CASE CONFERENCE
The Banner Alzheimer’s Institute Case Conference is a weekly event in which physicians and staff discuss challenging and/or teaching cases of patients seen in clinical settings. These conferences are attended by a multidisciplinary group that includes Banner Alzheimer’s Institute dementia specialists, community physicians (internal medicine, family medicine, and radiology), neuropsychologists, physician assistants, nurse practitioners, social workers, medical students, residents, and fellows. The Banner Alzheimer’s Institute is located in Phoenix, Arizona, and it has an ambitious mission: to end Alzheimer’s disease without losing a generation, set a new standard of care for patients and families, and forge a model of collaboration in biomedical research. The Institute provides high-level care and treatment for patients affected by Alzheimer’s disease, dementia, and related disorders. In addition, the Institute offers extensive support services for families and many unique and rewarding research opportunities.
HISTORY OF PRESENTING ILLNESS
Mr A is a 79-year-old man who presented to Banner Alzheimer’s Institute accompanied by his wife and daughter for an evaluation of cognitive complaints. He and his family report difficulty completing projects, such as home renovations that he previously had no difficulty with. This difficulty has progressively worsened over the past 3 years. During this time, they also report intermittent confusion, giving the example of Mr A getting lost during a trip to London. Mr A and his family deny recent changes in language. They deny recent falls but do note that he seems to be taking smaller steps.
The patient’s family report significant behavioral changes in Mr A. They note he has developed a lack of motivation and drive and is not engaging in the things he used to enjoy. The family reports impulsive behaviors such as interacting with strangers as if they are close friends. Mr A recently hugged a plumber he did not know and asked a waitress about her tattoos, which according to his family is not normal for him. His family states that over a recent 6-month period he had a significant craving for sweets and other foods uncharacteristic of previous behavior. His family notes a possible decline in his ability to express sympathy and empathy, but they do not feel this has been significant. Mr A’s family denies any new repetitive or ritualistic behaviors.
During the patient’s office visit, he reports his mood is “good.” Mr A denies sleep disturbance, dream enactment behavior, hallucinations, and delusions. He denies experiencing any recent change in weight or appetite.
From a functional standpoint, his wife manages their finances. She initiated this role about 1.5 years ago after noticing Mr A was having trouble with taxes and investments. They report Mr A stopped driving about 2 years ago. This was recommended by a previous memory care provider in 2018 after the patient’s wife reported he was driving too fast and failing to stop safely at stop signs and red lights. The patient had reported onset of memory difficulties including getting lost while driving around the same time. His wife manages his medications but has always done so. He can independently complete most activities of daily living (ADLs) but does wear pads for occasional urinary incontinence.
PAST MEDICAL HISTORY
Mr A has a history of hypothyroidism, asthma, and hyperlipidemia. He has a past surgical history of Mohs surgery on his neck and a tonsillectomy. There is no history of stroke, transient ischemic attack, seizure, tremor, exposure to toxins, psychiatric illness, or substance misuse.
Recent complete blood count; comprehensive metabolic panel; thyroid-stimulating hormone (TSH), vitamin B12, and folate levels; and lipid panel were within normal limits other than a slightly decreased estimated glomerular filtration rate of 55 and creatinine of 1.23.
ALLERGIES
There are no known allergies.
MEDICATIONS
Mr A’s current medications include donepezil 10 mg/d, a fluticasone furoate/vilanterol inhaler, montelukast 10 mg/d, atorvastatin 40 mg/d, and levothyroxine 50 mcg/d.
SOCIAL HISTORY
Mr A lives with his wife. His highest level of education is a bachelor’s degree, and he worked as an engineer and vice president of research and development.
SUBSTANCE USE HISTORY
Mr A has a nonrecent history of occasional pipe tobacco use. At the initial presentation, he reports drinking 1 to 2 times weekly. He denies lifetime history of other recreational drug use. No further information on substance use was obtained.
FAMILY HISTORY
Family history is significant for Alzheimer’s disease in his mother and stomach cancer in his father.
Lifetime risk for developing dementia for an individual with a family history of dementia is 20%, whereas lifetime risk for developing dementia in the general population is 10% (Loy et al, 2014). Based on “2021 Alzheimer’s Disease Facts and Figures,” a report detailing national statistics, Alzheimer’s disease accounts for 60%–80% of all dementia diagnoses in the US (Alzheimer’s Association, 2021). A person with a first-degree relative with Alzheimer’s disease is more likely to develop the disease than a person without a first-degree relative with Alzheimer’s disease. Having more than 1 first-degree relative puts a person at even higher risk for developing the disease. Additionally, having a parent with dementia increases the chance of a person carrying a known genetic risk factor of Alzheimer’s disease, such as the APOE-e4 allele (Green et al, 2002).
REFERENCES
Alzheimer’s Association Report. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17(3):327–406. PubMed CrossRef
Green RC, Cupples LA, Go R, et al; MIRAGE Study Group. Risk of dementia among White and African American relatives of patients with Alzheimer disease. JAMA. 2002;287(3):329–336. PubMed CrossRef
Loy CT, Schofield PR, Turner AM, et al. Genetics of dementia. Lancet. 2014;383(9919):828–840. PubMed CrossRef
PHYSICAL AND NEUROLOGIC EXAMINATION
Vitals: heart rate = 76 bpm, blood pressure: 96/68 mm Hg–113/77 mm Hg; and weight: 160 lb.
Mental status: Mr A was awake and cooperative with largely fluent language output and intact comprehension.
Cranial nerves: Pupils were round and reactive to light. Extraocular movements were in full range. The face was symmetric with normal sensation. Hearing was adequate for interview but decreased to finger rubbing on examination. Palate was elevated symmetrically. Shoulder shrug was symmetric and strong. The tongue protruded in midline.
Motor: Tone was difficult to assess due to the patient’s inability to relax but appeared to be grossly normal in bilateral upper and lower extremities with intact strength. No significant pronator drift was noted. There were mild postural and action tremors in the bilateral upper extremities.
Sensory: Sensation was intact to light touch and pinprick.
Reflexes: Deep tendon reflexes were 2 to 3+ throughout except at the ankles where they were trace. Plantar responses were flexor bilaterally.
Coordination: Finger-nose-finger and heel-to-shin were performed well without dysmetria.
Gait: Mr A had erect posture. His walk was slightly cautious but with adequate arm swings.
The patient scored 23/30 on the MMSE (Folstein et al, 1975), with impairment in orientation (3 points lost for missing date, floor, and county), delayed recall (1 point lost), naming (1 point lost), 3-step commands (1 point lost), and intersecting pentagons (1 point lost).
When interpreting MMSE results, it is important to remember that while the score can reflect cognitive impairment, it is also dependent on demographic factors, including age, education, and cultural background. Several studies have found education to be the most important demographic factor influencing the MMSE score. It has previously been proposed to adjust MMSE cutoff values for education level or to add a point to the scores of patients with lower education levels (Cardoso et al, 2022).
REFERENCES
Cardoso S, Barros R, Marôco J, et al. Different MMSE domains are associated to cognitive decline and education. Appl Neuropsychol Adult. 2022;1–7. PubMed CrossRef
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. PubMed CrossRef
Mr A’s MoCA yielded a score of 15/30, with impairments in visuospatial/executive function, attention, orientation, delayed recall, language, and abstraction. Notably, on delayed recall he was able to recognize 4 of the 5 words when provided with multiple choices, suggesting recall difficulties were less likely a reflection of a storage deficit and more likely a retrieval difficulty.
Both the MMSE and MoCA use various tasks to evaluate different cognitive domains. Looking at the patterns of deficits can help to identify which cognitive domains are affected in a particular patient. The patient in this case exhibited deficits in orientation in both screening tests. A lower score in orientation to time is associated with future cognitive decline (Cardoso et al, 2022). He demonstrated deficits in copying intersecting pentagons on the MMSE and copying a cube and clock drawing on the MoCA, suggesting visuospatial and executive function deficits, respectively. On the MMSE, Mr A had difficulty with naming and following a 3-step command, while on the MoCA he had difficulty with the phonemic fluency task, findings that suggest deficits in language and/or executive functioning. He demonstrated difficulty with delayed recall on both the MMSE and MoCA, suggesting memory impairment. On the MoCA, he had difficulty with the tapping task, which indicates problems in sustaining attention, and with the verbal abstraction task. Executive function is assessed in several different tasks, including phonemic fluency, verbal abstraction, and the alteration task in the MoCA. The patient demonstrated impairments in these tasks.
