ABSTRACT
Objective: To evaluate the impact of extended-release (ER) intramuscular naltrexone on readmission rates for hospitalized patients with alcohol use disorder (AUD).
Methods: This was a single-center, retrospective before and after study. Adult patients with AUD who received ER naltrexone prior to discharge between June 29, 2020, and November 30, 2020, were included in the study. The primary outcome measure was alcohol-related readmission 90 days after ER naltrexone administration. Secondary outcomes were the number of emergency department visits, length of hospital stay, and time between hospital admissions. Patients served as their own controls before and after ER naltrexone administration, and data were collected from the electronic medical records. Comparative analysis was performed using descriptive statistics and paired Student t test.
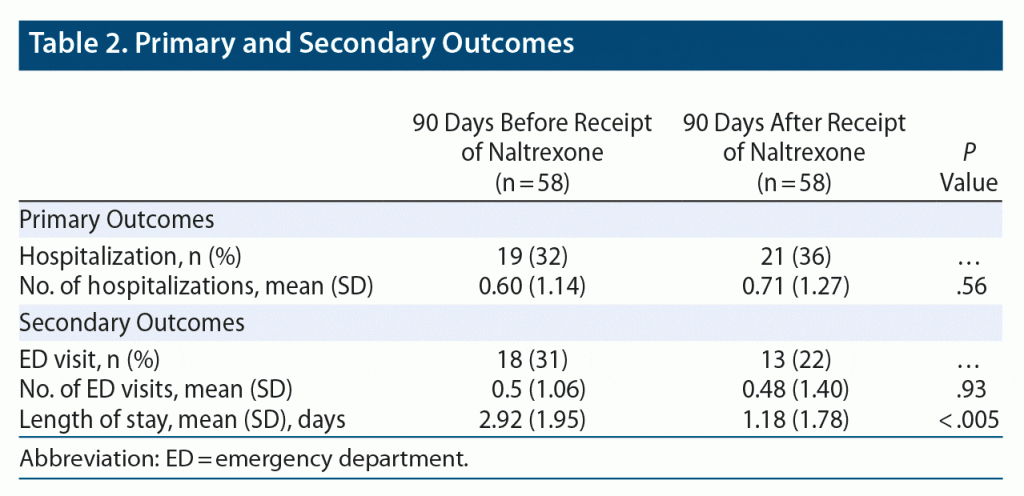
Results: 58 patients received ER naltrexone during the study period, with a mean (SD) pre and post 90-day admission rate of 0.60 (1.14) and 0.71 (1.27), respectively, P = .56. Number of emergency department visits before and after the intervention were 0.5 (1.06) and 0.48 (1.40), respectively, P = .93. Length of hospital stay decreased after naltrexone administration (2.92 [1.95] vs 1.18 [1.78] days, P < .005).
Conclusion: There was no major difference in the number of hospitalizations or emergency department visits, but there was a decreased length of hospital stay in patients who received ER naltrexone prior to hospital discharge for the treatment of AUD.
Prim Care Companion CNS Disord 2022;24(6):21m03213
To cite: Tigh J, Daniel K, Balasanova AA. Impact of hospital-administered extended-release naltrexone on readmission rates in patients with alcohol use disorder: a pilot study. Prim Care Companion CNS Disord. 2022;24(6):21m03213.
To share: https://doi.org/10.4088/PCC.21m03213
© 2022 Physicians Postgraduate Press, Inc.
aNebraska Medicine, Nebraska Medical Center, Omaha, Nebraska
bDepartment of Psychiatry, University of Nebraska Medical Center, Omaha, Nebraska
*Corresponding author: Alëna A. Balasanova, MD, Department of Psychiatry, Poynter Hall 5th Fl, University of Nebraska Medical Center, 985578 Nebraska Medical Center, Omaha, NE 68198-5578 ([email protected]).
Alcohol use disorder (AUD) is a chronic relapsing disease defined by an impaired ability to stop or control alcohol use despite negative consequences.1 It is a significant public health concern, affecting over 14.4 million adults in the United States with around 9% of adults who drink meeting the criteria for AUD.2 AUD contributes to over 335,000 hospitalizations and approximately 95,000 deaths per year, making it a leading cause of preventable death in the United States.3,4 The overall health cost associated with alcohol use was 249 billion dollars in 2010, with greater than 40% of the expenses being paid by public health programs.5 The mortality, morbidity, and cost associated with AUD encourage the development of effective evidence-based interventions that promote harm reduction; however, only 8% of individuals with AUD received treatment in 2018.2 There exists a disparity, as patients who would benefit from treatment are unlikely to receive evidence-based care, and opportunities to treat AUD during a hospitalization are missed.6 Harm reduction measures are those intended to reduce the harm of substance use whether it be to decrease use or increase safety around use. Regarding alcohol, harm reduction often entails reduction in quantity and frequency of alcohol consumed.
Medications are useful in the treatment of AUD and are included in guidelines as first-line treatment but are prescribed in less than 9% of those who would benefit.6,7 Three medications (disulfiram, acamprosate, and naltrexone oral and extended-release [ER] injectable formulations) are currently approved by the US Food and Drug Administration for the treatment of AUD. There are data to support the safety and efficacy of each of these medications. Still, naltrexone is considered a first-line treatment by many experts due to its safety and efficacy profile in both clinical trials and real-world settings.6 Naltrexone is an antagonist at the μ-, κ-, and δ-opioid receptors and was initially approved for the treatment of opioid use disorder. Naltrexone exerts its effect by reducing opioidergic activity, resulting in modulation of the dopamine-mediated rewarding effects of alcohol, thereby reducing cravings and alcohol consumption. Naltrexone is the only medication approved for treatment of AUD in patients in which the goal is not total abstinence, making it a critical medication utilized in harm reduction to reduce binge drinking.6
However, studies8 have shown that up to 50% of patients prescribed a new medication do not fill their first prescription, and drugs prescribed for the treatment of AUD are filled at even lower rates. Medication adherence is key to efficacy, and in 2006, once-monthly injectable ER naltrexone was approved by the FDA. In the randomized controlled trials that led to its approval, ER naltrexone was associated with a 25% decrease in heavy drinking days, longer time to first drink, and higher frequency of abstinent days versus placebo.9,10
An estimated 10%–20% of patients seen in primary care or hospital settings have diagnosable AUD. AUD is prevalent in hospitalized patients and often precedes and complicates their hospital course, but treatment is infrequently accessed after discharge.11 Multiple studies12–16 have demonstrated decreased ED visits, hospital readmissions, and lower cost when AUD discharge planning and oral drug therapy are carefully initiated, but few have looked at these outcomes in patients who received intramuscular ER naltrexone prior to discharge. Retrospective claims database analysis and meta-analysis demonstrate that ER naltrexone is associated with higher refill rates, fewer hospitalizations for any reason, and lower overall health care–related costs compared to oral medications for AUD.8,17,18
At our medical center, a new workflow implemented at the end of June 2020 increased access to ER naltrexone during hospitalization for qualifying admitted acute care patients. Patients were selected to receive ER naltrexone through addiction psychiatry consultation-liaison team evaluation and recommendation, which was based on the patient’s diagnosis of AUD and interest in receiving the medication. The administration of ER naltrexone in an inpatient setting capitalizes on the intervenable moment of hospitalization, and treatment begins prior to discharge, mitigating potential adherence concerns. This pilot study aims to analyze the frequency of hospital admission in patients with AUD who received an ER naltrexone injection before hospital discharge compared to routine care at an academic medical center in the midwestern United States.
METHODS
This was a retrospective descriptive study utilizing manual review of the electronic medical record. Nebraska Medicine’s Institutional Review Board reviewed and approved this study. Adult patients (≥ 18 years of age) who were admitted to the Nebraska Medical Center between June 29, 2020, and November 30, 2020, and received ER naltrexone for AUD treatment prior to hospital discharge were included. Patients were included if they received 1 or more doses of ER naltrexone during the study period. Patients were excluded if there was concurrent use of acamprosate or disulfiram, as defined by the discharge medication list or the use of naltrexone for diagnoses other than AUD (eg, opioid use disorder). Additional exclusion criteria included death during the data collection window.
Patients served as their own controls, with data collected for the 90 days prior to receiving ER naltrexone and the 90 days after administration. The primary outcome was the number of alcohol-related hospital admissions 90 days post discharge after receiving ER naltrexone compared to 90 days before receiving ER naltrexone. Data were analyzed at 30 days, 60 days, and 90 days after naltrexone administration, owing to ER naltrexone’s 4-week mechanism of action and literature supporting that treatment benefit may outlast the active treatment phase.8 Secondary outcomes included comparisons pre/post intervention in the rates of emergency department (ED) visits, frequency and time between hospital admissions, length of hospital stay, and subgroup analyses including patients experiencing or at risk for homelessness.
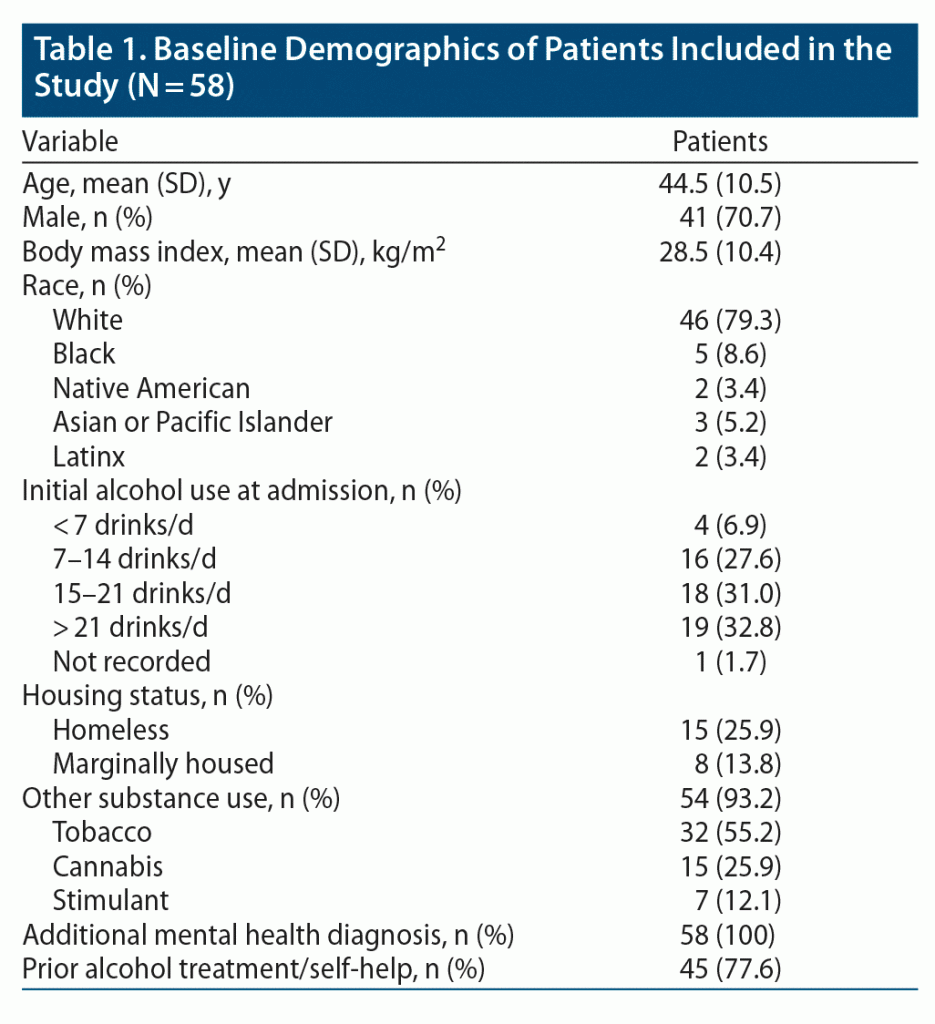
Baseline demographics were collected including age, sex, race, alcohol use at admission, housing status, prior alcohol treatment or self-help, and additional psychiatric diagnoses. Outcome data collected included the number of alcohol-related hospital admissions defined as alcohol-related liver problems, alcohol-related pancreatitis, alcohol withdrawal, or suicidal ideation in the context of alcohol intoxication. Additionally, the number of alcohol-related ED visits, time between hospital admissions, and length of hospital stay for each encounter were gathered.
Demographic data were analyzed using descriptive statistics. The primary and secondary outcomes were compared using the paired sample Student t test. All statistical analyses were performed in Excel.
RESULTS
A total of 59 patients were identified as having received a dose of ER naltrexone prior to hospital discharge during the study period. Of those, 1 patient was excluded for death before 90 days post intervention. Thus, 58 patients were eligible for inclusion. Four patients received 2 doses of naltrexone during the study period. Baseline demographics are described in Table 1. Patients were mostly male (70%), middle aged (mean age of 44 years), and White (79%). The majority of patients reported drinking ≥ 7 drinks/day (91%). Housing insecurity was common, with 40% being homeless or at risk of becoming homeless. Tobacco was the most co-used substance (55%). Also, 77.6% of patients reported prior alcohol treatment or self-help consisting of pharmacotherapy, residential rehabilitation, outpatient counseling, or peer support groups. All the included patients had ≥ 1 additional psychiatric diagnosis listed in their medical record primarily encompassing mood, anxiety, and psychotic disorders.
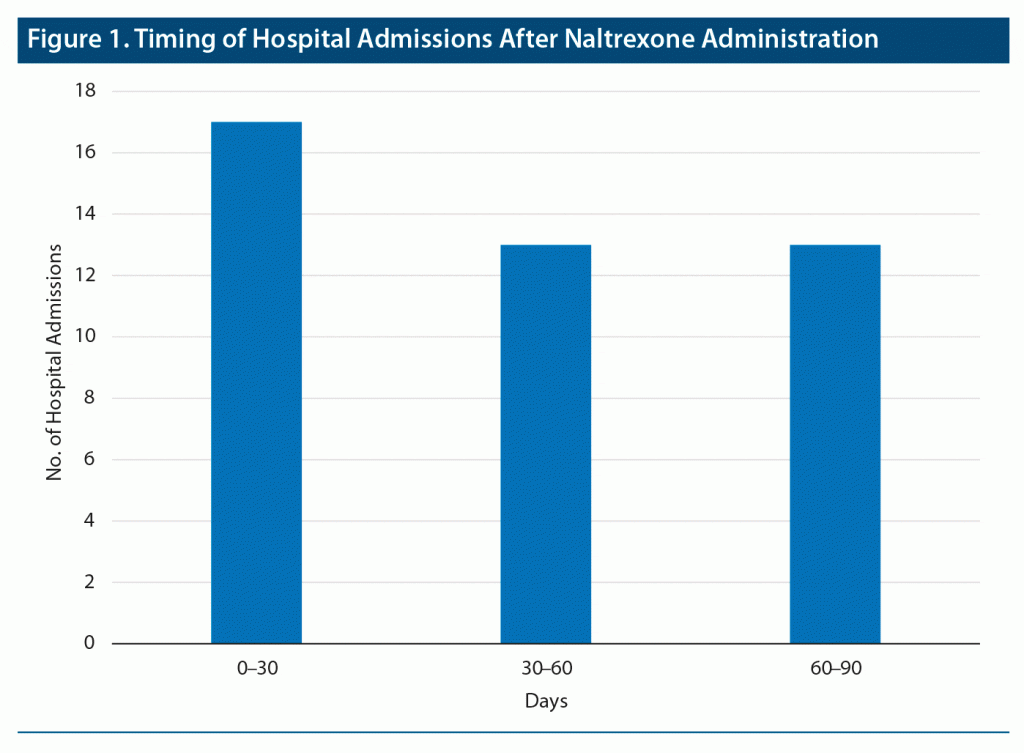
Primary and secondary outcomes are described in Table 2. Ninety-day hospitalization averages were not significantly different (t57 = −0.59, P = .56) before (mean = 0.60, SD = 1.14) and after (mean = 0.71, SD = 10.27) the intervention. ED visits also did not significantly differ (t57 = 0.089, P = .92) before (mean = 0.5, SD = 1.06) and after (mean = 0.48, SD = 1.40) the intervention. Average length of hospital stay was significantly shorter after the intervention (t57 = 5.58, P < .001). There was no significant difference in timing of hospital admission after naltrexone administration with 17, 13, and 13 hospitalizations occurring 0–30 days, 30–60 days, and 60–90 days after administration, respectively (Figure 1).
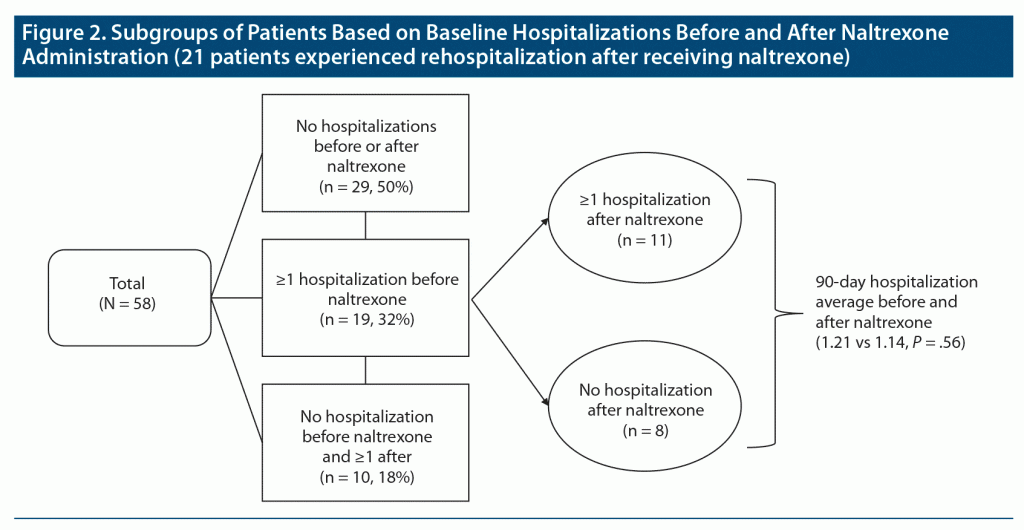
Figure 2 provides an analysis of baseline hospitalizations before and after naltrexone administration. Of the included patients, 29 (50%) had no admissions 90 days before or after ER naltrexone administration. Nineteen patients (32%) had ≥ 1 hospitalization before receiving naltrexone. After naltrexone administration, 11 of these patients had ≥ 1 hospitalization, and 8 had no hospitalizations. In this group, hospitalization averages did not significantly differ (t18 = 0.90, P = .38). Of the total population, 21 patients experienced rehospitalization after receiving naltrexone, and of those, 10 patients (17%) had 0 pre-naltrexone and ≥ 1 post-naltrexone admissions.
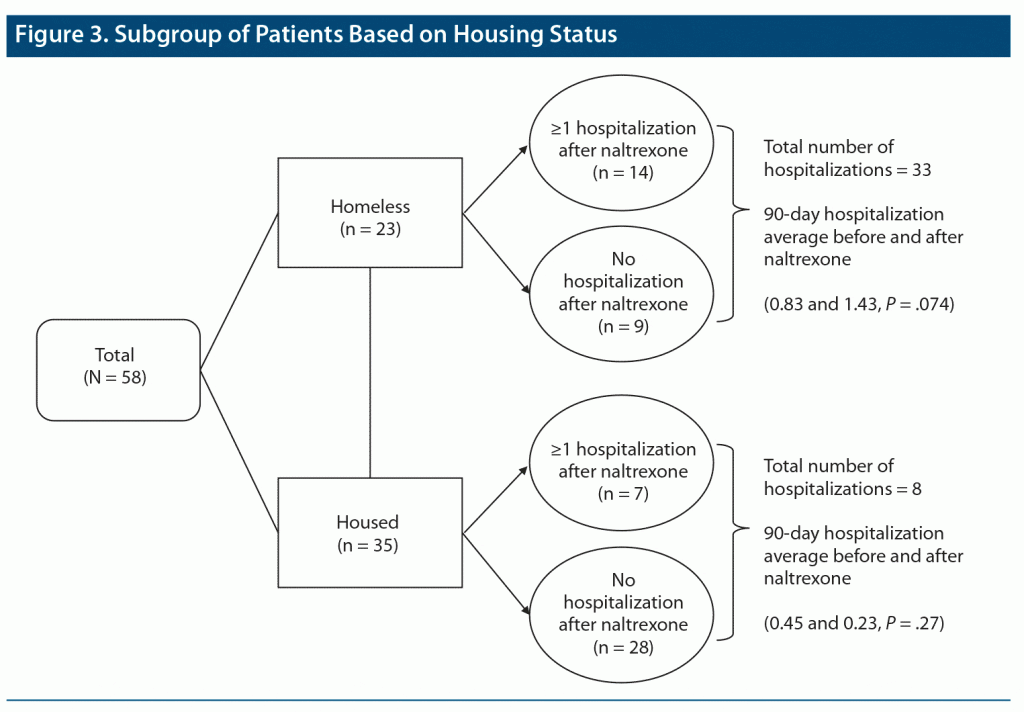
Further subgroup analysis based on housing status is provided in Figure 3. Patients experiencing or at risk for homelessness (n = 23) made up 67% (14/21 patients) of readmissions and accounted for 80% (33/41) of the total number of hospitalizations after naltrexone administration. Average 90-day hospitalization was increased, but not significantly (t22 = −1.87, P = .07), after naltrexone administration in this group (mean = 0.83, SD = 1.19 vs mean = 1.43, SD = 10.7). When excluding these patients from the overall sample, mean hospitalizations were lower before (mean = 0.45, SD = 1.1) and after (mean = 0.23, SD = 0.49) naltrexone in patients with housing security, although this was not statistically significant (t34 = 1.24, P = .27). In patients experiencing homelessness, 7 (30%) did not have hospitalizations before or after naltrexone administration. Ten patients (43%) had ≥ 1 previous admissions for comparison. Of these 10 patients, 2 did not have a readmission, and 8 had ≥ 1 readmissions. In this subgroup, 6 patients (26%) did not have a hospitalization before naltrexone and had ≥ 1 post-naltrexone admissions.
DISCUSSION
In this pilot retrospective study, no significant differences were found in the number of hospitalizations or ED visits in patients treated with ER naltrexone for AUD. Length of hospital stay was significantly decreased in patients who had readmissions after treatment. Notably, 50% of the patients included in this study had zero baseline hospitalizations and no readmissions during the study period. When analyzing various subgroups, patients experiencing housing insecurity had more than double the number of hospitalizations both before and after naltrexone administration compared to patients who were housed. This population was the biggest driver of hospitalization with two-thirds of patients with readmissions experiencing homelessness, representing 80% of the total number of hospitalizations. This population had increased hospital admissions before naltrexone compared to after naltrexone, whereas the housed population had fewer admissions after receiving treatment.
Studies looking at initiation of AUD treatment prior to hospital discharge have demonstrated varying results. In a retrospective claims database analysis, Baser et al8 noted meaningful outcomes when patients were treated with ER naltrexone, including fewer hospitalizations for any reason, greater refill persistence, and overall lower health care costs despite higher drug cost. They8 also showed that outcomes persisted over a 6-month timeframe, despite active treatment only lasting 2 to 3 months, suggesting that treatment benefit may outlast active treatment, which is why we included outcome data to 90 days. This analysis was limited, as it was unable to describe important demographic information, which setting patients received the medication from (inpatient versus outpatient), or how patients were selected for therapy. Conversely, Beatty and Stock15 performed a small retrospective study in veterans and showed increased readmission rates and health care utilization in patients who received ER intramuscular naltrexone (n = 14) versus oral naltrexone (n = 65). They included patients who received their first dose as either an inpatient or outpatient through the Veterans Affairs Healthcare System and did not include a control group. Their15 results suggest that the benefits of ER naltrexone likely do not outweigh the cost, but the study was limited, as factors that could affect outcomes such as homelessness and how patients were selected for treatment were not accounted for.
A pilot randomized controlled trial16 comparing oral and intramuscular ER naltrexone given prior to discharge in hospitalized veterans admitted for an acute physical or psychiatric illness with a diagnosis of AUD demonstrated lower alcohol usage at days 14 and 45 in both groups and similar medication adherence and treatment engagement, although hospital readmission rates were not evaluated. Generalizability was limited in that study,16 as most patients were White male veterans and housing status was not described. Wei et al12 showed that implementation of a discharge planning protocol including the use of oral naltrexone, but not ER, in patients admitted with an alcohol-related diagnosis decreased all-cause 30-day readmission rates from 23.4% to 8.2%. About 40% of patients included in that study12 were homeless, more closely resembling our study population. In a similar study, Stephens et al13 found that patients who received documented counseling about naltrexone prior to discharge had significantly lower 30-day ED revisits and rehospitalizations compared to patients who did not receive counseling in adjusted subgroup analysis (hospitalizations: 2.8% vs 26.2%). No differences were found in unadjusted analyses, highlighting the possibility of unmeasured confounders (ie, homelessness) that may have influenced outcomes.
During our literature review, only 1 study14 was identified that directly looked at readmission rates in patients with AUD who received a dose of ER naltrexone prior to discharge (n = 35) compared to standard of care (n = 358) over a 15-month period. In this retrospective observational study,14 30-day all-cause readmission rates were 3% in the naltrexone group and 25% in the standard of care group. Notably, utilizing public health database information, the author14 was able to capture readmissions that may have fallen outside of their hospital network. This study14 was limited, as demographics were not described, patients were not matched, and the study sample was compared to a much larger control population, possibly introducing bias and limiting conclusions.
Collins et al19 performed a randomized controlled trial evaluating harm reduction measures including combined behavioral and ER naltrexone treatment in patients with AUD experiencing homelessness. Compared to usual care, patients who received this combined treatment had improvement in several harm reduction outcomes including reduction in alcohol use and related harm and health-related quality of life for the 12-week treatment period. However, patients receiving placebo plus behavioral treatment also improved in outcomes, suggesting improvement could not be attributed to ER naltrexone alone. Notably, after active treatment ended (12 weeks), outcomes plateaued in patients receiving ER naltrexone or placebo, whereas patients receiving usual care improved in several measures. No significant differences in the number of hospitalizations or ED visits were found between the treatment and control groups. Also, 25% of patients in the combined behavioral and ER naltrexone group experienced hospital admission versus 29% in the combined behavioral and placebo group and 20% in the services as usual group.19
Our study is the only published report, to our knowledge, that utilized patients as their own controls to compare admission outcomes after receiving ER naltrexone during hospitalization. Results of our study augment prior work, as patients who were housed had numerically decreased hospitalizations after receiving naltrexone, but patients experiencing homelessness had increased utilization after treatment. Collins et al19 included outpatient treatment and showed improved harm reduction outcomes in patients receiving combined behavioral and pharmacologic treatment but no difference in hospitalizations between treatment groups. Our population included a large proportion of patients experiencing homelessness. These patients are often marginalized from traditional AUD treatment that aims for complete alcohol abstinence, making ER naltrexone a critical tool in reducing drinking.
Harm reduction outcomes such as reduced number of drinks are challenging to measure and capture retrospectively. Through chart review, baseline self-reported alcohol use was able to be gathered, but subsequent admissions after ER naltrexone administration often had missing data, making comparisons of the number of drinks unavailable. The rate of hospital admission is a critical cost-related variable and can represent a surrogate for morbidity, which is why we chose it as our primary outcome.8 ER naltrexone is traditionally viewed as an outpatient medication by payers. The results of this study did not show decreased health care utilization but did show decreased length of stay in patients with readmissions, which may provide support for policies allowing the use of ER naltrexone at discharge from hospital settings to improve patient-centered outcomes and reduce overall cost.
A large proportion (50%) of patients included in this study had only a single encounter (no hospitalizations before or after receiving a dose of ER naltrexone); to account for this, subgroup analyses were performed excluding this group. Not all patients may have access to outpatient ER naltrexone to continue treatment, which is dependent on follow-up and insurance status. It is important to note that follow-up care after discharge is limited in our community and was unable to be assessed in this study, as most of it is outside of our health care system. Likewise, we were unable to capture hospitalizations or health care utilization outside of our institution. These factors likely contributed to the results. Additionally, offering expensive medication at no cost may bias the findings, as patients who are more motivated to abstain and decrease their alcohol use may opt to receive the medication.20 Finally, given the observational study design, we were unable to determine a causal relationship with the intervention, though we did identify an association. The psychological effect of COVID-19 has had a significant impact on mental health in the general population, thus patients served as their own controls to limit confounding that may occur if we tried to match patients to a different time period. Additionally, the time period selected may have influenced the baseline admission rate, as overall hospitalizations at our institution were down amid the onset of COVID-19 during March–June 2020.
CONCLUSIONS
The administration of ER naltrexone prior to hospital discharge in acute care patients with AUD did not show a significant decrease in health care utilization in either the primary or subgroup analysis but did show a decreased length of hospital stay during readmission. Patients experiencing or at risk for homelessness accounted for the majority of readmissions, and more research is needed to evaluate the impact of homelessness on hospital admission rates for patients with AUD.
Submitted: December 9, 2021; accepted March 2, 2022.
Published online: October 25, 2022.
Relevant financial relationships: None.
Funding/support: None.
ORCID: Alëna A. Balasanova: https://orcid.org/0000-0001-9735-2712
Clinical Points
- Hospitalization offers an intervenable moment to address alcohol use disorder (AUD) in patients.
- More evidence is needed before broadly recommending extended-release naltrexone as a harm reduction tool in hospitalized patients with AUD.
- Social determinants of health, such as housing insecurity, should be considered when evaluating outcomes for hospitalized patients with AUD.
References (20)

- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 2013.
- Substance Abuse and Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health (HHS Publication No. PEP19-5068, NSDUH Series H-54). SAMHSA website. Published online 2019. Accessed September 20, 2022. https://www.samhsa.gov/data/
- Esser MB, Sherk A, Liu Y, et al. Morbidity and Mortality Weekly Report (MMWR). Deaths and Years of Potential Life Lost From Excessive Alcohol Use-United States, 2011–2015. 2020;69(39):1428–1433. CDC website. Accessed September 20, 2022. https://www.cdc.gov/mmwr/volumes/69/wr/mm6939a6.htm
- Russo CA. Elixhauser A. Hospitalizations for Alcohol Abuse Disorders, 2003. HCUP Statistical Brief #4. AHRQ website. Published online 2006. Accessed September 20, 2022. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb4.pdf
- Sacks JJ, Gonzales KR, Bouchery EE, et al. 2010 national and state costs of excessive alcohol consumption. Am J Prev Med. 2015;49(5):e73–e79. PubMed CrossRef
- Kranzler HR, Soyka M. Diagnosis and pharmacotherapy of alcohol use disorder: a review. JAMA. 2018;320(8):815–824. PubMed CrossRef
- Reus VI, Fochtmann LJ, Bukstein O, et al. The American Psychiatric Association practice guideline for the pharmacological treatment of patients with alcohol use disorder. Am J Psychiatry. 2018;175(1):86–90. PubMed CrossRef
- Baser O, Chalk M, Rawson R, et al. Alcohol Dependence Treatments: Comprehensive Healthcare Costs, Utilization Outcomes, and Pharmacotherapy Persistence. 2011. Accessed September 20, 2022. AJMC website. www.ajmc.com
- Kranzler HR, Wesson DR, Billot L; DrugAbuse Sciences Naltrexone Depot Study Group. Naltrexone depot for treatment of alcohol dependence: a multicenter, randomized, placebo-controlled clinical trial. Alcohol Clin Exp Res. 2004;28(7):1051–1059. PubMed CrossRef
- Garbutt JC, Kranzler HR, O’Malley SS, et al; Vivitrex Study Group. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293(13):1617–1625. PubMed CrossRef
- Borg B, Douglas IS, Hull M, et al. Alcohol misuse and outpatient follow-up after hospital discharge: a retrospective cohort study. Addict Sci Clin Pract. 2018;13(1):24. PubMed CrossRef
- Wei J, Defries T, Lozada M, et al. An inpatient treatment and discharge planning protocol for alcohol dependence: efficacy in reducing 30-day readmissions and emergency department visits. J Gen Intern Med. 2015;30(3):365–370. PubMed CrossRef
- Stephens JR, Moore C, Stepanek KV, et al. Implementation of a process for initiating naltrexone in patients hospitalized for alcohol detoxification or withdrawal. J Hosp Med. 2018;13(4):221–228. PubMed CrossRef
- Espiridion ED. A retrospective study of hospital recidivism among patients with alcohol use disorders treated with intramuscular naltrexone. Cureus. 2019;11(12):e6287. PubMed CrossRef
- Beatty A, Stock C. Efficacy of long-acting, injectable versus oral naltrexone for preventing admissions for alcohol use disorder. Ment Health Clin. 2018;7(3):106–110. PubMed CrossRef
- Busch AC, Denduluri M, Glass J, et al. Predischarge injectable versus oral naltrexone to improve postdischarge treatment engagement among hospitalized veterans with alcohol use disorder: a randomized pilot proof-of-concept study. Alcohol Clin Exp Res. 2017;41(7):1352–1360. PubMed CrossRef
- Hartung DM, McCarty D, Fu R, et al. Extended-release naltrexone for alcohol and opioid dependence: a meta-analysis of healthcare utilization studies. J Subst Abuse Treat. 2014;47(2):113–121. PubMed CrossRef
- Mark TL, Montejano LB, Kranzler HR, et al. Comparison of healthcare utilization among patients treated with alcoholism medications. Am J Manag Care. 2010;16(12):879–888. PubMed CrossRef
- Collins SE, Duncan MH, Saxon AJ, et al. Combining behavioral harm-reduction treatment and extended-release naltrexone for people experiencing homelessness and alcohol use disorder in the USA: a randomized clinical trial. Lancet Psychiatry. 2021;8(4):287–300. PubMed CrossRef
- Malone M, McDonald R, Vittitow A, et al. Extended-release vs oral naltrexone for alcohol dependence treatment in primary care (XON). Contemp Clin Trials. 2019;81:102–109. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite