Objective: To identify potential correlations between baseline plasma insulin level and shifts in metabolic status in psychiatric inpatients.
Methods: A population of 75 patients was identified for this single-center, retrospective study conducted at a state psychiatric hospital in Kansas City, Missouri. Patients were selected on the basis of presence of a baseline fasting plasma insulin level drawn at admission; duration of stay ≥ 12 weeks between August 1, 2013, and December 31, 2015; and presence of metabolic laboratory and weight measurements at baseline and 3 months. Total initial plasma insulin level (≥ 19 µIU/mL or < 19 µIU/mL) was compared to shifts in metabolic laboratory measurements and weight. A secondary analysis was performed to detect association between the numerical values of assessed parameters and the numerical values for plasma insulin measurements.
Results: The primary analysis found no correlation between plasma insulin level and changes in any metabolic parameter category at 3 (n = 75) or 6 months (n = 30) after admission. Secondary analysis found significant correlations between the numerical values of baseline total plasma insulin level and fasting plasma glucose level at baseline (r = 0.492, P < .001), 3 months (r = 0.247, P = .035), and 6 months (r = 0.723, P < .001). Secondary analysis demonstrated a significant correlation between baseline total plasma insulin level and hemoglobin A1c values at baseline (r = 0.329, P = .004), 3 months (r = 0.455, P < .001), and 6 months (r = 0.517, P = .003).
Conclusions: Baseline total plasma insulin levels were strongly correlated with parameters affected directly by alterations in glucose up to 6 months after admission but were weakly correlated or not correlated with other metabolic parameters. The results do not support routine use of plasma insulin as a predictor for shifts in metabolic parameters in patients receiving antipsychotic medications.
Plasma Insulin Level Correlation to Metabolic Parameter Shifts in Hospitalized Psychiatric Patients

ABSTRACT
Objective: To identify potential correlations between baseline plasma insulin level and shifts in metabolic status in psychiatric inpatients.
Methods: A population of 75 patients was identified for this single-center, retrospective study conducted at a state psychiatric hospital in Kansas City, Missouri. Patients were selected on the basis of presence of a baseline fasting plasma insulin level drawn at admission; duration of stay ≥ 12 weeks between August 1, 2013, and December 31, 2015; and presence of metabolic laboratory and weight measurements at baseline and 3 months. Total initial plasma insulin level (≥ 19 µIU/mL or < 19 µIU/mL) was compared to shifts in metabolic laboratory measurements and weight. A secondary analysis was performed to detect association between the numerical values of assessed parameters and the numerical values for plasma insulin measurements.
Results: The primary analysis found no correlation between plasma insulin level and changes in any metabolic parameter category at 3 (n = 75) or 6 months (n = 30) after admission. Secondary analysis found significant correlations between the numerical values of baseline total plasma insulin level and fasting plasma glucose level at baseline (r = 0.492, P < .001), 3 months (r = 0.247, P = .035), and 6 months (r = 0.723, P < .001). Secondary analysis demonstrated a significant correlation between baseline total plasma insulin level and hemoglobin A1c values at baseline (r = 0.329, P = .004), 3 months (r = 0.455, P < .001), and 6 months (r = 0.517, P = .003).
Conclusions: Baseline total plasma insulin levels were strongly correlated with parameters affected directly by alterations in glucose up to 6 months after admission but were weakly correlated or not correlated with other metabolic parameters. The results do not support routine use of plasma insulin as a predictor for shifts in metabolic parameters in patients receiving antipsychotic medications.
Prim Care Companion CNS Disord 2017;19(2):16m02086
https://doi.org/10.4088/PCC.16m02086
© Copyright 2017 Physicians Postgraduate Press, Inc.
aCenter for Behavioral Medicine, Kansas City, Missouri
bUniversity of Missouri-Kansas City School of Pharmacy, Kansas City
*Corresponding author: Leigh Anne Nelson, PharmD, BCPP, University of Missouri-Kansas City School of Pharmacy, 2464 Charlotte St, Kansas City, MO 64108 ([email protected]).
The struggle to identify patients most at risk for metabolic complications of antipsychotic use has been ongoing since the introduction of second-generation antipsychotics (SGAs) into common practice. In psychiatric patient populations, metabolic complications of SGAs and other select psychotropic medications compound with preexisting risks of cardiovascular disease to create a greatly increased risk of morbidity and mortality.1 Metabolic abnormalities include development of insulin resistance, elevated triglycerides, elevations in low-density lipoprotein (LDL) and very LDL cholesterol, weight gain, and increased body mass index. The yet uncharacterized mechanism behind these changes predisposes patients to development of prediabetes and type 2 diabetes.
The characterization of metabolic syndrome began in the 1980s with examinations of blood glucose, cholesterol parameters, body mass index, and insulin resistance that together indicate what was termed dysmetabolic syndrome.2 As part of the research evaluating metabolic syndrome, insulin levels were examined for possible correlation with the development of cardiovascular disease, diabetes, and hypertension.2 Several studies3,4 specifically evaluated general populations of patients for a correlation between fasting plasma insulin levels and future development of these same complications. The studies3,4 found that these otherwise "normal populations" (eg, those with no record of diabetes or other metabolic complications) experienced elevations in plasma insulin levels prior to the development of more advanced metabolic complications. Despite the apparent causal link between therapy with psychotropic medications and metabolic complications, the relationship between plasma insulin levels and development of metabolic complications in psychiatric patients has not yet been explored.
The primary objective of this study was to identify correlations between baseline plasma insulin levels of either ≥ 19 µIU/mL or < 19 µIU/mL and development of shifts in metabolic or weight parameter categories during hospitalization in psychiatric patients with a length of stay ≥ 12 weeks. The secondary objective of the study was to identify associations between the numerical values of baseline plasma insulin levels and the numerical values of associated metabolic laboratory values and weight.
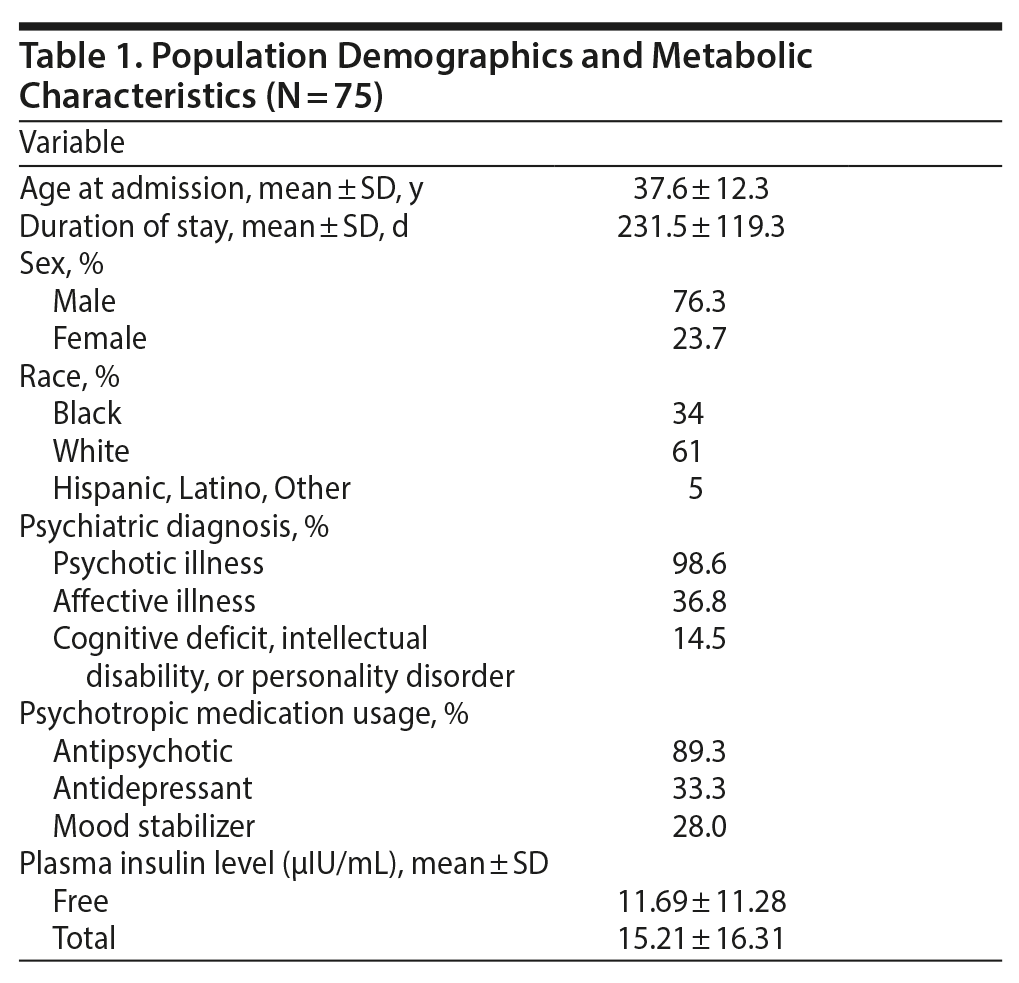
Demographic data including age, sex, race, medications, and diagnosis were collected for descriptive purposes (Table 1). Age, sex, and race were analyzed to determine if baseline plasma insulin levels correlated significantly with any of these factors.
METHODS
This single-center, retrospective chart review was conducted at the Center for Behavioral Medicine, a state psychiatric hospital in Kansas City, Missouri. The protocol for the study was approved by the University of Missouri-Kansas City Institutional Review Board and the Missouri Department of Mental Health.
Study inclusion required patients to be aged ≥ 18 years with a duration of stay ≥ 12 weeks between August 1, 2013, and December 31, 2015; a baseline laboratory assessment including a fasting plasma insulin level, fasting lipid panel, and fasting plasma glucose or hemoglobin A1c (HbA1c) measurement; a second laboratory assessment including a fasting lipid panel and a fasting plasma glucose or HbA1c measurement; and at least 2 weight measurements recorded during their stay. Patients were excluded if they were admitted with a diagnosis of metabolic syndrome, type 1 or 2 diabetes, familial hypercholesterolemia, or other familial metabolic abnormality.

- Plasma insulin level is correlated primarily with metabolic parameters that reflect glucose homeostasis at baseline, 3 months, and 6 months.
- Plasma insulin levels do not appear to be highly correlated with clinically significant changes in metabolic parameters in the short-term inpatient forensic setting.
Study data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at University of Missouri-Kansas City.5 REDCap is a secure, web-based application designed to support data capture for research studies as well as to perform basic descriptive statistical analysis and to de-identify patient data prior to analysis.5
Statistical Analysis
Data analysis was conducted using Minitab 17 Statistical Software.6 Baseline total and free plasma insulin levels were initially compared to each other using the Pearson product moment correlation test. Analysis for primary outcome measures was performed comparing baseline total plasma insulin level categorized as < 19 µIU/mL or ≥ 19 µIU/mL to clinically significant variables. These categories were determined on the basis of evidence suggesting that a cutoff between 2 and 8 times that of patients with insulin concentrations at a "normal level" of ≤ 4 µIU/mL roughly corresponds to a risk of developing prediabetes,3,7 and the clinical laboratory cutoff is 19 µIU/mL.
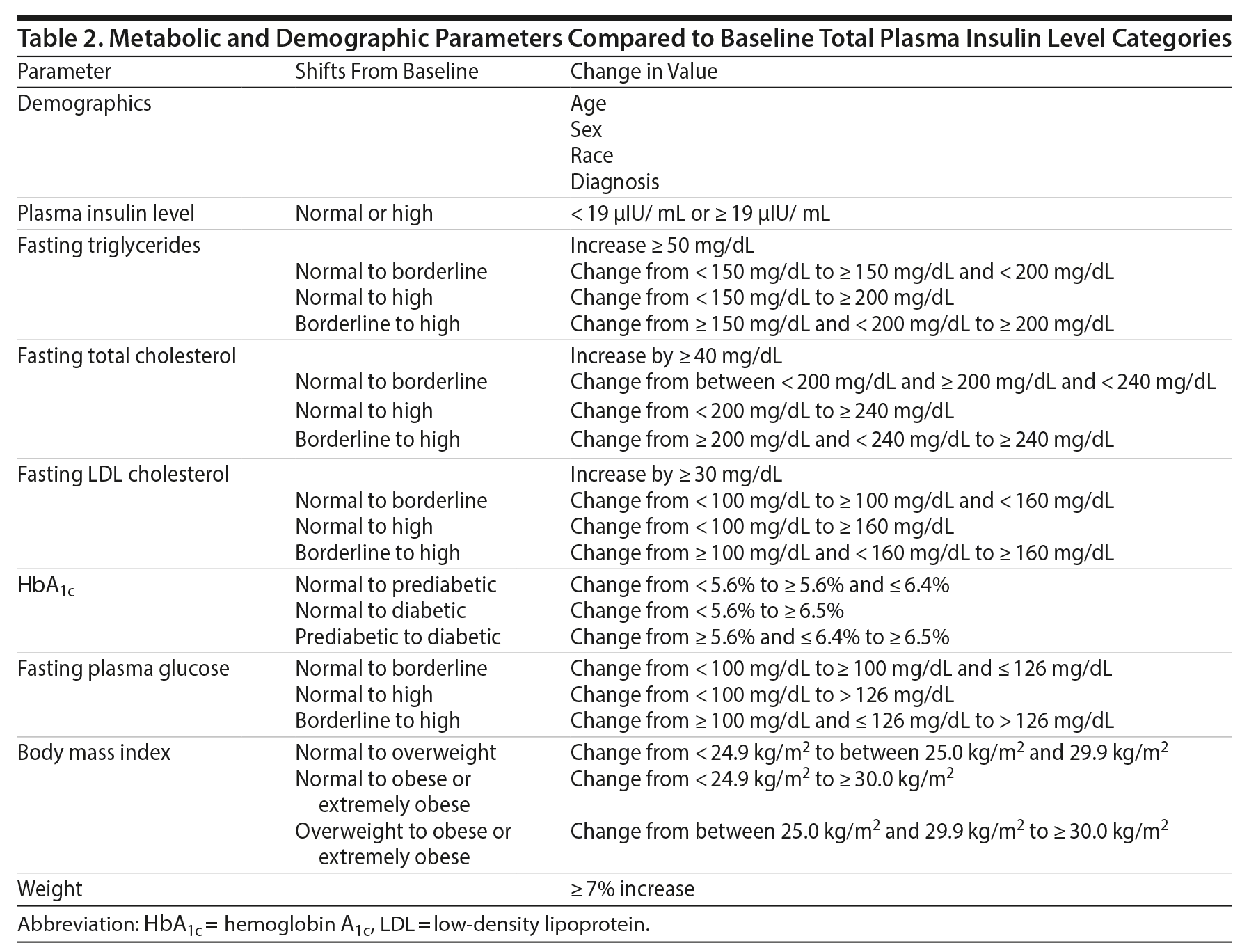
Metabolic parameters that were compared to the baseline total plasma insulin level categories are listed in Table 2. The parameters were derived from US Food and Drug Administration requirements for manufacturer reporting of psychotropic medication-induced metabolic side effects and represent standard cutoffs for clinically significant shifts in metabolic parameters. The designated parameters were compared to the baseline total plasma insulin level cutoff of < 19 µIU/mL or ≥ 19 µIU/mL using the Pearson χ2 test for association. If a given variable occurred < 5 times per group, the variables were compared using Fisher exact test, which is considered to be more appropriate in populations with low rates of event occurrence.
Numerical values of the metabolic parameters presented in Table 3 were compared to the baseline numerical values for total plasma insulin level using the Pearson product moment correlation test. For all comparisons between the variables, significance was assumed if the statistical testing demonstrated P < .05. All calculated P values were 2-sided, and 95% confidence intervals were calculated for each individual value.
RESULTS
An initial group of 119 patients was admitted during the study timeframe. Forty-one patients were excluded due to insufficient laboratory or weight measurements or insufficient duration of stay, and 3 were excluded due to patient duplication. Ultimately, 75 patients met criteria for inclusion in the study. Demographic data for the patient population is summarized in Table 1. Starting from baseline, laboratory and weight assessments were recorded every 3 months until the patient was discharged. The patient population that met the criteria for inclusion most consistently had data available at 3 months from baseline (n = 75). Too few admissions lasted more than 6 months to allow detection of a meaningful difference in metabolic shifts. Because of this limitation, data analysis was not conducted on data collected 6 months after admission.
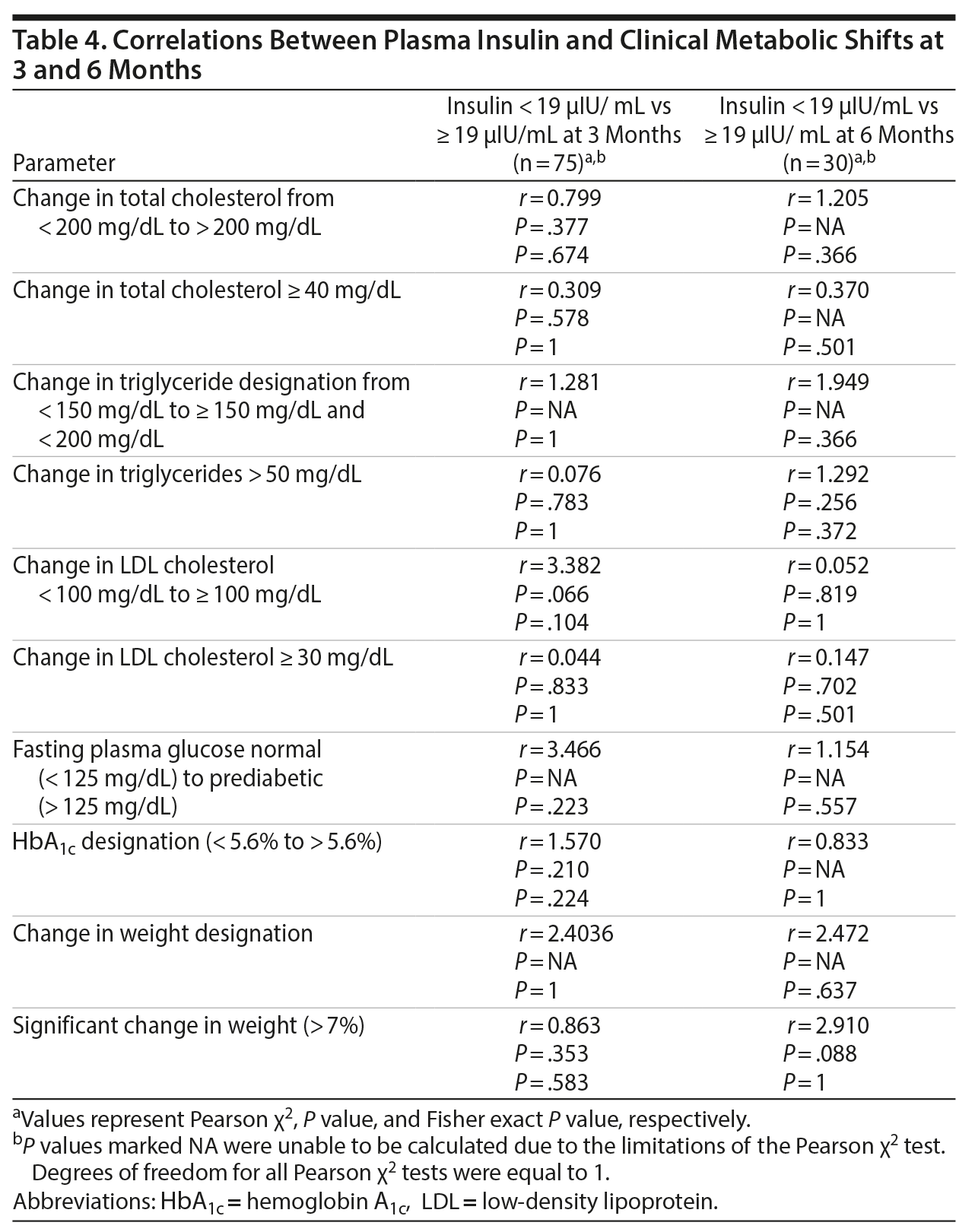
The correlation between total and free insulin levels was statistically significant and very highly linear (r = 0.986, P < .001). Because of the strength of the correlation, only total plasma insulin level measurements were compared to other variables. Baseline total plasma insulin level (< 19 µIU/mL or ≥ 19 µIU/mL) did not demonstrate statistically significant correlations with any of the shifts in metabolic parameters at 3 (n = 75) or 6 (n = 30) months after baseline (Table 4). The parameters presented in Table 4 are representative of the variables in which at least 1 event occurred. There were no significant relationships between age or race and baseline plasma insulin level. Female sex was associated with a significant but seemingly incidental relationship with plasma insulin levels ≥ 19 µIU/mL (r1 = 6.62, P = .010).
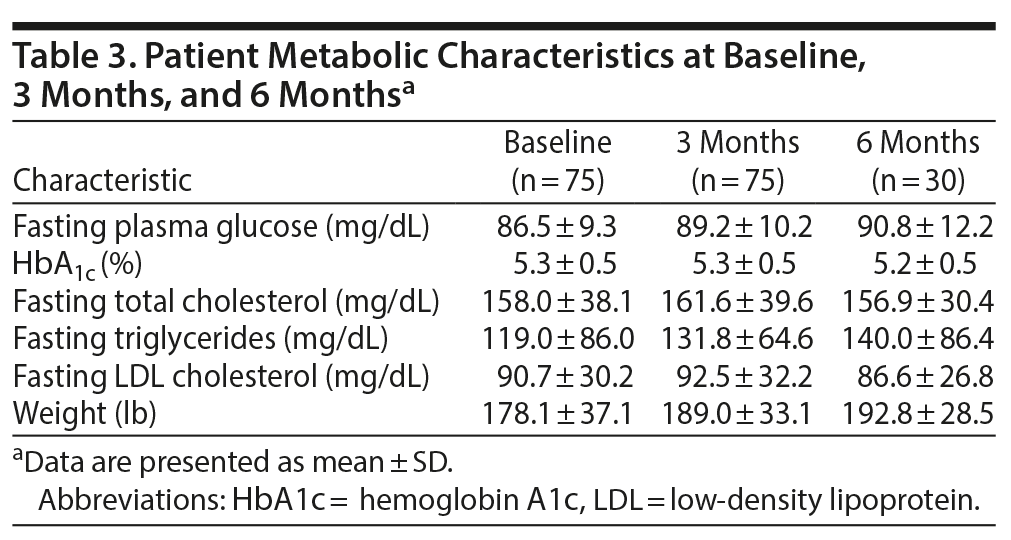
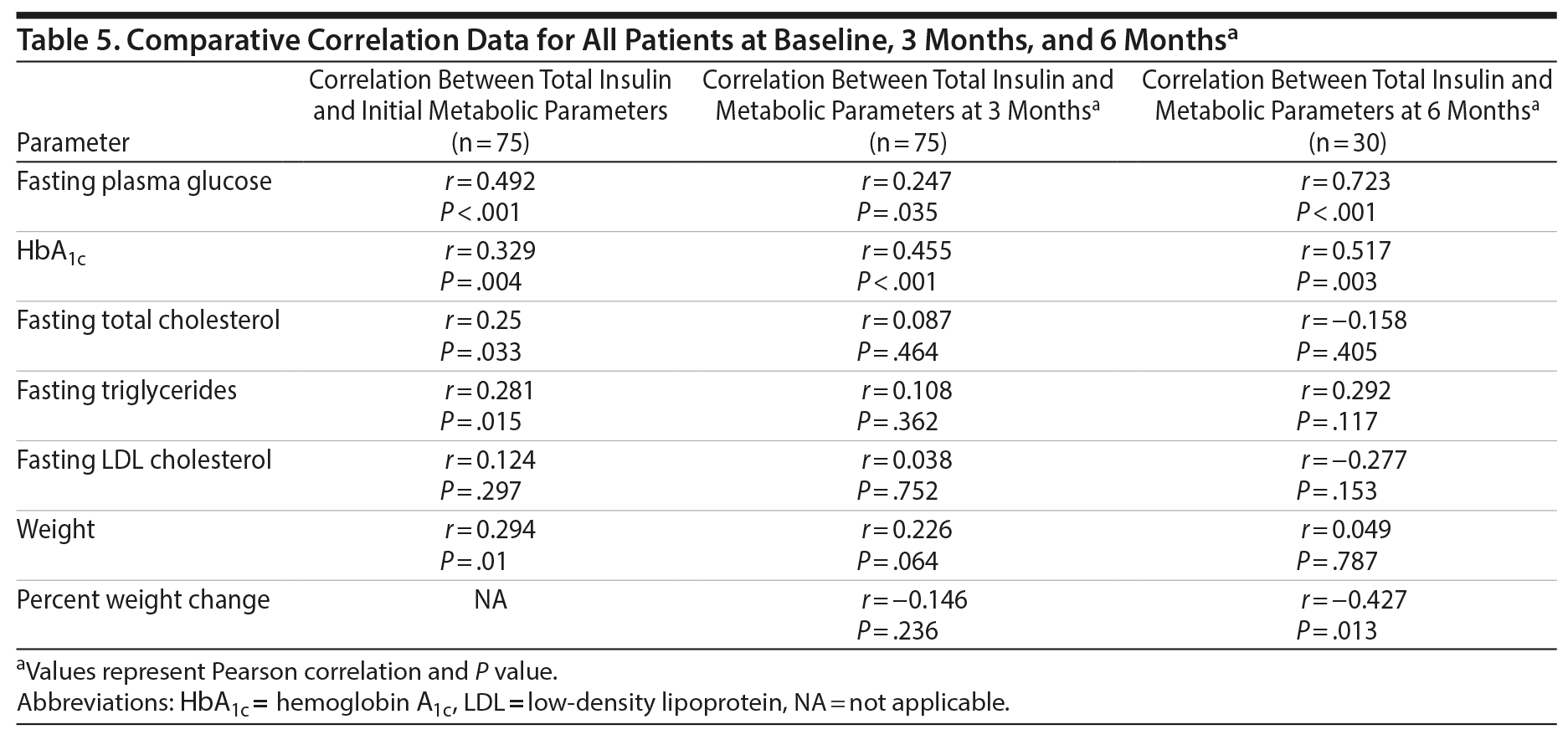
Correlations between the numerical values of total plasma insulin levels and the numerical values of metabolic and weight parameters at baseline (n = 75), 3 months (n = 75), and 6 months (n = 30) are summarized in Table 5. There were significant correlations at 3 and 6 months between baseline total plasma insulin levels and parameters directly reflective of plasma glucose including fasting plasma glucose (3 months: r = 0.247, P = .035 and 6 months: r = 0.723, P < .001) and HbA1c (3 months: r = 0.455, P < .001 and 6 months: r = 0.517, P = .003). Fasting total cholesterol (r = 0.25, P = .033) and fasting triglycerides (r = 0.281, P = .015) were significantly correlated with baseline total plasma insulin level, but the correlations were not maintained at 3 or 6 months. Fasting LDL cholesterol was not correlated with baseline total plasma insulin level.
Weight correlated significantly with baseline total plasma insulin level (r = 0.294, P = .01), but the correlation was not maintained at 3 months or 6 months. Percent change in weight was not correlated at 3 months but was correlated with baseline total plasma insulin level at 6 months.
DISCUSSION
Early identification of metabolic complications presents a particularly vexing problem. The actual mechanism by which psychotropic medications induce metabolic complications has been theorized to involve a variety of different receptor-based mechanisms8,9 and many other potential disturbances in carbohydrate and fatty acid metabolism.10 Studies11 have examined the development of increasing weight and cholesterol parameters in relation to insulin resistance in select patient populations without reaching a clear consensus regarding the cause of these abnormalities. The sequence of events precipitating classic metabolic complications is incompletely understood but is thought to occur most frequently between 1 and 3 months after initiation of select psychotropic medications.12 In 1 study,13 a small group of patients with severe, persistent mental illness presenting with elevated cortisol levels and severe psychiatric illness appeared to have a form of stress-induced insulin resistance, which remitted with effective treatment despite the elevated risk of metabolic complications associated with the medication.
Our study is the first known to examine plasma insulin levels as they relate to changes in metabolic parameters in patients exposed to psychotropic medications. We observed no significant relationships between baseline total plasma insulin level (< 19 µIU/mL or ≥ 19 µIU/mL) and clinically significant shifts in metabolic parameters. There was, however, a significant correlation for the numerical values of the metabolic parameters and the plasma insulin level, suggesting a relationship between insulin and metabolic parameters directly affected by plasma glucose levels from baseline until 6 months.
Our study is limited primarily by small sample size. For many of the parameters assessing clinically significant changes, there were not enough changes during the study period to allow for meaningful comparisons between the parameters and the baseline total plasma insulin level. The study is also limited by a design-based inability to statistically evaluate correlations between initial metabolic parameter classifications and baseline total plasma insulin levels. Our study was designed only to analyze change from baseline values, so patients who had aberrant metabolic parameters at baseline were not considered to meet the requirements for metabolic shifts. This limitation was partially remedied by exclusion criteria specifying metabolic and glucose abnormalities and our assessment of clinically significant shifts in parameters independent of other metabolic alterations (change in total cholesterol ≥ 40 mg/dL, change in triglycerides by > 50 mg/dL, and change in LDL by ≥ 30 mg/dL).
The patient population for the study is derived from patients who may have been receiving psychotropic medications prior to admission, as well as patients who were relatively psychotropic medication naive. Because the study is retrospective in nature, there was no reasonable way to determine the extent of medication use or adherence to medications prior to entering our facility.
CONCLUSION
Baseline total plasma insulin level designations of < 19 µIU/mL or ≥ 19 µIU/mL were not significantly correlated with clinically significant shifts in metabolic parameters. Numerical baseline total plasma insulin level values were significantly associated with parameters directly or partially affected by changes in glucose metabolism including fasting plasma glucose, HbA1c, and triglycerides.
In our patient population, baseline plasma insulin levels do not appear to correlate strongly enough with shifts in metabolic or weight parameters to suggest that total plasma insulin level would be a useful tool to indicate the presence or potential for future development of metabolic abnormalities. However, studies that evaluate multiple insulin levels during the patient’s stay, categorize patients on the basis of their history of antipsychotic use, or target patients with higher risks for metabolic effects may demonstrate more consistent relationships.
Submitted: December 21, 2016; accepted February 17, 2017.
Published online: April 6, 2017.
Potential conflicts of interest: None.
Funding/support: None.
Previous presentation: Poster presented at the College of Psychiatric and Neurologic Pharmacists Annual Meeting; April 18, 2016; Colorado Springs, Colorado.
REFERENCES
1. De Hert M, Correll CU, Bobes J, et al. Physical illness in patients with severe mental disorders, I: prevalence, impact of medications and disparities in health care. World Psychiatry. 2011;10(1):52-77. PubMed doi:10.1002/j.2051-5545.2011.tb00014.x
2. Reaven GM. Banting lecture 1988: role of insulin resistance in human disease. Diabetes. 1988;37(12):1595-1607. PubMed doi:10.2337/diab.37.12.1595
3. Zavaroni I, Bonini L, Gasparini P, et al. Hyperinsulinemia in a normal population as a predictor of non-insulin-dependent diabetes mellitus, hypertension, and coronary heart disease: the Barilla factory revisited. Metabolism. 1999;48(8):989-994. PubMed doi:10.1016/S0026-0495(99)90195-6
4. Laakso M. How good a marker is insulin level for insulin resistance? Am J Epidemiol. 1993;137(9):959-965. PubMed doi:10.1093/oxfordjournals.aje.a116768
5. Harris PA, Taylor R, Thielke R, et al. Research Electronic Data Capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381. PubMed doi:10.1016/j.jbi.2008.08.010
6. Minitab 17 Statistical Software [computer program]. Version 17. State College, PA: Minitab.
7. Johnson JL, Duick DS, Chui MA, et al. Identifying prediabetes using fasting insulin levels. Endocr Pract. 2010;16(1):47-52. PubMed doi:10.4158/EP09031.OR
8. Weston-Green K, Huang X-F, Deng C. Second generation antipsychotic-induced type 2 diabetes: a role for the muscarinic M3 receptor. CNS Drugs. 2013;27(12):1069-1080. PubMed doi:10.1007/s40263-013-0115-5
9. Miron IC, Baroană VC, Popescu F, et al. Pharmacological mechanisms underlying the association of antipsychotics with metabolic disorders. Curr Health Sci J. 2014;40(1):12-17. PubMed
10. Paredes RM, Quinones M, Marballi K, et al. Metabolomic profiling of schizophrenia patients at risk for metabolic syndrome. Int J Neuropsychopharmacol. 2014;17(8):1139-1148. PubMed doi:10.1017/S1461145714000157
11. Dasgupta A, Singh OP, Rout JK, et al. Insulin resistance and metabolic profile in antipsychotic naׯve schizophrenia patients. Prog Neuropsychopharmacol Biol Psychiatry. 2010;34(7):1202-1207. PubMed doi:10.1016/j.pnpbp.2010.06.011
12. Lieberman JA 3rd. Metabolic changes associated with antipsychotic use. Prim Care Companion J Clin Psychiatry. 2004;6(suppl 2):8-13. PubMed
13. Brown TM. Atypical antipsychotics and insulin resistance in acute mental illness: a case series. Prim Care Companion CNS Disord. 2011;13(1):doi:10.4088/PCC.10l00985. PubMed doi:10.4088/pcc.10l00985yel
Please sign in or purchase this PDF for $40.00.
Save
Cite