The MoCA has been shown to have a better sensitivity and specificity in detecting subtle cognitive impairments, such as mild cognitive impairment (MCI), when compared to the MMSE. Nasreddine et al (2005) found that the MMSE had a sensitivity of 18% to detect MCI, whereas the MoCA detected 90% of MCI subjects. In the mild Alzheimer’s disease group, the MMSE had a sensitivity of 78%, whereas the MoCA detected 100%. Specificity was excellent for both the MMSE and MoCA (100% and 87%, respectively). Therefore, in patients with a borderline low MMSE score, proceeding to the MoCA can be informative.
REFERENCES
Cardoso S, Barros R, Marôco J, et al. Different MMSE domains are associated to cognitive decline and education. Appl Neuropsychol Adult. 2022;1–7. PubMed CrossRef
Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. PubMed CrossRef
The DSM-5 (American Psychiatric Association, 2013) defines a major neurocognitive disorder as follows:
- Evidence of significant cognitive decline from a previous level of performance in 1 or more areas of cognitive domains (complex attention, executive function, learning and memory, language, perceptual-motor, or social cognition) based on:
(1) Concern of the individual, a knowledgeable informant, or the clinician that there has been a significant decline in cognitive function and
(2) Substantial impairment in cognitive performance, preferably documented by standardized neuropsychological testing or, in its absence, another quantified clinical assessment.
- The cognitive deficits interfere with independence in everyday activities.
- The cognitive deficits do not occur exclusively in the context of a delirium.
- The cognitive deficits are not better explained by another mental disorder.
Mr A had experienced significant cognitive and functional decline as reported by his family, causing difficulties in his ability to be independent in everyday activities. His current level of functioning was a significant deterioration from his previous level of functioning. In addition, he demonstrated impairments in multiple cognitive domains on bedside evaluation. The deficits did not occur exclusively in the context of a delirium. There appeared to be no other mental disorder present. Given this information, the presence of a major neurocognitive disorder is likely.
REFERENCE
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. Fifth Edition. Arlington, VA: American Psychiatric Association; 2013.
PRIOR EVALUATIONS
The patient was previously evaluated at another local institution about 18 months prior to his presentation to Banner Alzheimer’s Institute. Laboratory studies showed no significant abnormalities, including TSH, vitamin B12, and folate levels within normal limits.
He had a neuropsychological evaluation that demonstrated most prominent impairment in aspects of executive functioning including perseverative problem-solving, impaired cognitive flexibility, impaired lexical verbal fluency, and low average working memory and processing speed. Memory was approximated to be below his estimated baseline but at the low end of normal range and did not exhibit a classically amnestic pattern. He was given the diagnosis of mild cognitive impairment, primarily in executive functioning.
He was started on donepezil by a previous provider, which was subsequently tapered off and replaced with memantine. However, he had gastrointestinal issues on memantine, and donepezil was restarted.
Alzheimer’s disease is the most common type of dementia, accounting for 60%–80% of all cases in the US. Dementia due to mixed etiology accounts for more than 50% of cases. About 5% of individuals with dementia show evidence of dementia with Lewy body disease alone, but most people with dementia with Lewy bodies also have Alzheimer’s disease pathology. Frontotemporal lobar dementia is a common cause of early-onset dementia. A systemic review found that frontotemporal lobar dementia accounts for about 3% of dementia patients in those older than age 65 and about 10% of dementia cases in those presenting younger than 65 years old (ie, early-onset dementia), and 5%–10% of dementia cases are of vascular etiology alone (Alzheimer’s Association, 2021).
REFERENCES
Alzheimer’s Association Report. 2021 Alzheimer’s disease facts and figures. Alzheimers Dement. 2021;17(3):327–406. PubMed CrossRef
IMAGING
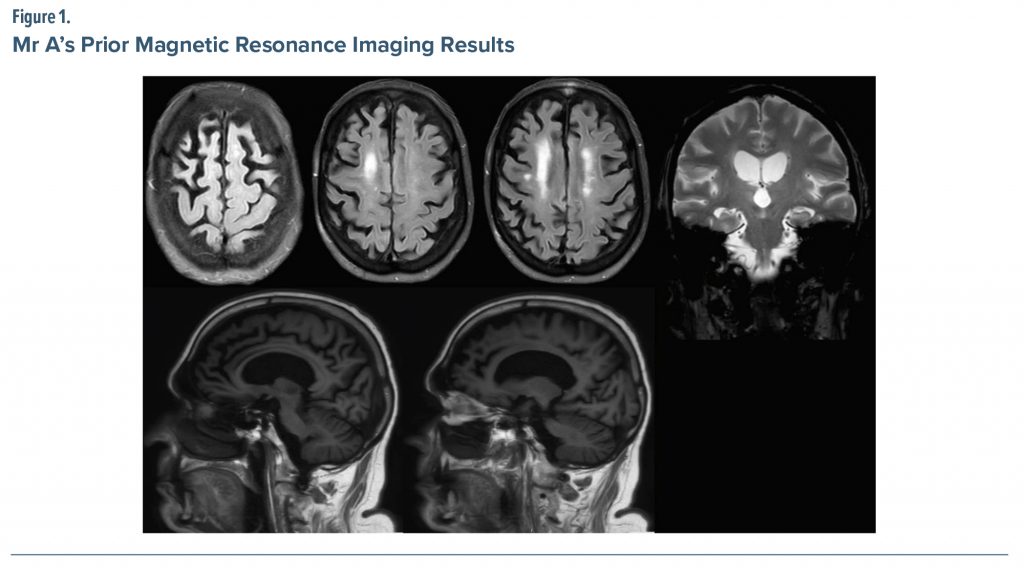
Brain magnetic resonance imaging (MRI) (Figure 1) performed about 1 year prior to presentation was interpreted as showing the following: (1) multifocal brain volume loss (mild generalized cerebral and cerebellar volume loss, including atrophy of the medial temporal lobes with ex vacuo dilatation of the temporal horns, right greater than left), (2) mild chronic small vessel ischemic changes, and (3) no evidence of acute intracranial abnormality. The treating physician’s review of an MRI performed at another institution, however, noted the atrophy seems to be most notable in the frontal lobes and worse in the right hemisphere.
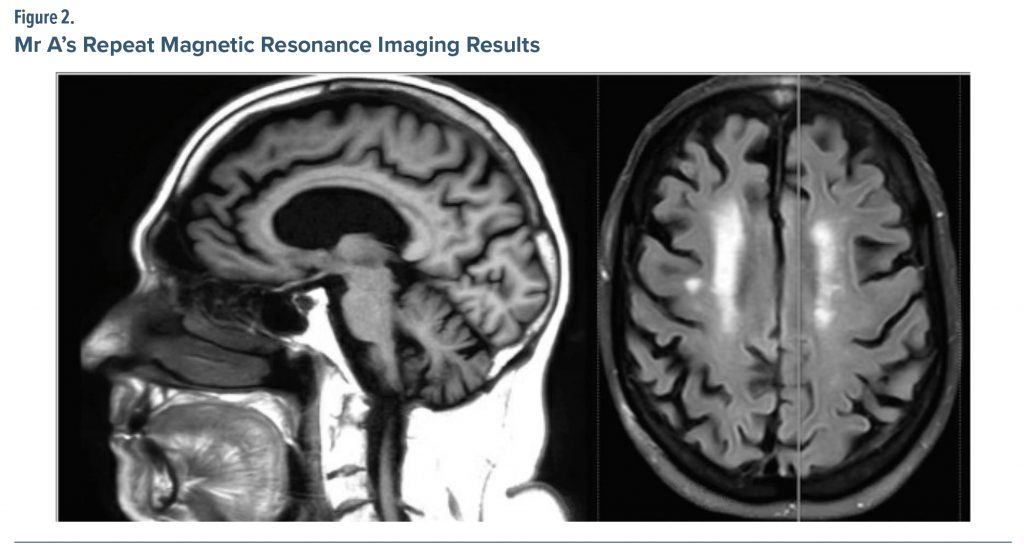
A repeat MRI was ordered by the treating physician (Figure 2) showing moderate diffuse cerebral volume loss with moderate small vessel ischemic change present. The treating physician further noted the cerebral atrophy was generalized, though most notable in the right frontal region.
THE TREATING PHYSICIAN’S IMPRESSION
The patient presented with approximately 3 years of cognitive decline and behavioral changes. His physical examination was nonfocal with no significant signs of parkinsonism. On cognitive assessment with the MoCA, he demonstrated deficits in a frontal-dysexecutive pattern with impaired mini-trail B, clock number/hands placement, digital span reverse, vigilance, and lexical verbal fluency. He showed poor memory performance with some benefit from cueing. Functionally, he had a decline as well. Clinically, he meets criteria for dementia.
The etiology of his symptoms is not entirely clear. He seems to be experiencing deficits commonly associated with frontal lobe pathology. Given the associated behavioral changes (apathy, impulsiveness, and dietary change), behavioral variant frontotemporal dementia is a concern; however, his age at onset is older than typical presentation. Given his age, a “frontal-variant” of Alzheimer’s disease is possible.
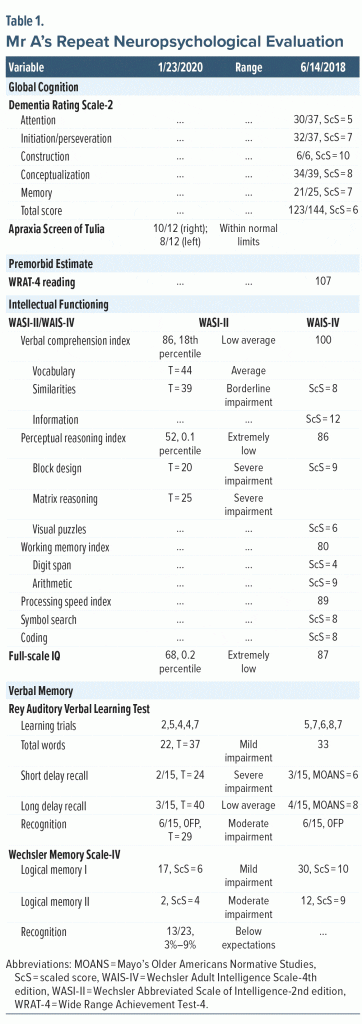
The treating physician ordered a repeat neuropsychological evaluation (Table 1). Mr A’s neuropsychological profile demonstrated significant impairments across most domains: memory (verbal, visual), attention, processing speed, executive functioning, and language (semantic fluency). Relative to his last neuropsychological evaluation, his overall performance in most of these domains showed notable declines: verbal memory for unstructured stimuli (average to low average), narrative memory (average to moderate impairment), visual memory (low average to severe impairment), attention (moderate impairment to severe impairment), processing speed (low average to borderline/severe impairment), and semantic fluency (mild to severe impairment). Set switching (severe) and inhibition of distracting stimuli (mild) were impaired in the prior evaluation, but he was unable to complete these tests with the current evaluation due to significant comprehension difficulties, reflecting significant decline. Phonemic fluency remained stable at severe impairment. Confrontation naming and visuospatial abilities remained intact and were stable relative to the last evaluation. There was a substantial decline (average to extremely low) in intellectual functioning relative to the estimated premorbid level from the prior neuropsychological evaluation.
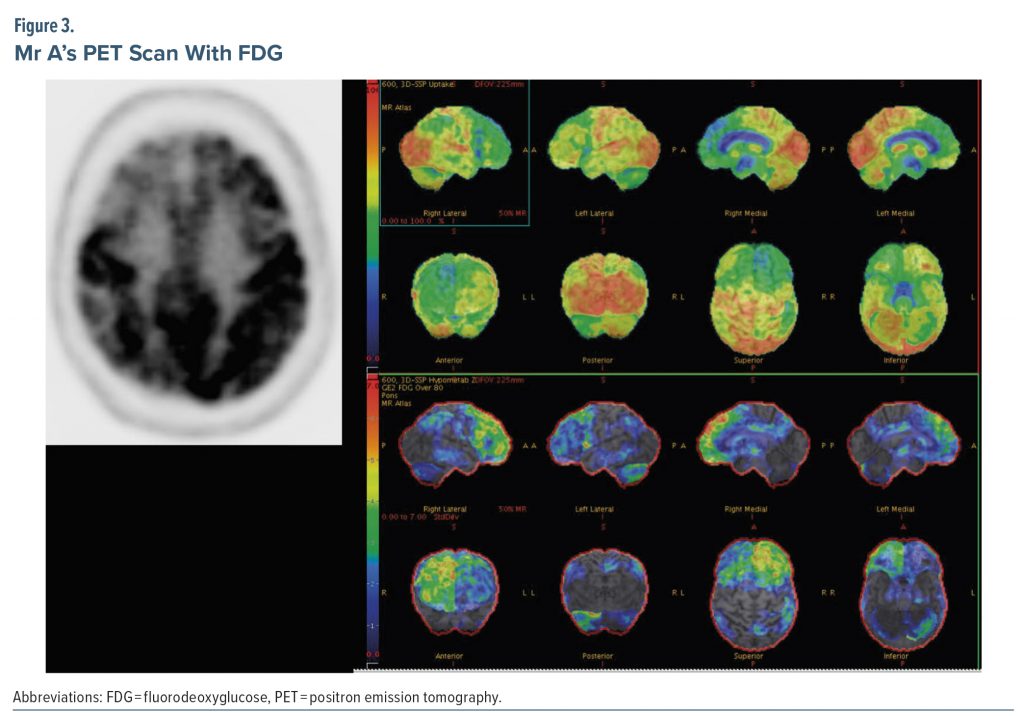
Due to the unclear etiology of dementia at this stage, the treating provider ordered a PET scan with FDG. FDG-PET can be useful in differentiating Alzheimer’s disease from frontotemporal dementia. Patterns of hypometabolism help distinguish between the 2 diagnoses, as well as between amnestic and nonamnestic Alzheimer’s disease (Murray et al, 2021). CT angiography head and fMRI brain are not indicated for routine evaluations of cognitive impairment.
Figure 3 shows results of the PET scan with FDG. The radiologist interpreted the study as demonstrating multiple areas of relatively decreased F-18 FDG labeling, indicating hypometabolism in the following areas: the convex surface of the frontal lobe bilaterally but more evident on the right side, the anterior cingulate cortices bilaterally, a small area of the most superior-posterior aspect of the convex surface of the right parietal lobe, and the anterior inferior aspect of the right temporal lobe in a patchy pattern. The rest of the brain cortical regions, basal ganglia, thalami, and cerebellar hemispheres were of a normal pattern comparatively. Due to involvement of the medial surfaces of the bilateral frontal lobes, convex surface of the right frontal lobe, bilateral anterior cingulate cortices, and right temporal lobe, the radiologist posited the findings to be most consistent with frontotemporal dementia. The radiologist further stated that Alzheimer’s dementia was unlikely given sparing of the left parietal cortex, major parts of the right parietal cortex, bilateral posterior cingulate cortices, and bilateral precuneus regions.
DIAGNOSIS
Based on initial symptoms of a frontally predominant dysexecutive process with associated behavioral changes (apathy, impulsive/disinhibited behaviors, and lack of empathy and dietary changes) and findings of frontal atrophy and hypometabolism noted on MRI and FDG-PET, respectively, a diagnosis of behavioral variant frontotemporal dementia was made. Of note, during the course of diagnostic evaluation, Mr A was started on sertraline by an outside physician.
DISCUSSION
For elderly patients presenting with new-onset behavioral disturbances or executive dysfunction, frontotemporal dementia should be high on a practitioner’s differential diagnosis. The treating physician in this case also appropriately considered Alzheimer’s disease despite the presentation being atypical. PET-FDG scan confirmed a diagnosis of behavioral variant frontotemporal dementia, which was initially made based on his presentation and neuropsychological testing. Recent literature describes multiple variants of nonamnestic Alzheimer’s disease that present with symptoms mimicking other neurodegenerative diseases. A recent review estimates that 7%–20% of patients with clinically diagnosed behavioral variant frontotemporal dementia show Alzheimer’s disease neuropathology on autopsy (Graff-Radford et al, 2021). This serves as a reminder to avoid making a rigid diagnosis of a specific neurodegenerative disease before a full workup is completed.
The review by Graff-Radford et al (2021) describes at least 5 nonamnestic variants of Alzheimer’s disease. Two of these variants, dysexecutive Alzheimer’s disease and behavioral Alzheimer’s disease, were previously characterized as frontal variant Alzheimer’s disease (Graff-Radford et al, 2021). Johnson et al (1999) presented 3 patients with predominantly executive dysfunction, who all had amyloid plaque and neurofibrillary tangle pathology consistent with Alzheimer’s disease on autopsy. The plaque and tangle pathology were most notable in these patients’ frontal lobes. Since that publication, similar autopsy findings have been described in patients who initially presented with primarily behavioral disturbances. Graff-Radford et al (2021) described 3 other variant presentations with later confirmed Alzheimer’s disease pathology including posterior cortical atrophy Alzheimer’s disease, logopenic variant primary progressive aphasia, and corticobasal syndrome Alzheimer’s disease. Patients with each variant present with features of a different neurodegenerative disease. This leaves high likelihood for misdiagnosis and inappropriate management, which is especially pertinent now as treatments for specific dementia subtypes are rapidly developing.
