ABSTRACT
Objective: To describe a novel case of imipramine-induced hyperpigmentation and characterize the literature pertaining to imipramine-induced hyperpigmentation to this point.
Data Sources: PubMed and Google Scholar were searched through July 2021 utilizing various combinations of imipramine, discoloration, and hyperpigmentation. The references of initial articles were searched for more case reports and imipramine-related literature. Also, articles that cited the references identified in the literature search were reviewed using Google Scholar. Only articles published in English were included.
Study Selection: A total of 19 cases of imipramine-induced hyperpigmentation were found in 15 publications to date. All cases were included to determine the variation in clinical presentations of this rare condition.
Data Extraction: The case reports were reviewed in their entirety for information concerning patient demographic, clinical presentation, histologic findings on biopsy, and treatment options for imipramine-induced hyperpigmentation.
Results: This presentation, to our knowledge, represents the third case of imipramine-induced iris hyperpigmentation, the first case of iris hyperpigmentation occurring in a blue-eyed individual, and the first report to include pictures of the hyperpigmented irides. A novel proposed pathophysiologic mechanism is also provided.
Conclusions: Imipramine is a tricyclic antidepressant with a rare side effect of cutaneous and iris hyperpigmentation. Granular dermal deposits in microscopy appear to be the cause of this discoloration. Treatment primarily focuses on discontinuing imipramine or laser therapy.
Prim Care Companion CNS Disord 2022;24(2):21r03161
To cite: Coffey N, Ali Z, Grau J. Imipramine-induced cutaneous and iris hyperpigmentation: a case report and literature review. Prim Care Companion CNS Disord. 2022;24(2):21r03161.
To share: https://doi.org/10.4088/PCC.21r03161
© Copyright 2022 Physicians Postgraduate Press, Inc.
aUniversity of Kentucky College of Medicine, Bowling Green, Kentucky
bThe Medical Center of Bowling Green, Bowling Green, Kentucky
*Corresponding author: Nicholas Coffey, MS, University of Kentucky College of Medicine, 399 US 31 W Bypass, Bowling Green, KY 42101 ([email protected]).
Imipramine is a tricyclic antidepressant (TCA) that was first developed in the 1950s and was a first-line treatment for depression until replaced by newer antidepressants, such as selective serotonin reuptake inhibitors. However, tricyclic antidepressants are still very powerful medications used by many experts today for treatment-resistant depression. Common side effects of imipramine include sedation, tremors, weight gain, orthostatic hypotension, constipation, and dry mouth. A few patients may rarely develop cutaneous hyperpigmentation on sun-exposed areas. This discoloration can be distressing to many patients. It is crucial for primary care physicians, psychiatrists, and dermatologists to be familiar with imipramine-induced hyperpigmentation to provide an accurate diagnosis and proper treatment. We report a rare case of cutaneous and iris hyperpigmentation in an 80-year-old female patient who used imipramine for approximately 35 years.
CASE REPORT
The patient is an 80-year-old White woman with a past psychiatric history of major depressive disorder and generalized anxiety disorder who was admitted to our hospital due to passive suicidal ideations, such as not wanting to wake up in the morning again. During our evaluation, the patient reported that she had been stabilized on imipramine 200 mg/d and diazepam 5 mg/d for approximately 35 years until her imipramine was discontinued 4 months ago due to recurrent falls and orthostatic hypotension. The patient reported a relapse in her depression symptoms for the past 3 months. She was then started on mirtazapine 30 mg/d, to which her symptoms did not respond and pigmentation continued. The patient reported a persisting depressed mood, anhedonia, loss of appetite, reduced energy, and insomnia, which began 6 weeks after imipramine cessation and continued until her presentation to our emergency department.
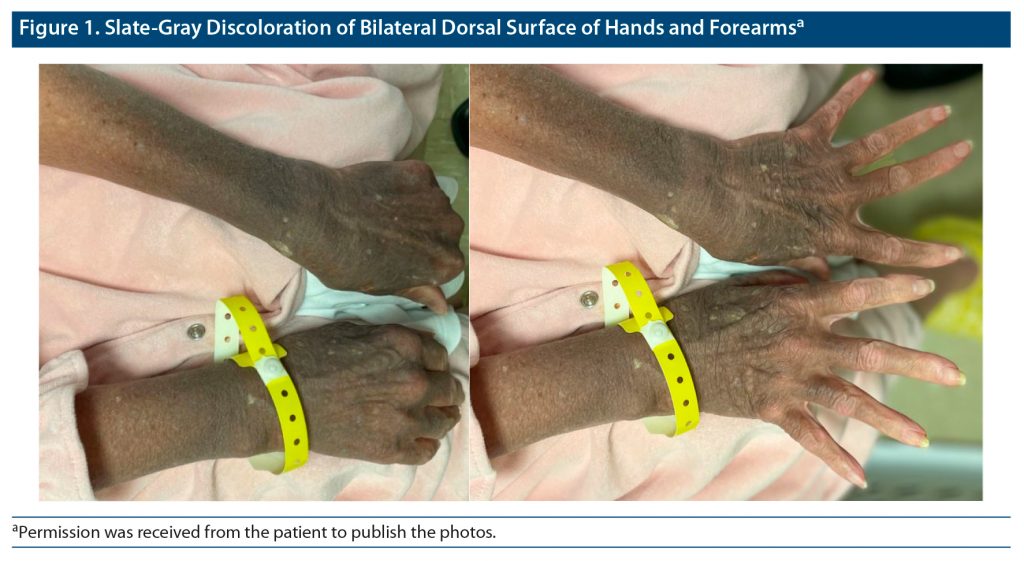
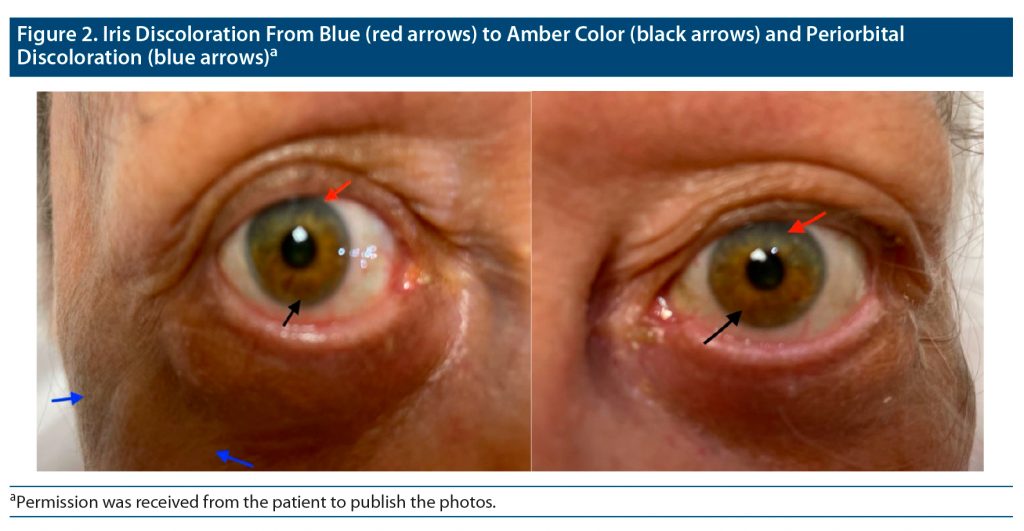
During physical examination, the patient was found to have slate gray discoloration on the dorsal surface of her bilateral hands and forearms in a sun-exposed distribution pattern. In addition, she had similar bilateral facial discoloration in the periorbital and temporal areas. The patient mentioned that she first noticed the discoloration about 18 years ago. She described the initial presentation of her discoloration as a patchy gray on her hands and forearms that slowly progressed to the diffuse pigmentation displayed at presentation as shown in Figure 1. The patient also reported a rapid progression of periorbital and temporal gray macular and patch discoloration that occurred approximately 3 years after the onset of her hand discoloration. Upon questioning about any ocular discoloration, she reported that her iris color had changed from blue to amber at approximately the same time as her facial pigmentation developed, as seen in Figure 2.
The patient sought a dermatologic consultation at that time and was diagnosed with imipramine-induced hyperpigmentation based on clinical presentation. The dermatologist recommended to discontinue her imipramine at that time, but the patient had previously attempted to stop imipramine use, resulting in returning depression symptoms. The patient had tried other medications as well as electroconvulsive therapy but had found only imipramine to be effective. Thus, she continued imipramine use despite the recommendation from her dermatologic therapy consult. The patient reported some distress secondary to the discoloration and that she previously covered her hyperpigmentation with makeup. The patient reported use of no other medications that are linked to her presenting hyperpigmentation, such as amiodarone or chlorpromazine. Apart from orthostatic hypotension, the patient was medically stable and had only been taking multivitamins. Routine laboratories, including vitamin levels and thyroid-stimulating hormone, were unremarkable. Skin biopsy and histologic examination could not be done at that time.
Due to the patient’s severe depression, a decision was made to gradually restart imipramine. However, the patient continued to have orthostatic hypotension, which was resistant to treatment and required discontinuing imipramine again. Thus, the patient was switched to desvenlafaxine 50 mg/d, and she reported partial response after 2 weeks. Upon discharge, the patient was referred to our outpatient psychiatry clinic for continuation of treatment. The patient will be monitored for possible improvement in the discoloration after discontinuing imipramine.
METHODS
References for this literature review were found by searching PubMed and Google Scholar through July 2021 utilizing various combinations of imipramine, discoloration, and hyperpigmentation. From these initial searches, case reports and imipramine basic science literature were obtained, and their references were checked for more case reports and imipramine-related literature. Finally, a Google Scholar function was utilized to find all works that had cited the articles we had obtained to that point for more case reports and imipramine-related literature. Ultimately, we found 19 cases of imipramine-induced hyperpigmentation in 15 publications.1–15 All articles were reviewed for any information regarding clinical presentation, diagnostic methods, and treatment. This information from these articles was utilized if relevant to the discussion. Only articles published in English were included.
RESULTS
Case Reports
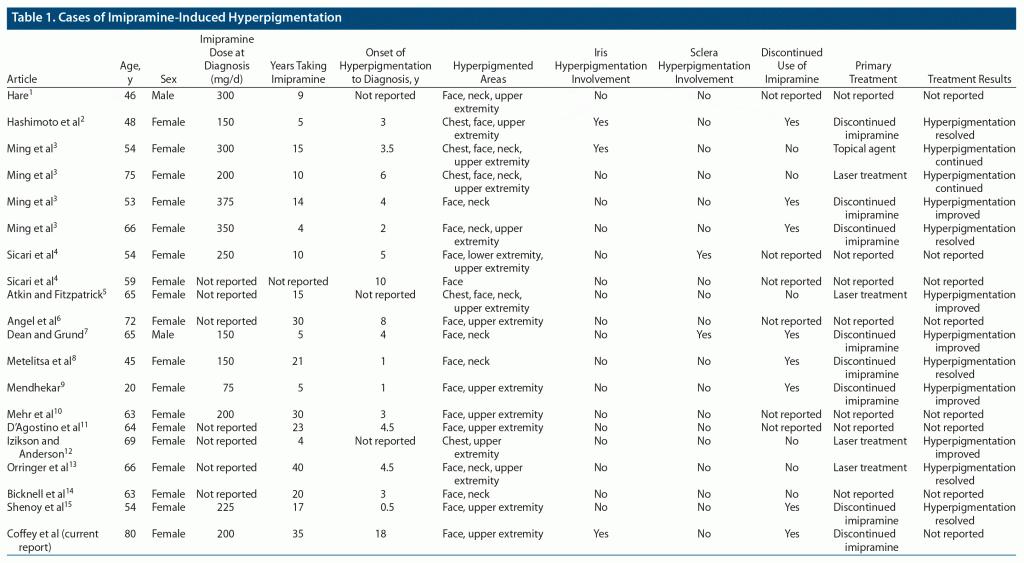
The first known case report was published in 1970,1 and all subsequent case reports to 2020 are summarized in Table 1. Of those, the patients were primarily female (90% or 18/20) with a mean age of 59 years at diagnosis.1–15 At the time of diagnosis, the dosage of imipramine was between 75 and 375 mg/d.1–4,7–10,15 Imipramine was taken by patients approximately 16 years before hyperpigmentation was noticed by the patient.1–15 Imipramine hyperpigmentation was diagnosed approximately 5 years after initial hyperpigmentation was noted by the patient.2–4,6,11,13–15 Of the reports that provided the patient’s primary reason for imipramine use, the most cited causes were depression, major depressive disorder, or dysthymia (12/15 or 80%), with other reasons being panic disorder, paranoid schizophrenia, and “severe psychiatric symptoms.”1–3,5,7–11,13–15
Clinical Characteristics of Imipramine Hyperpigmentation
The presentation of the patient in our report is consistent with topical imipramine-induced hyperpigmentation on visual inspection as described in previous case reports. The most common presentation is distinct or coalescing macular and patch hyperpigmentation, usually found on sun-exposed areas or in a photo-distributive pattern.3,4,6,10,11,13–15 The color of the macular and patch hyperpigmentation varied greatly, with 58% (11/19) of the cases reporting the coloration being gray, slate-gray, or gray with a bluish tint.2,3,6,9–12,14,15 Other monochromatic presentations included purple or bluish-purple hyperpigmentation (11% or 2/19)1,7 and brown or golden-brown discoloration (11% or 2/19).3 The other case reports (16% or 3/19) were polychromatic in presentation with gray and brown macules being observed in various distributions on each patient.4,5,8
The most common area presenting with hyperpigmented macular and patch discoloration was the face (95% or 19/20).1–11,13–15 Next in frequency was the upper extremities (75% or 15/20), with areas typically appearing on the dorsal hand and forearm.1–6,9–13,15 Other areas on which hyperpigmented macules and patches appeared included the neck (50% or 10/20),1,3,5,7,8,13,14 chest (25% or 5/20),2,3,5,12 and lower extremities (5% or 1/20).4
Ocular imipramine hyperpigmentation (25% or 5/20) was noted in the literature as well as in our case. Iris hyperpigmentation was noted in 2 cases,2,3 while 2 other cases4,7 had sclera hyperpigmentation. One patient’s iris changed from a “greenish brown” to amber with the superior iris retaining more of the patient’s original greenish-brown coloration and the inferior iris changing to the amber coloration.2 The other patient with reported iris involvement went from a brown to a darker brown.3 Similarly, the “original blue” eye color of our patient could still be observed in the superior iris, and the inferior iris had undergone the coloration change to amber. Unlike previous case reports, our patient reported that she originally had blue eyes. The melanin in blue eyes has been shown to have approximately 25% less affinity for imipramine compared to brown eyes, which further exemplifies the rarity of our patient’s findings.16 The other 2 cases with ocular hyperpigmentation reported sclera hyperpigmentation, with amber hyperpigmentation adjacent to the irides in 1 patient and brownish granular deposits reported in the other.4,7
Biopsy Microscopy and Histologic Findings
Unlike the visual presentation of imipramine hyperpigmentation, the microscopic biopsy results were generally uniform across the 16 case reports2–11,13,14 that obtained skin biopsies for microscopic testing. Various shades of amber-colored granules, globoids, or deposits were noted intracellularly or extracellularly on analysis by light microscopy of the skin biopsies. These deposits were found within the papillary, superficial, midreticular, and middermis.2–8,10,11,13,14 The epidermis was prototypically found without granules or hypermyelination.4,6,8,11,14 The intracellular granules were primarily found in dermal phagocytic cells, such as dendrocytes, macrophages, and fibroblasts.2,6,13 However, these granules were also found perivascular as well and were primarily contained in histocytes.2,4,8,14 None of the granules were birefringent or polarizable; however, the larger and more peripherally located granules were observed to have a reflective, shiny property that led to the presence of double border rings.2–6 The granules were shown to be iron free, but contained melanin by either MEL-5, which is a monoclonal antibody used to label melanosomes, or Fontana-Masson stain.2–6,8–11,14
Four case reports2–4,8 conducted electron microscopy on the obtained skin biopsies. All electron microscopies revealed electron-dense inclusion bodies within phagocytic cells, such as fibroblasts, dendrocytes, and macrophages.2–4,8 These inclusion bodies would start as separate small dense granules independent of phagocytized melanosomes. The dense granules would have toxic effects on the host cell and lead to its degeneration and release of the dense granule, thus extracellularly seen on microscopy.2 For comparison, a skin biopsy taken from an unaffected area of the patient was shown to rarely have phagocytes with dense granules or inclusion bodies.2
Other laboratory procedures, such as a microspectroscopic analysis, an energy dispersive x-ray microanalysis (EDXA), a backscattered electron image, and an electron dispersive spectrograph, were conducted.3,4 The microspectroscopic analysis found that the granules did not contain imipramine. The EDXA revealed that the granules primarily consisted of carbon, oxygen, and sulfur. The backscattered electron image observed a structure within the granule most consistent with a central sulfur-containing material surrounded by carbon-containing material.3 The electron dispersive spectrograph found sulfur and copper within the granule.4
Pathologic Mechanism of Imipramine Hyperpigmentation
The exact mechanism of imipramine hyperpigmentation is unknown; however, the hypothesized mechanism of previous case reports is based on chlorpromazine. Chlorpromazine has a similar chemical structure to imipramine along with similar clinical presentation, histologic presentation, and EDXA findings for drug-induced hyperpigmentation.2,3,6,17 These similarities make it likely that the 2 drugs share a similar pathophysiology. It is hypothesized that ultraviolet light reacts with chlorpromazine to produce purple free radicals, which activate tyrosinases in melanocytes to increase melanin. These free radicals become trapped by combination with melanin to form an amorphous material. This mechanism is theorized to be occurring in imipramine as well.4,18–21
The mechanism proposed for imipramine may also disrupt normal dermal melanogenesis producing phaeomelanin, a melanin pigment that contains sulfur. Phaeomelanin is known to be associated with red, red-brown, and brown coloration and to be easily destroyed in the presence of UV light and oxygen.3,6,22 The presence of phaeomelanin in the granules could help explain the sulfur found on EDXA, the difference in clinical presentation between case reports, and the difference in treatment success of macules and patches in imipramine hyperpigmentation due to the varying composition of red/brown phaeomelanin, melanin, and purple imipramine/chlorpromazine free radicals.
However, Ni-Komatsu and Orlow23 studied imipramine’s effect on melanin and melanocytes. They found that imipramine did not significantly affect tyrosinase activity and expression or the activity of its key related proteins, such as tyrosinase-related protein 1 or Dct proteins, within melanocytes in vivo or in vitro. Instead, the location of tyrosinase and its key related proteins were altered due to altered intracellular trafficking controlled by the protein HPS1. Normally, tyrosinase and its key proteins were found distributed throughout the cytoplasm in small granules without the presence of large granules. In imipramine-exposed melanocytes, they were found concentrated in large granules within the melanocyte. The secretion of these granules resulted in increased secretion of tyrosinase. This resulted in a 1.6- to 2.9-fold increase in tyrosinase extracellular activity and could explain the increase in extracellular melanin seen in these studies compared to control.23 This mechanism could help explain various pathological aspects of imipramine-induced hyperpigmentation, such as the electron microscopy results and excess copper found in granules by the electron dispersive spectrograph, since tyrosinase uses copper as a cofactor.
On the basis of the current data, we propose that a combination of the previous 2 theories with a direct mode of action for imipramine on melanocytes is the most likely mechanism. Ni-Komatsu and Orlow23 showed how imipramine led to altered intracellular trafficking of tyrosinase and its key proteins to create the large melanin-containing granules seen in those with hyperpigmentation due to imipramine. Also, imipramine has been shown to have a high affinity for melanin and melanosomes. Specifically, imipramine has been shown to have a higher affinity for melanin granules than albumin, red blood cells, or any other plasma content, thus the idea of a direct mechanism.24,25 Imipramine is likely broken down by UV light exposure into the proposed purple free radical from the chlorpromazine mechanism within the melanocyte or the released granule. This free radical combines with melanin to form an amorphous material that could explain why imipramine is not found in the granules themselves on microspectroscopic analysis, why these granules are found only on biopsies from sun-exposed areas, and why phagocytic cells have such difficulty breaking these granules down. The dysregulation of trafficking induced by imipramine on tyrosinase and its key proteins presented by Ni-Komatsu and Orlow23 serves as a potential mechanism by which phaeomelanin occurs in these granules as well.
Treatment
Generally, treatment options are determined based on the patient’s willingness to stop using imipramine. Of the case reports citing imipramine use status after diagnosis, only 8 of 14 (57%) patients were willing to stop using imipramine.2,3,5,7–9,12–15 Many patients prefer to have hyperpigmentation and not risk a recurrence of their psychiatric symptoms.
Patients who discontinued imipramine reported either complete resolution or improvement in hyperpigmentation.2,3,7–9,15 Of the case reports that provided treatment results, 4 of 7 (57%) reported the complete resolution of symptoms, including iris discoloration, within a year of imipramine discontinuation.2,3,8,15 The other 3 (43%) cases reported mild to significant clearance within 6 months to 2 years of imipramine cessation.3,7,9 Of interest, 1 patient with “significant clearance” at 6 months was switched to desipramine and had a return of hyperpigmentation within 1 month.3
Some medical agents were utilized in an attempt to reduce hyperpigmentation, including tretinoin cream, hydroquinone cream, topical retinoid, vitamin C serum, and photoprotective agents, but there was limited to no success.3,5,14 Laser therapy has been used to treat imipramine hyperpigmentation with varying levels of success.3,5,12,14 Ming et al3 reported a case wherein a patient received a combination therapy of a bleaching cream and neodymium:YAG laser therapy that was unsuccessful. Some cases reported using Q-switched alexandrite and ruby laser therapy with satisfactory improvement in the clinical and microscopic hyperpigmentation.5,12,13
The most important factor that may affect the therapeutic value of laser therapy is the depth of skin penetration by the laser. For example, erbium:YAG laser therapy was utilized in one study but found to be ineffective since it mainly lightens superficial hyperpigmentation and does not reach the depth required to affect the imipramine hyperpigmentation granules seen in the dermis.5 In contrast, laser therapies that penetrate deeper into the skin and can target melanin with appropriate wavelengths, such as the Q-switched alexandrite and ruby laser, have been successful.5,12,13,26 Laser therapies of inappropriate depth should be generally avoided, as it is hypothesized that such therapies could result in paradoxical photochemical or photothermal modification by subsequent laser therapies, inducing chromophores and triggering tissue trauma and inflammation, leading to delayed hyperpigmentation following treatment.12 Paradoxical darkening was present in less than 1% of treated skin surface areas and was resolved by subsequent laser therapy. Thus, paradoxical darkening is not recommended to be a contraindication to treatment.13
CONCLUSION
Imipramine is a tricyclic antidepressant with a rare side effect of cutaneous and iris hyperpigmentation. Despite the reduced popularity of imipramine use, it is still crucial for physicians to be familiar with this rare adverse effect that is usually distressing to patients. Microscopy findings in individuals using imipramine consistently show granular dermal deposits that cause the discoloration observed. The hyperpigmentation is hypothesized to be the result of imipramine’s direct effect on melanosomes and dysregulation of essential melanosome proteins. Imipramine discontinuation is first-line treatment. For those unwilling to discontinue or exhibiting residual imipramine-induced hyperpigmentation, laser therapy with appropriate length and depth might be considered as an alternative treatment option.
Submitted: September 29, 2021; accepted November 11, 2021.
Published online: March 29, 2022.
Relevant financial relationships: None.
Funding/support: None.
Patient consent: The patient was counseled, and consent forms were signed by the patient that allowed release of de-identified health information and accompanying pictures for use in this case report.
Clinical Points
- Imipramine-induced hyperpigmentation generally occurs after administration of imipramine for several years.
- First-line treatment is discontinuation of imipramine and switching to a different antidepressant agent.
- Stabilization of depressive symptoms on imipramine and concerns regarding relapse of depression usually hinder many patients and medical providers from making decisions on imipramine cessation.
- Laser therapy has been shown to improve hyperpigmentation in several cases.
References (26)

- Hare PJ. “Visage mauve” (? from imipramine). Br J Dermatol. 1970;83(3):420. PubMed
- Hashimoto K, Joselow SA, Tye MJ. Imipramine hyperpigmentation: a slate-gray discoloration caused by long-term imipramine administration. J Am Acad Dermatol. 1991;25(2 Pt 2):357–361. PubMed CrossRef
- Ming ME, Bhawan J, Stefanato CM, et al. Imipramine-induced hyperpigmentation: four cases and a review of the literature. J Am Acad Dermatol. 1999;40(2 Pt 1):159–166. PubMed CrossRef
- Sicari MC, Lebwohl M, Baral J, et al. Photoinduced dermal pigmentation in patients taking tricyclic antidepressants: histology, electron microscopy, and energy dispersive spectroscopy. J Am Acad Dermatol. 1999;40(2 Pt 2):290–293. PubMed CrossRef
- Atkin DH, Fitzpatrick RE. Laser treatment of imipramine-induced hyperpigmentation. J Am Acad Dermatol. 2000;43(1 Pt 1):77–80. PubMed CrossRef
- Angel TA, Stalkup JR, Hsu S. Photodistributed blue-gray pigmentation of the skin associated with long-term imipramine use. Int J Dermatol. 2002;41(6):327–329. PubMed CrossRef
- Dean CE, Grund FM. Imipramine-associated hyperpigmentation. Ann Pharmacother. 2003;37(6):825–828. PubMed CrossRef
- Metelitsa AI, Nguyen GK, Lin AN. Imipramine-induced facial pigmentation: case report and literature review. J Cutan Med Surg. 2005;9(6):341–345. PubMed CrossRef
- Mendhekar DN. Imipramine monotherapy-induced Hyperpigmentation in an adolescent girl. Indian J Med Sci. 2005;59(9):405–406. PubMed CrossRef
- Mehr N, Wu JJ, Dyson SW, et al. Imipramine-induced hyperpigmentation of the skin. Dermatol Online J. 2007;13(4):8. PubMed CrossRef
- D’Agostino ML, Risser J, Robinson-Bostom L. Imipramine-induced hyperpigmentation: a case report and review of the literature. J Cutan Pathol. 2009;36(7):799–803. PubMed CrossRef
- Izikson L, Anderson RR. Delayed darkening of imipramine-induced hyperpigmentation after treatment with a Q-switched Nd:YAG laser followed by a Q-switched ruby laser. Dermatol Surg. 2009;35(3):527–529. PubMed CrossRef
- Orringer JS, Lowe L, Cha KB. Treatment of imipramine-induced dyspigmentation with Q-switched alexandrite laser therapy. Dermatol Surg. 2010;36(9):1469–1472. PubMed CrossRef
- Bicknell LM, McFaddin CL, Fernandez M, et al. Imipramine-induced hyperpigmentation. Cutis. 2017;100(3):E8–E10. PubMed
- Shenoy S, Shenoi SD, Sharma PSVN. Imipramine-induced hyperpigmentation of skin: a case report. Eur J Psychiatry. 2020;34(4):225–226. CrossRef
- Persad S, Menon IA, Basu PK, et al. Binding of imipramine, 8-methoxypsoralen, and epinephrine to human blue and brown eye melanins. J Toxicol Cutaneous Ocul Toxicol. 1986;5(2):125–132. CrossRef
- Benning TL, McCormack KM, Ingram P, et al. Microprobe analysis of chlorpromazine pigmentation. Arch Dermatol. 1988;124(10):1541–1544. PubMed CrossRef
- Lerner EA, Sober AJ. Chemical and pharmacologic agents that cause hyperpigmentation or hypopigmentation of the skin. Dermatol Clin. 1988;6(2):327–337. PubMed CrossRef
- Satanove A, McIntosh JS. Phototoxic reactions induced by high doses of chlorpromazine and thioridazine. JAMA. 1967;200(3):209–212. PubMed CrossRef
- Srebrnik A, Hes JP, Brenner S. Adverse cutaneous reactions to psychotropic drugs. Acta Derm Venereol suppl (Stockh). 1991;158:1–12. PubMed
- Zelickson AS. Skin changes and chlorpromazine: some hazards of long-term drug therapy. JAMA. 1966;198(4):341–344. PubMed CrossRef
- Chedekel MR, Post PW, Deibel RM, et al. Photodestruction of phaeomelanin. Photochem Photobiol. 1977;26(6):651–653. PubMed CrossRef
- Ni-Komatsu L, Orlow SJ. Chemical genetic screening identifies tricyclic compounds that decrease cellular melanin content. J Invest Dermatol. 2008;128(5):1236–1247. PubMed CrossRef
- Salazar M, Rahwan RG, Patil PN. Binding of 14C-imipramine by pigmented and non-pigmented tissues. Eur J Pharmacol. 1976;38(2):233–241. PubMed CrossRef
- Bickel MH. Binding of chlorpromazine and imipramine to red cells, albumin, lipoproteins and other blood components. J Pharm Pharmacol. 1975;27(10):733–738. PubMed CrossRef
- Goyal S, Arndt KA, Stern RS, et al. Laser treatment of tattoos: a prospective, paired, comparison study of the Q-switched Nd:YAG (1064 nm), frequency-doubled Q-switched Nd:YAG (532 nm), and Q-switched ruby lasers. J Am Acad Dermatol. 1997;36(1):122–125. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite