ABSTRACT
Objective: To evaluate real-world treatment patterns for patients initiating benztropine and to understand treatment approaches in patients with drug-induced movement disorders from a health care provider perspective.
Methods: A retrospective claims analysis was conducted among patients with evidence of benztropine initiation from January 2017 through March 2020 to assess treatment patterns and patient health care resource utilization. Subsequently, a 30-minute, United States–based online survey fielded from December 2021 to January 2022 was sent to physicians, nurse practitioners, and physician assistants who reported a primary care or psychiatry specialty currently treating drug-induced movement disorders and prescribed benztropine.
Results: The health care claims analysis included 112,542 patients. Polypharmacy and multiple comorbidities were frequent characteristics in this population; 54.1% of patients had ≥ 2 comorbidities at baseline, and 59.1% had claims for > 10 medications. Benztropine was used for > 3 months in > 50% of the population. Health care costs and resource utilization were high, with mean all-cause pharmacy and outpatient costs totaling $11,755. Survey results from 349 primary care or psychiatry health care providers indicated that benztropine is often used in non–tardive dyskinesia drug-induced movement disorders but frequently continued for > 3 months or used in tardive dyskinesia. In this study, psychiatry providers prescribed benztropine in line with guideline recommendations more often than primary care providers; however, < 40% indicated familiarity with 2020 American Psychiatric Association Practice Guideline for the Treatment of Patients with Schizophrenia.
Conclusions: These complementary analyses suggest that benztropine is used long-term in non–tardive dyskinesia drug-induced movement disorders and in tardive dyskinesia despite risks of worsening tardive dyskinesia or adverse effects.
Prim Care Companion CNS Disord 2023;25(4):22m03472
Author affiliations are listed at the end of this article.
Drug-induced movement disorders (DIMDs) are a subgroup of movement disorders that have been linked with certain medications and include acute dystonia, drug-induced parkinsonism, tardive dystonia, and tardive dyskinesia (TD).1 Causal medications of DIMDs include antiemetics, antimicrobials, antipsychotics, mood stabilizers, and antidepressants.2–4
TD is a persistent and potentially disabling movement disorder associated with prolonged exposure to dopamine receptor–blocking agents that are prescribed to treat psychiatric conditions such as schizophrenia, major depressive disorder, or bipolar disorder.5,6 Diagnostic criteria for TD include involuntary athetoid or choreiform movements, which can occur in any part of the body, but most commonly in the tongue, lower face, jaw, and extremities, that last for at least 3 months in adults or 1 month in geriatric patients with dopamine receptor–blocking agent use.1 TD can occur during drug exposure or within weeks of discontinuation or dose reduction.3,4 TD is often underdiagnosed due to the overlap between DIMDs, historical categorization of TD and other DIMDs as extrapyramidal symptoms (EPS), and limited awareness of the number of drugs that can cause TD. As such, patients may receive suboptimal or delayed treatment and experience reduced quality of life due to TD symptoms.3
Historical TD treatment options included dose reduction of the offending medication or changing medication. However, because recurrent symptoms or relapse in the mental health condition being treated can occur, these options are not recommended by the American Academy of Neurology (AAN) or the American Psychiatric Association (APA).7–9 Clonazepam has been used with some success, although it is associated with side effects and is recommended only for short-term treatment as tolerance can develop.8 The reversible vesicular monoamine transporter 2 (VMAT2) inhibitors, valbenazine and deutetrabenazine, are recommended as first-line treatment in TD by both APA guidelines and the AAN guideline update recommendations and are the only US Food and Drug Administration (FDA)–approved TD treatments.7,9–11 The APA recommends a VMAT2 inhibitor in patients with moderate-to-severe or disabling TD or for consideration in patients with mild TD if functional impact is present.7
Benztropine, a medication with anticholinergic properties, can be used short-term for a subset of other DIMDs, such as parkinsonism or dystonia.7,12 Evidence has shown that benztropine can worsen TD symptoms.7,9,13 Indeed, in the benztropine prescribing information, TD is included under precautions, and benztropine is specifically not recommended for use in TD.12 As such, the differential diagnosis of TD from other DIMDs is essential in providing appropriate treatment. Because of the partial overlap between some DIMD symptoms, health care providers (HCPs) may use benztropine when it is not beneficial for the patient. Potential examples include using benztropine long-term (eg, > 3 months), which may be associated with serious side effects, particularly in the geriatric population; increasing anticholinergic burden, which can worsen cognitive function in schizophrenia and add to the risk of TD; and use in TD, which can worsen TD symptoms.7,14,15
Given these concerns, we sought to evaluate real-world treatment patterns for patients initiating benztropine and to understand the approach to treatment in patients receiving benztropine from an HCP perspective.
METHODS
Two complementary studies were conducted to assess how and why benztropine is being used in patients with psychiatric disorders. An advisory board of 9 active clinicians specializing in psychiatry was convened in June 2021 to understand benztropine use in DIMDs and to align on recommendations for use of medications with anticholinergic properties in DIMDs. The generated advisory board consensus statements were used to inform the health care claims analysis and the HCP survey.16
Health Care Claims Analysis
The first study aimed to explore HCPs’ real-world treatment and prescribing patterns of benztropine using a retrospective health care claims analysis of patients initiating benztropine in the United States (US) who had commercial insurance, Medicare/Medicare Part D, or Medicaid. Data were obtained from IQVIA’s New Data Warehouse, which includes prescription, medical, and hospital charge detail master (CDM) databases. The prescription database includes patient demographics and prescription details on dispensed prescriptions from retail, mail, long-term care, and specialty pharmacies. The medical claims database includes patient demographics, diagnoses, and medical and service procedures on pre-adjudicated claims from 800,000 physician and specialist offices. The CDM database includes service order records from inpatient and outpatient procedures that occurred during hospitalization and contains data related to diagnoses, procedures, and drugs and devices used. Patients were linked between databases via a deterministic matching algorithm that used patient information such as age, gender, and number of visits to ensure continuity across datasets.
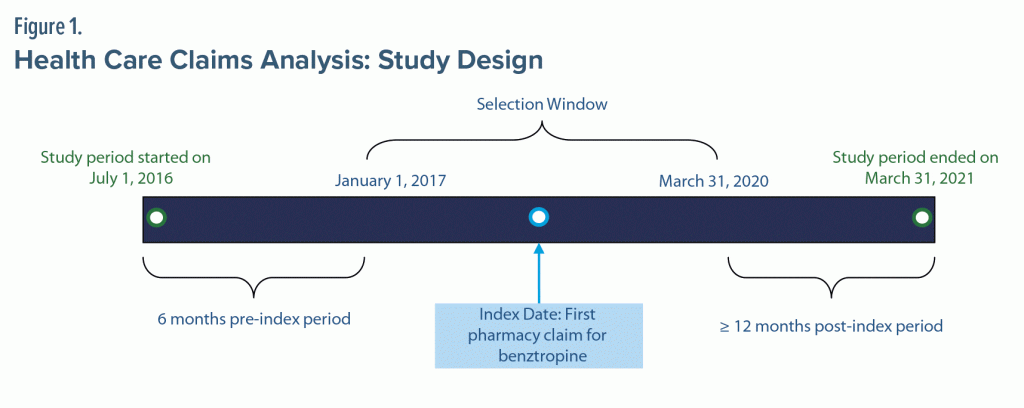
The study period began July 1, 2016, and continued through March 31, 2021 (Figure 1). Patients were identified for inclusion from January 1, 2017, through March 31, 2020. The index date was the first pharmacy claim for benztropine. Adult patients (≥ 18 years old) were included if they had ≥ 2 pharmacy claims for benztropine occurring on different days, continuous enrollment in benefits for at least 6 months prior to the index date (pre-index) and at least 12 months after the index date (post-index period; defined as ≥ 1 office visit or pharmacy claim in pre- and post-index periods and ≥ 1 claim beyond both intervals), consistent reporting from pharmacy most frequently used, and ≥ 1 claim for an antipsychotic 6 months before or on the index date. Cohorts were identified with minimum post-index durations of 12 months and 24 months; patients with up to 24 months of data were included as a subgroup. Patients were excluded if there was evidence of benztropine use before the index date or if there were data quality issues (eg, invalid date of birth, missing gender).
Outcomes assessed included demographic and clinical characteristics, duration of benztropine use and prescription fills, anticholinergic burden, all-cause health care resource utilization (HCRU), and all-cause costs associated with all medical and pharmacy claims. Demographics and clinical characteristics were assessed at baseline for the total population. Prescription data, HCRU, and costs were assessed in the post-index period (≥ 12 months). Anticholinergic burden was calculated using the anticholinergic burden calculator (ACB; https://www.acbcalc.com/).17 Medications with anticholinergic properties are scored as 1 (mild anticholinergic burden [eg, diazepam]), 2 (moderate anticholinergic burden), or 3 (severe anticholinergic burden [eg, benztropine]). As patients could receive multiple medications with anticholinergic properties, the categories were not mutually exclusive. All-cause costs per patient for 12- and 24-month post-index periods were calculated and reported as 2021 US dollars (USD). HCRU and costs of emergency department (ED) visits, outpatient visits, and inpatient admissions were also reported. Inpatient stays were based on patients with linkage to the CDM data, which included 30% of the total patients. Inpatient results were extrapolated to the full population by dividing by 0.3. Results are reported as descriptive statistics.
Health Care Provider Survey
To gain insight into the management of patients receiving benztropine from an HCP’s perspective, US HCPs specializing in primary care or psychiatry were invited to respond to a 30-minute, internet-based, double-blinded quantitative survey from December 2021 to January 2022. Physicians, nurse practitioners (NPs), and physician assistants (PAs) were included if they treated ≥ 2 patients with DIMDs (primary care physicians) or ≥ 3 patients with DIMDs (psychiatric physicians and all NPs and PAs) and prescribed benztropine. Additionally, their practice must have been ≥ 70% adults with ≥ 60% of time dedicated to seeing patients in the office, clinic, or telemedicine. Survey participants had to provide consent to participate and were compensated for completing the survey. The study was exempted from the WCG institutional review board approval, as the research included only survey procedures and there were adequate provisions to protect the privacy of the participants and confidentiality of the data.18 Results are reported as descriptive statistics, and significant difference between groups was determined using 95% confidence intervals.
RESULTS
Health Care Claims Analysis
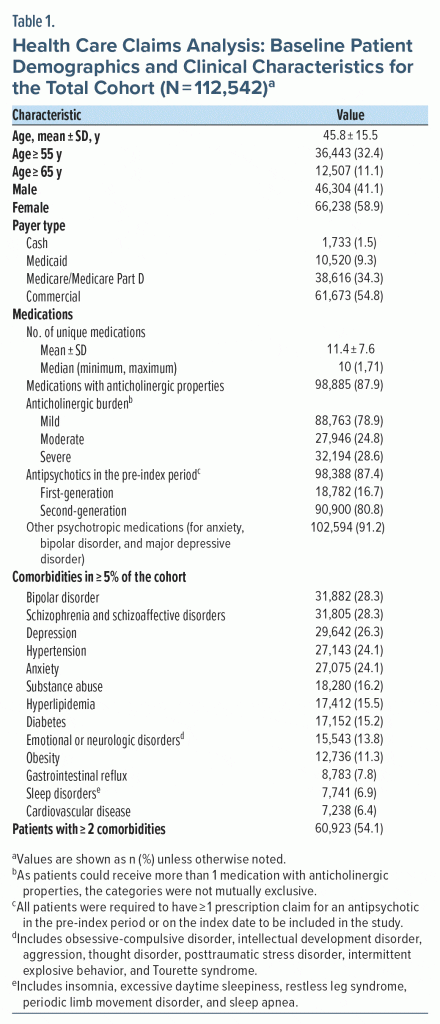
A total of 738,207 patients with ≥ 2 non–same-day pharmacy claims for benztropine were identified between January 1, 2017, and March 31, 2020. After the study criteria were applied, 112,542 patients were included in the 12-month analysis (total cohort), with 73,836 having at least 24 months of post-index data (24-month cohort) (Supplementary Table 1). The mean age of the total cohort was 45.8 years, with 32% of patients aged 55 years or older (Table 1). Most patients were female (58.9%) and had commercial insurance (54.8%). Over half (54.1%) of patients had ≥ 2 comorbid conditions at baseline; the most common conditions were bipolar disorder (28.3%), schizophrenia and schizoaffective disorders (28.3%), and depression (26.3%). Additionally, 87.9% of patients were receiving at least 1 medication with anticholinergic properties at baseline. Moderate-to-severe anticholinergic burden was calculated in 53.4% of patients (groups were not mutually exclusive).17 Polypharmacy was frequent, with 59.1% of the total cohort having claims for > 10 medications, and of those, 19.8% had claims for > 20 medications. Similarly, psychotropic medications for anxiety, bipolar disorder, or depression were used by 91.2% of the total cohort in the pre-index period.
In the total cohort, the median number of prescription fills for benztropine was 5, though 31.4% of patients had > 8 claims for benztropine. Benztropine was received for up to 3 months by 44.3% of patients, > 3 to 6 months by 17.4%, > 6 to 12 months by 25.6%, and > 12 to 24 months by the remaining 12.7% patients. Benztropine was most commonly prescribed by physicians with a psychiatry specialty (52.6%) and NPs (31.0%). Results were similar in the 24-month cohort (Supplementary Table 2).
In the 6-month pre-index period, mean ± SD all-cause total health care costs were $5,355 ± $8,413. In the 12-month post-index period, mean all-cause total health care costs (ie, pharmacy and outpatient costs) were $11,755 ± $22,577), with the majority of costs attributed to pharmacy ($9,229 ± $21,526) (Supplementary Table 2). In patients with available inpatient CDM data (n = 33,717), inpatient stays occurred in 4.0% (13.3% when extrapolated to all patients) with a mean cost of $34,669 ($56,979). ED visits occurred in 47.3% and physician office visits in 78.9% of the total cohort. Mean total health care costs in the 24-month cohort were $23,128 ($30,914), with pharmacy remaining the highest contributor to costs. Inpatient stays occurred in 21.9% of patients with 24-month data when extrapolated to all patients.
Health Care Provider Survey
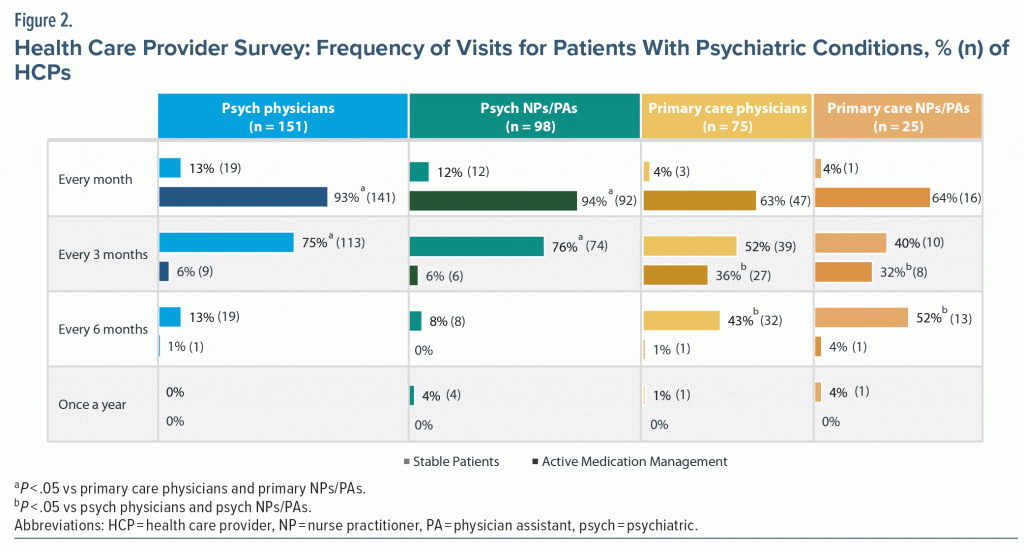
A total of 151 physicians and 98 NP/PAs in psychiatry and 75 physicians and 25 NP/PAs in primary care (N = 349) completed the survey (demographic information in Supplementary Table 3). Among all HCPs, tremor (24.5%), TD (24.4%), and akathisia (20.7%) were the most commonly reported DIMD diagnoses. HCPs were asked about the frequency of visits and evaluation and monitoring of their patients with psychiatric conditions based on if active medication management was needed or if the patient was stable (ie, effective and well-tolerated regimen) on their medication. Patients with active medication management were typically seen monthly, whereas stable patients were more likely to be seen every 3 months (Figure 2). Frequency of DIMD evaluation and monitoring was more common among psychiatric HCPs, with psychiatric physicians evaluating DIMD in 77% of visits and psychiatric NPs/PAs in 87% of visits versus 69% of visits with primary care HCPs. Psychiatric HCPs were also more likely to have a policy in place for recommended frequency of DIMD evaluation (77% vs 13% in primary care settings).
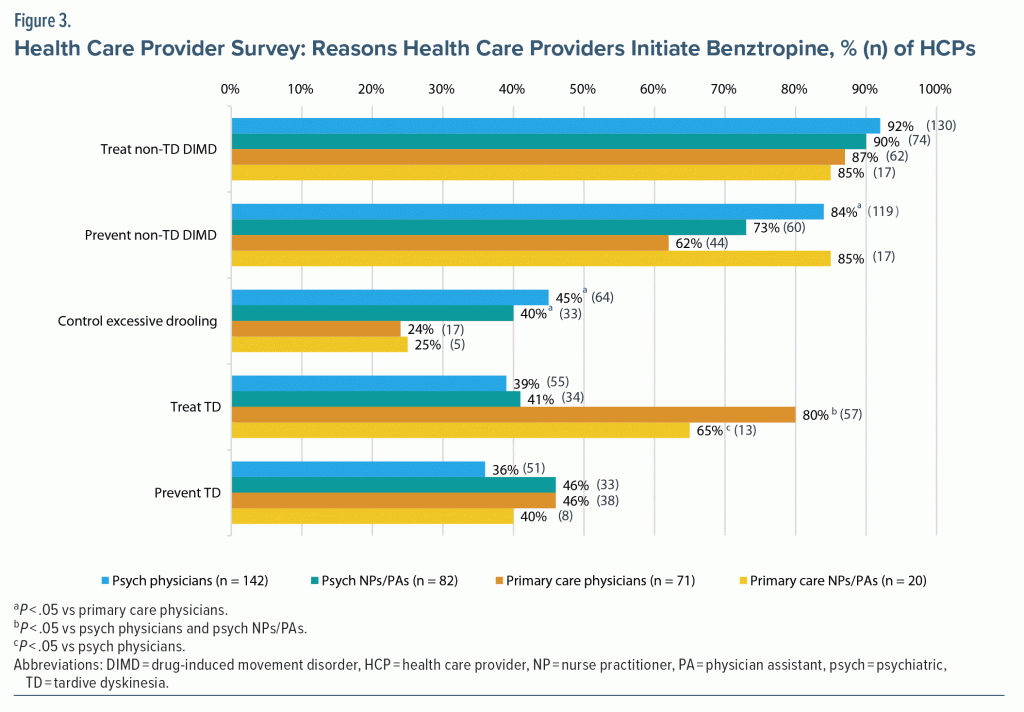
When asked about benztropine use in DIMD, HCPs reported the most common reasons for initiating benztropine were treatment or prevention of non-TD DIMD (ie, EPS); treatment of TD was also a common reason, ranging from 39% (n = 55) of psychiatric physicians to 80% (n = 57) of primary care physicians (Figure 3). When given patient characteristics that can be associated with DIMD and the likelihood to use benztropine, HCPs in primary care were significantly more likely than psychiatric HCPs to use benztropine in patients with TD (P < .05; Supplementary Figure 1). Approximately 60% to 70% of primary care physicians and 30% to 40% of psychiatric physicians responded that they were likely to use benztropine in patients ≥ 65 years or those with cognitive decline or a history of falls.
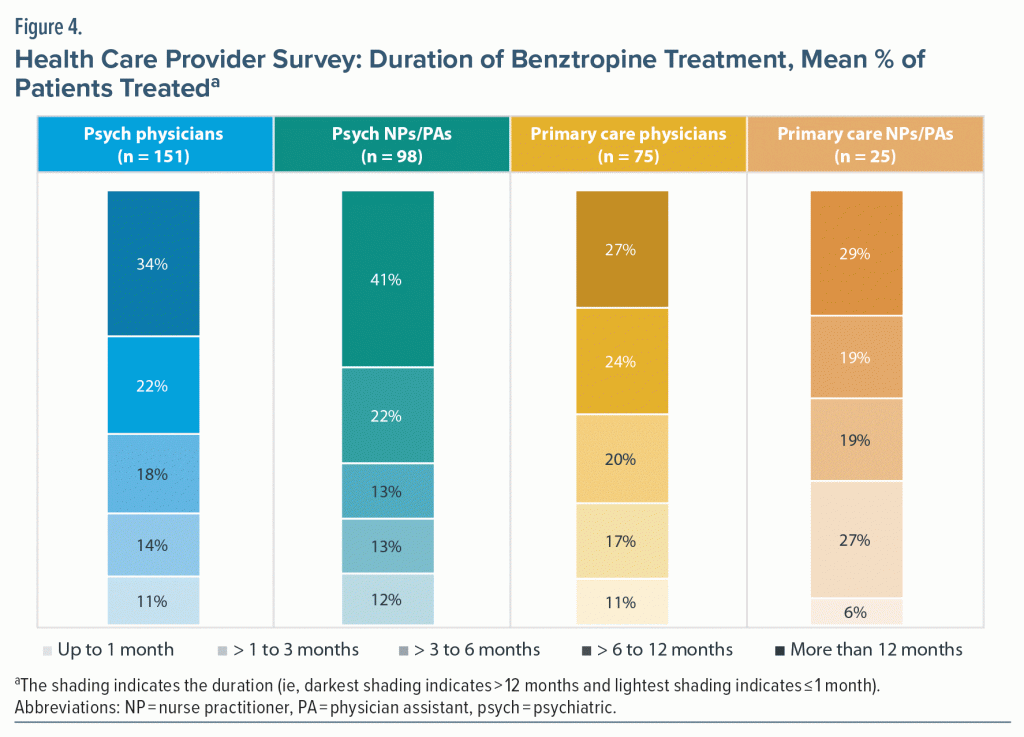
Psychiatric HCPs continued benztropine in more patients for > 6 months versus primary care HCPs (Figure 4), with a significantly higher percentage continuing benztropine for > 12 months (36.4% vs 27.5%; P < .05). The most common reasons reported by all HCPs for continuing benztropine for > 3 months included patient stability on all medications (range, 62%–75%) and prevention of non-TD DIMD (51%–69%). Over half of primary care HCPs (56.3%) also reported using it to prevent TD. Most HCPs agreed that patients treated with medications with anticholinergic properties should be reevaluated every 3 months for discontinuation (68%–84%). The most common reasons for discontinuing benztropine among all HCPs were development of a comorbid condition exacerbated by anticholinergics (65%–77%) or high anticholinergic burden (46%–62%). HCPs reported that few of their practices (< 15%) had a standard protocol or guideline for discontinuing benztropine. When asked specifically about the recommendations contained in the 2020 APA Practice Guideline for the Treatment of Patients with Schizophrenia, 39.0% of psychiatric HCPs and 29.0% of primary care HCPs reported being “somewhat” or “very” familiar with the recommendations. Of HCPs who reported being at least somewhat familiar with the 2020 APA guidelines (n = 126), 80.2% agreed that following the guidelines led to optimal treatment of DIMDs and that VMAT2 inhibitors are first-line treatment for TD. HCPs were interested in learning more about topics related to DIMDs such as the rationale for discontinuing benztropine (30.4%), guidance on dose tapering of medications with anticholinergic properties (43.6%), and differential diagnosis of DIMDs via telehealth (52.7%).
DISCUSSION
Results from the health care claims analysis and HCP survey of psychiatric and primary care physicians, NPs, and PAs indicated that benztropine is commonly prescribed for non-TD DIMD but may be used longer than recommended. In the health care claims analysis, over half of the cohort received benztropine for > 3 months, which is longer than the FDA recommendation; approximately 50% of primary care HCPs and 60% of psychiatric HCPs surveyed use benztropine for > 6 months in patients with DIMDs. Additionally, reasons for benztropine use did not fully align with the APA guideline and the AAN guideline update recommendations. Although treating non-TD DIMDs (ie, parkinsonism or dystonia) was the most common reason for initiating benztropine for all HCPs, prevention and treatment of TD were also common reasons, particularly for primary care HCPs.
As TD and non-TD DIMDs can occur within weeks to years, consistent monitoring and evaluation of patients with DIMDs is essential. The HCP survey results revealed psychiatric HCPs evaluate stable patients (ie, on effective and well-tolerated regimens) for DIMDs only during ~75% of visits and primary care HCPs only over half of the time (55%), yet nearly all HCPs agreed that patients prescribed antipsychotics should be monitored regularly for DIMDs. Monitoring should include an assessment of risk factors for non-TD DIMDs and TD.7 In the health care claims analysis, a majority of patients were receiving medications with anticholinergic properties, and over 50% had calculated moderate-to-severe anticholinergic burden. Use of medications with anticholinergic properties, older age, and female sex increase the risk of TD.19,20 Additionally, anticholinergic burden has been found to affect cognition and daily functioning in patients with schizophrenia,14,21–23 particularly in older adults, for whom deficits were similar to those observed in early Alzheimer’s disease.24 The Beers Criteria suggest avoiding combining medications with anticholinergic effects in patients aged > 65 years due to increased risk of cognitive decline.25 Despite this, HCPs responded in the survey that they are likely to prescribe benztropine in patients with these characteristics.
Few HCPs reported working in settings with protocols or policies in place for discontinuing benztropine. This finding is not surprising, as there is no relevant clinical trial evidence to inform the process, nor are there commonly accepted guidelines from standardized bodies on the best practices for discontinuing benztropine. However, smaller studies have examined discontinuing medications with anticholinergic properties, including benztropine.21,26–28 Results from these studies suggest that gradually discontinuing anticholinergics may have a positive impact on quality of life and cognition without worsening DIMD symptoms in most patients with schizophrenia.21,26,27
Providing education and increasing awareness of the 2020 APA guidelines related to the management of DIMDs may help HCPs understand when benztropine should be used in DIMDs. Less than 40% of all HCPs reported being somewhat or very familiar with the 2020 APA guidelines. HCPs that used the 2020 APA guidelines agreed that the recommendations led to optimal management of patients with DIMDs, including TD. HCPs seemed interested in learning more about benztropine use as well as diagnosing DIMDs correctly, particularly within telehealth visits. Patients with mental illness are often a complex population, with multiple medications and comorbidities that can further complicate diagnosis and treatment of DIMDs and increase the risk for greater HCRU and health care costs.29–31 Although benztropine can be an appropriate short-term treatment for certain DIMDs, the risks need to be considered, particularly in patients who are older, have TD, or are at high risk for TD.
While claims data are valuable for the efficient and effective examination of health care outcomes, treatment patterns, HCRU, and costs, all claims databases have certain inherent limitations. Although most insurance types (commercial insurance, Medicare/Medicare Part D, and Medicaid) were represented in the data, results and conclusions are limited to the studied patient population and may not be generalizable to other managed care populations in the US. An incorrect diagnosis code or a diagnosis included as a rule-out criterion is possible, although the risk of rule-out diagnosis is low since patients were included based on pharmacy codes. Patients had to have continuous eligibility before and after the index period, and patients were not censored. As such, patients who did not meet this criterion may have different results. The HCP survey included only physicians and NP/PAs who reported primary care or psychiatry specialty and were part of the IQVIA primary intelligence panel. While the panel includes > 200,000 HCPs in the US, responses may not be generalizable to HCPs who did not respond or were not part of the IQVIA panel.
Although benztropine may be a valuable treatment in the short-term for some DIMDs, it is often used long-term in non-TD DIMDs and in TD despite the risk of adverse events or exacerbation of TD symptoms. Evidence from these 2 complementary study methods demonstrated that additional education on the recommendations for treating DIMDs and appropriate benztropine use would be welcomed by HCPs and may benefit this complex patient population.
Article Information
Published Online: August 29, 2023. https://doi.org/10.4088/PCC.22m03472
© 2023 Physicians Postgraduate Press, Inc.
Submitted: December 20, 2022; accepted April 18, 2023.
To Cite: Chepke C, Benning B, Cicero S, et al. Investigating real-world benztropine usage patterns in movement disorders: claims analysis and health care provider survey results. Prim Care Companion CNS Disord. 2023;25(4):22m03472.
Author Affiliations: Excel Psychiatric Associates, Huntersville, North Carolina (Chepke); SUNY Upstate Medical University, Syracuse, New York (Chepke); IQVIA, Durham, North Carolina (Benning, Hull, Yeaw, Coyle); Neurocrine Biosciences Inc., San Diego, California (Cicero, Giraldo, Jen, Bron).
Corresponding Author: Morgan Bron, PharmD, MS, 12780 El Camino Real, San Diego, CA 92130 ([email protected]).
Relevant Financial Relationships: Ms Benning, Mr Hull, Mr Yeaw, and Ms Coyle are consultants of Neurocrine Biosciences Inc. and are employees of IQVIA and have no other disclosures. Dr Cicero, Ms Giraldo, Dr Jen, and Dr Bron are employees and stockholders of Neurocrine Biosciences Inc. and have no other disclosures. Dr Chepke is an employee of Excel Psychiatric Associates and is supported by grants from the following: Acadia, Axsome, Biohaven, Harmony, Neurocrine, and Teva. In addition, he is a paid consultant to AbbVie, Acadia, Alkermes, Axsome, Biogen, BioXcel, Corium, Eisai, Genomind, Intra-Cellular, Janssen, Jazz, Karuna, Lundbeck, MedinCell, Neurocrine, Noven, Otsuka, Sage, Sunovion, and Teva and has received speaking fees/travel grants/writing fees from AbbVie, Acadia, Alkermes, Axsome, Corium, Eisai, Genomind, Intra-Cellular, Ironshore, Janssen, Jazz, Lundbeck, Merck, Neurocrine, Noven, Otsuka, Sunovion, Takeda, and Teva. He is on advisory boards for AbbVie, Acadia, Alkermes, Axsome, Corium, Eisai, Idorsia, Intra-Cellular, Ironshore, Janssen, Jazz, Karuna, Lundbeck, Neurocrine, Noven, Otsuka, Sunovion, Takeda, and Teva. He has no stock/ownership interest to disclose. He is an active member of the Psych Congress Steering Committee and is Scientific Director of Psych Congress.
Funding/Support: Neurocrine Biosciences Inc. provided funding for this study and development of the manuscript. Neurocrine develops therapies for movement disorders, including the vesicular monoamine transporter 2 (VMAT2) inhibitor valbenazine.
Role of the Funders/Sponsors: Neurocrine Biosciences Inc. assisted in the conduct of the study, study design, analysis, and interpretation of the data and in the preparation, review, and approval of the manuscript.
Previous Presentations: Poster presented at: NEI Congress 2022; Colorado Springs, Colorado; November 3–6, 2022 • Psych Congress 2022; New Orleans, Louisiana; September 17–20, 2022
Acknowledgments: We would like to acknowledge Charles Yonan, PharmD, of Neurocrine Biosciences Inc. for his thoughtful insights throughout the studies and critical review of this manuscript. Dr Yonan is an employee and stockholder of Neurocrine Biosciences, Inc. We would also like to acknowledge Andi Gundlach, PharmD, MPH, CMPP, and Bridgette Schroader, PharmD, MPA, BCOP, of Xcenda LLC, supported by Neurocrine Biosciences Inc. for their aid in medical writing and strategic support. Dr Gundlach and Dr Schroader have no relevant financial relationships to disclose.
Additional Information: There is a data use agreement in place between IQVIA and Neurocrine for these databases, and they are not publicly available.
Supplementary Material: See accompanying pages.
Clinical Points
- Use of benztropine in the long-term treatment of non–tardive dyskinesia drug-induced movement disorders (non-TD DIMDs) and TD is common despite the risk of adverse events and the exacerbation of TD symptoms.
- Improving familiarity with the 2020 APA guidelines related to recommended treatment options for non-TD DIMDs and TD, as well as the appropriate use of benztropine, can help clinicians effectively manage DIMDs, including TD.
References (31)

- American Psychiatric Association (APA). Medication-Induced Movement Disorders and Other Adverse Effects of Medication. Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association Publishing; 2022.
- Chouksey A, Pandey S. Clinical spectrum of drug-induced movement disorders: a study of 97 patients. Tremor Other Hyperkinet Mov (N Y). 2020;10(1):48. PubMed CrossRef
- Hauser RA, Meyer JM, Factor SA, et al. Differentiating tardive dyskinesia: a video-based review of antipsychotic-induced movement disorders in clinical practice. CNS Spectr. 2022;27(2):208–217. PubMed CrossRef
- Zádori D, Veres G, Szalárdy L, et al. Drug-induced movement disorders. Expert Opin Drug Saf. 2015;14(6):877–890. PubMed CrossRef
- Lerner PP, Miodownik C, Lerner V. Tardive dyskinesia (syndrome): current concept and modern approaches to its management. Psychiatry Clin Neurosci. 2015;69(6):321–334. PubMed CrossRef
- Waln O, Jankovic J. An update on tardive dyskinesia: from phenomenology to treatment. Tremor Other Hyperkinet Mov (N Y). 2013;3:tre-03-161-4138-1. PubMed CrossRef
- American Psychiatric Association (APA). Guideline Statements and Implementation. The American Psychiatric Association Practice Guideline for the Treatment of Patients With Schizophrenia. American Psychiatric Association Publishing; 2020.
- Bhidayasiri R, Fahn S, Weiner WJ, et al; American Academy of Neurology. Evidence-based guideline: treatment of tardive syndromes: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81(5):463–469. PubMed
- Bhidayasiri R, Jitkritsadakul O, Friedman JH, et al. Updating the recommendations for treatment of tardive syndromes: a systematic review of new evidence and practical treatment algorithm. J Neurol Sci. 2018;389:67–75. PubMed CrossRef
- Deutetrabenazine [prescribing information]. Parsippany, NJ: Teva Neuroscience, Inc; Last updated May 2022.
- Valbenazine [prescribing information]. San Diego, CA: Neurocrine Biosciences, Inc; Last updated August 2022.
- Benztropine [prescribing information]. Warren, NJ: Cipla USA, Inc; Last updated February 2020.
- Bergman H, Soares-Weiser K. Anticholinergic medication for antipsychotic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2018;1(1):CD000204. PubMed
- Joshi YB, Thomas ML, Braff DL, et al. Anticholinergic medication burden-associated cognitive impairment in schizophrenia. Am J Psychiatry. 2021;178(9):838–847. PubMed CrossRef
- Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15(1):31. PubMed CrossRef
- Vanderhoef D, Vanegas-Arroyave N, Stroup L, et al. Should anticholinergics be used to treat tardive dyskinesia? Insights from an expert panel of psychiatry and neurology healthcare professionals Poster presented at: American Association of Nurse Practitioners National Conference; June 21–26, 2022; Orlando, FL.
- King R, Rabino S. ACB calculator. ACB calculator website. Last updated November 7, 2022. Accessed December 20, 2022. https://www.acbcalc.com/
- WCG IRB. IRB Review Solutions. WCG website. Last updated 2022. Accessed October 4, 2022. https://www.wcgirb.com/
- Miller DD, McEvoy JP, Davis SM, et al. Clinical correlates of tardive dyskinesia in schizophrenia: baseline data from the CATIE schizophrenia trial. Schizophr Res. 2005;80(1):33–43. PubMed CrossRef
- Solmi M, Pigato G, Kane JM, et al. Clinical risk factors for the development of tardive dyskinesia. J Neurol Sci. 2018;389:21–27. PubMed CrossRef
- Georgiou R, Lamnisos D, Giannakou K. Anticholinergic burden and cognitive performance in patients with schizophrenia: a systematic literature review. Front Psychiatry. 2021;12:779607. PubMed CrossRef
- Khan WU, Ghazala Z, Brooks HJ, et al. The impact of anticholinergic burden on functional capacity in persons with schizophrenia across the adult life span. Schizophr Bull. 2021;47(1):249–257. PubMed CrossRef
- Eum S, Hill SK, Rubin LH, et al. Cognitive burden of anticholinergic medications in psychotic disorders. Schizophr Res. 2017;190:129–135. PubMed CrossRef
- Tsoutsoulas C, Mulsant BH, Kumar S, et al. Anticholinergic burden and cognition in older patients with schizophrenia. J Clin Psychiatry. 2017;78(9):e1284–e1290. PubMed CrossRef
- The 2019 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674–694. PubMed CrossRef
- Lupu AM, MacCamy KL, Gannon JM, et al. Less is more: deprescribing anticholinergic medications in persons with severe mental illness. Ann Clin Psychiatry. 2021;33(2):80–92. PubMed
- Desmarais JE, Beauclair L, Annable L, et al. Effects of discontinuing anticholinergic treatment on movement disorders, cognition and psychopathology in patients with schizophrenia. Ther Adv Psychopharmacol. 2014;4(6):257–267. PubMed CrossRef
- Ogino S, Miyamoto S, Tenjin T, et al. Effects of discontinuation of long-term biperiden use on cognitive function and quality of life in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(1):78–83. PubMed CrossRef
- McEvoy J, Park T, Schilling T, et al. The burden of tardive dyskinesia secondary to antipsychotic medication use among patients with mental disorders. Curr Med Res Opin. 2019;35(7):1205–1214. PubMed CrossRef
- Kadakia A, Brady BL, Dembek C, et al. The incidence and economic burden of extrapyramidal symptoms in patients with schizophrenia treated with second generation antipsychotics in a Medicaid population. J Med Econ. 2022;25(1):87–98. PubMed CrossRef
- Abouzaid S, Tian H, Zhou H, et al. Economic burden associated with extrapyramidal symptoms in a Medicaid population with schizophrenia. Community Ment Health J. 2014;50(1):51–58. PubMed CrossRef
This PDF is free for all visitors!
Save
Cite