Behavioral variant Alzheimer’s disease is still an uncommon presentation of Alzheimer’s disease. In a study of 532 patients with confirmed Alzheimer’s disease, only 2% reported predominantly behavioral features. Of these patients, early onset and male gender were characteristic (Snowden et al, 2007). Early onset is a typical feature of all nonamnestic variants of Alzheimer’s disease. Nonamnestic presentations represent a substantial proportion of patients with early-onset Alzheimer’s disease at 33% compared to late-onset Alzheimer’s disease, in which only 6% of patients exhibit nonamnestic presentations (Graff-Radford et al, 2021). However, it remains an important consideration in the diagnostic evaluation in older populations, as the prevalence of Alzheimer’s disease increases with age, thereby increasing the absolute number of nonamnestic variants.
A retrospective study by Ossenkoppele et al (2015) included 75 patients who presented with behavioral disturbance or executive dysfunction and showed evidence of amyloid pathology on PET scan or in CSF studies or on autopsy, correlating with a diagnosis of Alzheimer’s disease. Investigators further separated study participants into those presenting with behavioral dysfunction, those presenting with dysexecutive difficulties, and those with a mix of these symptomatologies. They found differences in structural MRI between behavioral or dysexecutive variant Alzheimer’s disease and behavioral variant frontotemporal dementia, with the former showing a typical pattern consistent with classic Alzheimer’s disease findings in the temporoparietal cortex and the latter group showing more anterior brain atrophy. Recently, research criteria for behavioral variant Alzheimer’s disease have been proposed, noting that behavioral variant Alzheimer’s disease is clinically most similar to behavioral variant frontotemporal dementia while sharing most pathophysiologic features with typical amnestic-onset Alzheimer’s disease (Ossenkoppele et al, 2022).
In conclusion, clinical findings, imaging, and neuropathologic features can all be useful in distinguishing between behavioral/dysexecutive variant Alzheimer’s disease and behavioral variant frontotemporal dementia. Establishing an accurate diagnosis is important for treatment planning with disease-modifying agents, for management of symptoms, and to support families and caregivers.
REFERENCES
Graff-Radford J, Yong KXX, Apostolova LG, et al. New insights into atypical Alzheimer’s disease in the era of biomarkers. Lancet Neurol. 2021;20(3):222–234. PubMed CrossRef
Johnson JK, Head E, Kim R, et al. Clinical and pathological evidence for a frontal variant of Alzheimer disease. Arch Neurol. 1999;56(10):1233–1239. PubMed CrossRef
Ossenkoppele R, Pijnenburg YA, Perry DC, et al. The behavioural/dysexecutive variant of Alzheimer’s disease: clinical, neuroimaging and pathological features. Brain. 2015;138(Pt 9):2732–2749. PubMed CrossRef
Ossenkoppele R, Singleton EH, Groot C, et al. Research criteria for the behavioral variant of Alzheimer disease: a systematic review and meta-analysis. JAMA Neurol. 2022;79(1):48–60. PubMed CrossRef
Snowden JS, Stopford CL, Julien CL, et al. Cognitive phenotypes in Alzheimer’s disease and genetic risk. Cortex. 2007;43(7):835–845. PubMed CrossRef
CLINICAL POINTS
- Clinicians should include variants of nonamnestic Alzheimer’s disease in their differential diagnosis, as they present with symptoms mimicking other neurodegenerative diseases.
- Nonamnestic presentations represent a substantial proportion of patients with early onset Alzheimer’s disease and are more common in men.
- Accurate diagnosis is essential for appropriate management of neurodegenerative diseases.
Article Information
Published Online: October 10, 2023. https://doi.org/10.4088/PCC.22alz03400
© 2023 Physicians Postgraduate Press, Inc.
Prim Care Companion CNS Disord. 2023;25(5):22alz03400
Submitted: August 22, 2022; accepted February 16, 2023.
To Cite: Adams C, Hendrie K, James M, et al. It may not be so typical: distinguishing frontotemporal dementia from behavioral variant Alzheimer’s disease. Prim Care Companion CNS Disord. 2023;25(5):22alz03400.
Author Affiliations: Banner University Medical Center, Phoenix, Arizona (Adams, Hendrie, James, Tsai)
Corresponding Author: Claire Adams, MD, UACOMP, 1300 N 12th St, Ste 320, Phoenix, AZ 85014 ([email protected]).
Funding/Support: None.
Disclaimer: The opinions expressed are those of the authors, not of Banner Health or Physicians Postgraduate Press.
Please sign in or purchase this PDF for $40.
Save
Cite