Because this piece does not have an abstract, we have provided for your benefit the first 3 sentences of the full text.
To the Editor: The antidepressant vilazodone is a serotonin partial agonist reuptake inhibitor. It is reported to have mild adverse effects like gastrointestinal symptoms and very few sexual side effects, though the literature regarding the same is scarce. While some report the presence of sexual side effects at higher doses (up to 40 mg), there have been no case reports of decreased libido with vilazodone at lower doses.
Vilazodone-Induced Decrease in Libido: A Case Report
To the Editor: The antidepressant vilazodone is a serotonin partial agonist reuptake inhibitor. It is reported to have mild adverse effects like gastrointestinal symptoms1 and very few sexual side effects,2 though the literature regarding the same is scarce.3 While some report the presence of sexual side effects at higher doses (up to 40 mg), there have been no case reports of decreased libido with vilazodone at lower doses.1
Here, we present a case of a woman who reported onset of decreased libido following initiation of vilazodone.
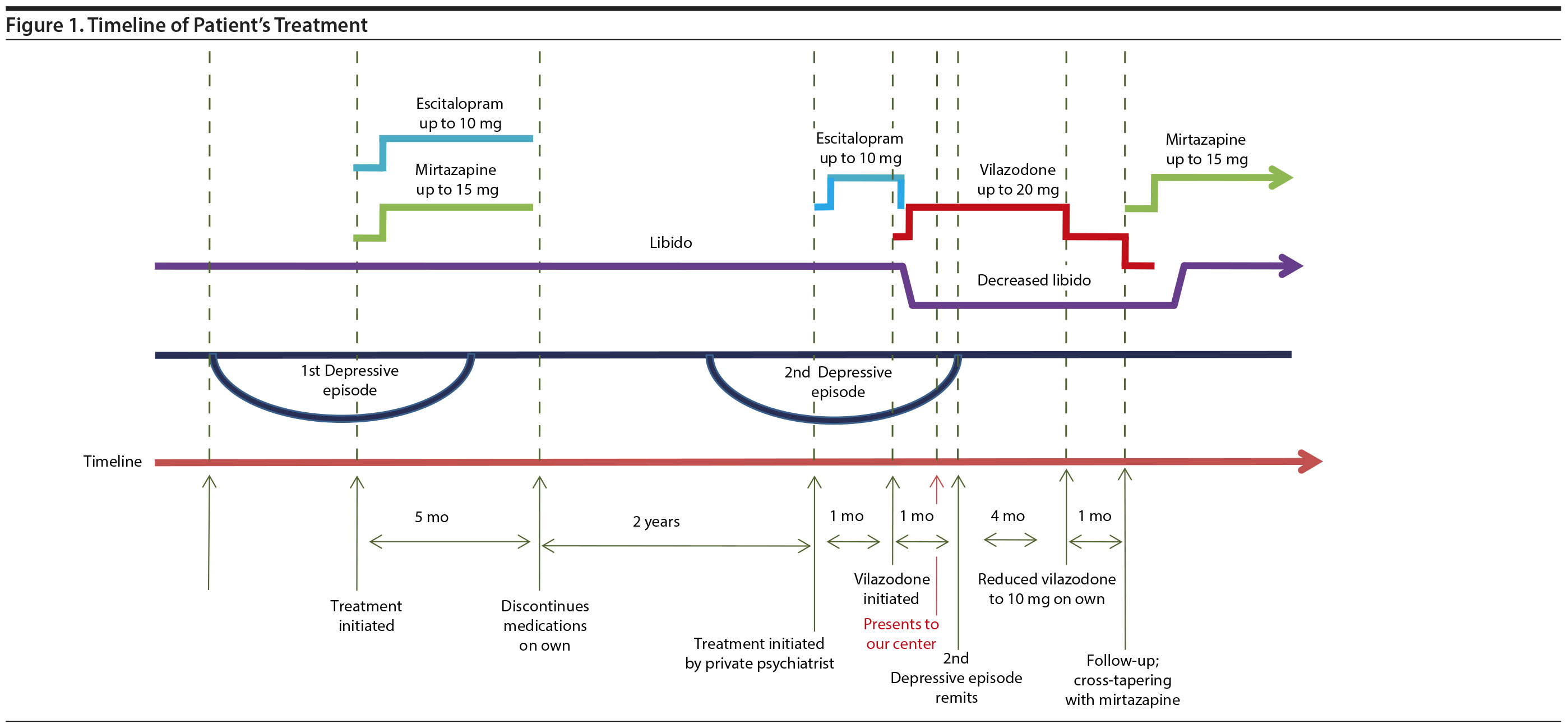
Case report. A 32-year-old woman presented to our outpatient department with a history of severe depressive and anxiety symptoms for about a year. She was diagnosed with recurrent depressive disorder (ICD-10) in light of a similar episode in the past (Figure 1).

During that first episode, 2 years prior, she had been prescribed escitalopram (up to 10 mg) and mirtazapine (up to 15 mg). Her symptoms remitted completely after 2 months of treatment, and she discontinued the medications on her own 3 months later. She denied any loss of libido during that time. In the current episode, she initiated treatment with a private psychiatrist 1 month after onset of the current depressive episode and anxiety symptoms. She was prescribed tablet escitalopram (up to 10 mg) and clonazepam (up to 0.5 mg), but she showed no significant improvement, and hence, escitalopram was tapered to 5 mg and stopped. Vilazodone was started at 10 mg and increased to 20 mg over 2 weeks. She presented to our center 3 weeks after starting vilazodone for subsyndromal depressive and anxiety symptoms. She was advised to continue the same medications in view of improvement in her symptoms. Her depressive symptoms remitted completely within 1 month; her anxiety symptoms persisted.
A few days after starting vilazodone, the patient noticed a decrease in libido while relating to her fiancé, with whom she had been romantically attached for more than 6 months. Unlike her usual self, she reported loss of interest or desire for initiating or responding to physical intimacy and loss of physiological arousal or pleasure when she was physically intimate with her fiancé despite sustained efforts for the same by both of them. She could not comment on reduced genital sensations during sexual activity as they had not participated in sexual intercourse at any time. She became increasingly distressed with these symptoms due to their approaching marriage and reduced vilazodone to 10 mg 5 months after its initiation and presented to our center for follow-up.
Due to persistence of decreased libido and in view of good response to mirtazapine in the past, vilazodone was cross-tapered with mirtazapine. Vilazodone was decreased from 10 mg to 5 mg and then stopped completely after 2 weeks. Mirtazapine was started at 7.5 mg and increased to 15 mg after 2 weeks. After 2-3 weeks of stopping vilazodone, the patient’s libido increased to her usual levels, although subsyndromal anxiety symptoms continued for a few more weeks despite continued mirtazapine. She married her fiancé 3 months after presentation to our center.
Vilazodone is often compared favorably with other antidepressants because of its purported lower propensity for sexual side effects.4,5 However, no head-to-head trials are available to compare its side effect profile with other antidepressants.2 The scarce literature available suggests that the sexual side effects, if present, with vilazodone are too mild to affect compliance.4,5 However, in the present case, we observed that the patient reduced her medication below therapeutic levels because of loss of sexual arousal and desire. It is important to be vigilant regarding such side effects because they cause considerable distress, reduce quality of life, and negatively affect compliance and management of patients.
References
1. Cruz MP. Vilazodone HCl (Viibryd): a serotonin partial agonist and reuptake inhibitor for the treatment of major depressive disorder. P&T. 2012;37(1):28-31. PubMed
2. Deardorff WJ, Grossberg GT. A review of the clinical efficacy, safety and tolerability of the antidepressants vilazodone, levomilnacipran and vortioxetine. Expert Opin Pharmacother. 2014;15(17):2525-2542. PubMed CrossRef
3. Wang SM, Han C, Lee SJ, et al. Vilazodone for the treatment of depression: an update. Chonnam Med J. 2016;52(2):91-100. PubMed CrossRef
4. Hopkins CR. ACS chemical neuroscience molecule spotlight on Viibryd (vilazodone). ACS Chem Neurosci. 2011;2(10):554. PubMed CrossRef
5. Hellerstein DJ, Flaxer J. Vilazodone for the treatment of major depressive disorder: an evidence-based review of its place in therapy. Core Evid. 2015;10:49-62. PubMed CrossRef
aDepartment of Psychiatry, All India Institute of Medical Sciences (AIIMS), Ansari Nagar, Delhi, India
Potential conflicts of interest: Dr Sharan was a principal investigator in a project funded by Eli Lilly on fibromyalgia that ended in January 2015. Drs Kathiresan and Chawla report no conflicts of interest related to the subject of this letter.
Funding/support: None.
Patient consent: Written permission was received from the patient to publish this case, and information has been de-identified to protect anonymity.
Published online: February 8, 2018.
Prim Care Companion CNS Disord 2018;20(1):17l02150
To cite: Sharan P, Kathiresan P, Chawla N. Vilazodone-induced decrease in libido: a case report. Prim Care Companion CNS Disord. 2018;20(1):17l02150.
To share: https://doi.org/10.4088/PCC.17l02150
© Copyright 2018 Physicians Postgraduate Press, Inc.
Please sign in or purchase this PDF for $40.00.
Save
Cite