanova M. Strategies for prevention and management of second-generation antipsychotic-induced metabolic side effects. Ment Health Clin. 2013;3(3):154–161. CrossRef
- Katz IR, Rogers MP, Lew R, et al; Li+ plus Investigators. Lithium treatment in the prevention of repeat suicide-related outcomes in veterans with major depression or bipolar disorder: a randomized clinical trial. JAMA Psychiatry. 2022;79(1):24–32. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite