Prim Care Companion CNS Disord 2022;24(2):21cr02994
To cite: Nendumba G, Boland L, Haufroid V, et al. Intravenous lipid emulsion in a case of trazodone overdose. Prim Care Companion CNS Disord. 2022;24(2):21cr02994.
To share: https://doi.org/10.4088/PCC.21cr02994
© Copyright 2022 Physicians Postgraduate Press, Inc.
aDepartment of Intensive Care, Cliniques St-Luc, Université Catholique de Louvain, Brussels, Belgium
bDepartment of Clinical Chemistry, Cliniques St-Luc, Université Catholique de Louvain, Brussels, Belgium
cLouvain Centre for Toxicology and Applied Pharmacology, Université Catholique de Louvain, Brussels, Belgium
*Corresponding author: Philippe Hantson, MD, PhD, Department of Intensive Care, Cliniques St-Luc, Ave Hippocrate, 10, 1200 Brussels, Belgium ([email protected]).
Major trazodone overdose may be followed by prolonged hypotension and sustained central nervous system (CNS) depression.1 The treatment of trazodone overdose is mainly supportive, but, as the molecule is lipophilic, we used intravenous lipid emulsion (ILE) in a recent case to minimize the risk of cardiovascular and CNS toxicity.
Case Report
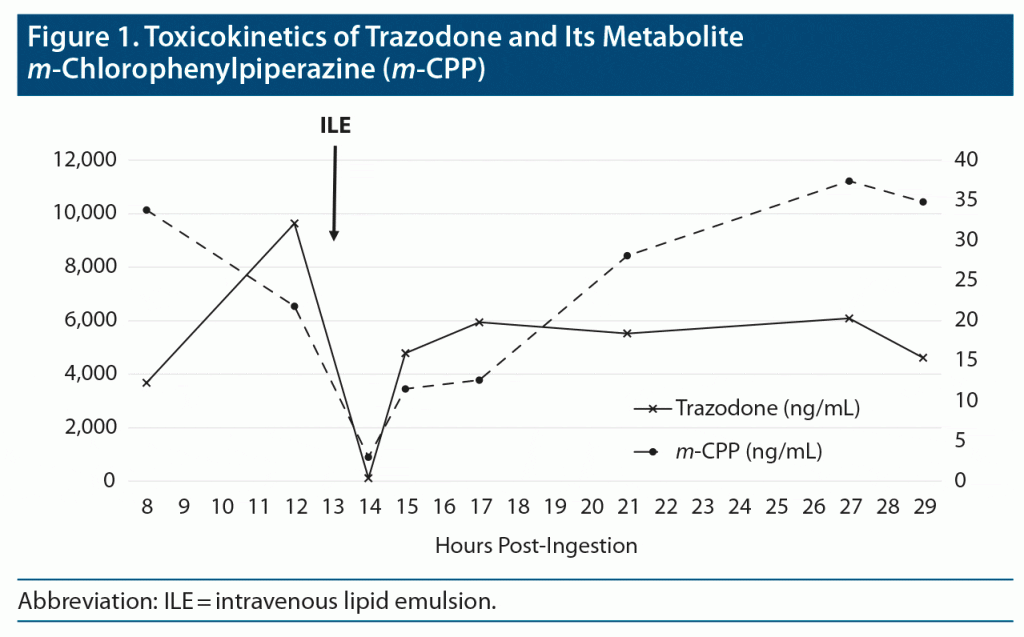
A 49-year-old man was admitted to the hospital 3 hours after the voluntary ingestion of 5,000 mg of immediate-release trazodone with a Glasgow Coma Scale score fluctuating between 11 and 13/15. He had a history of chronic ethanol abuse and depression. His arterial blood pressure was 109/63 mm Hg, heart rate was 65 beats/minute, respiratory rate was 11 breaths/minute, and body temperature was 34.3°C (93.74°F). Routine laboratory investigations were unremarkable. The admission electrocardiogram revealed a corrected QT interval of 489 msec. Due to the perceived risk of further cardiocirculatory or neurologic deterioration, he was transferred to the intensive care unit (ICU), wherein the plasma trazodone concentration 8 hours post ingestion was 3,678 ng/mL (therapeutic range, 500–2,500 ng/mL), but was measured as 9,634 ng/mL 4 hours later (Figure 1). The patient’s physical state was unchanged compared with the first examination in the emergency department. As prolonged absorption/distribution was suspected, a 20% lipid emulsion solution (Intralipid, Fresenius Kabi, India) was administered intravenously as an initial bolus dose of 1.5 mL/kg followed by a 0.25 mL/kg/minute infusion over 30 minutes. Thirty minutes after the end of the ILE infusion, the plasma trazodone concentration had dropped significantly to 111.6 ng/mL, with a rebound up to 4,784 ng/mL 1 hour later. Clinically, arterial blood pressure rose to 139/72 mm Hg, respiratory rate to 18 breaths/minute, and temperature to 35.6°C (96.08°F), while the patient became fully responsive. He experienced no further complications and was discharged from the ICU on day 2. No adverse effects of ILE were recorded.
The terminal elimination half-life of trazodone calculated on 4 data points after the rebound was 32.8 hours (Figure 1). The metabolic ratio of the metabolite m-chlorophenylpiperazine (m-CPP) to the parent compound appeared quite low (0.002–0.009) in comparison with the reference range (0.04–0.22) proposed by Hiemke et al,2 but large interindividual variations in trazodone metabolism are described.3 The genotyping results (cytochrome P450 [CYP] 3A4 *1/*1, CYP2D6 *1/*1) suggested that the patient was an extensive metabolizer.
Discussion
Lethargy and drowsiness are the main presenting symptoms following trazodone overdose, with also the possibility of QT prolongation complicated by torsades de pointes.1 The treatment for trazodone poisoning is mainly supportive. Trazodone is a lipophilic drug with a octanol-water partition coefficient (log P) described at 2.82.4 There is limited evidence for the administration of ILE for trazodone overdose in contrast with the literature available for local anesthetic agents.5,6 Its potential benefit has been mainly investigated for the prevention of cardiotoxicity, but ILE has also been shown to improve consciousness in some patients intoxicated by olanzapine, quetiapine, zopiclone, or sertraline.7 In a previous case8 of trazodone overdose, significant neurologic improvement was observed following the same protocol. Interestingly, the same toxicokinetic profile was noted, with an initial drop in plasma concentration followed by a rebound. This was interpreted as a sequestration of trazodone in a lipid phase (“lipid sink” theory), followed by a redistribution either from tissue or lipid compartment. The rebound may also be explained by the shorter elimination half-life of long-chain triglyceride contained in ILE in comparison with that of trazodone.9 It remains to be determined in other cases of serious trazodone overdose if ILE is useful to prevent CNS or cardiovascular toxicity when administered before the absorption/distribution phase is complete.
Published online: March 8, 2022.
Potential conflicts of interest: None.
Funding/support: None.
Patient consent: Written consent was obtained from the patient to publish the case report, and information has been de-identified to protect anonymity.
References (9)

- de Meester A, Carbutti G, Gabriel L, et al. Fatal overdose with trazodone: case report and literature review. Acta Clin Belg. 2001;56(4):258–261. PubMed CrossRef
- Hiemke C, Bergemann N, Clement HW, et al. Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry. 2018;51(1-02):9–62. PubMed CrossRef
- Ishida M, Otani K, Kaneko S, et al. Effects of various factors on steady state plasma concentrations of trazodone and its active metabolite m-chlorophenylpiperazine. Int Clin Psychopharmacol. 1995;10(3):143–146. PubMed CrossRef
- Pallicer JM, Rosés M, Ràfols C, et al. Evaluation of log Po/w values of drugs from some molecular structure calculation software. ADMET & DMPK. 2014;2(2):107–114. CrossRef
- Taylor R, Burg J, Mullen J. A case of trazodone overdose successfully rescued with lipid emulsion therapy. Cureus. 2020;12(10):e10864. PubMed CrossRef
- Paneta M, Waring WS. Literature review of the evidence regarding intravenous lipid administration in drug-induced cardiotoxicity. Expert Rev Clin Pharmacol. 2019;12(7):591–602. PubMed CrossRef
- Taftachi F, Sanaei-Zadeh H, Sepehrian B, et al. Lipid emulsion improves Glasgow coma scale and decreases blood glucose level in the setting of acute non-local anesthetic drug poisoning–a randomized controlled trial. Eur Rev Med Pharmacol Sci. 2012;16(suppl 1):38–42. PubMed
- Warnant A, Gerard L, Haufroid V, et al. Coma reversal after intravenous lipid emulsion therapy in a trazodone-poisoned patient. Clin Neuropharmacol. 2020;43(1):31–33. PubMed CrossRef
- Bae SI, Sohn JT. Lipid emulsion treatment for trazodone toxicity-induced coma. Clin Neuropharmacol. 2020;43(6):201. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite