ABSTRACT
Importance: Disorders of the autonomic nervous system are relatively common and have a significant impact on quality of life, offer very subtle diagnostic clues, and often mimic other disease processes, including certain psychiatric disorders. Pharmacologic treatment for psychiatric conditions in this group of patients can also be complicated by the pathophysiology of the various syndromes. Postural orthostatic tachycardia syndrome (POTS) is the final common pathway of a heterogenous group of underlying disorders that display similar characteristics.
Observations: The current literature regarding the association between POTS and psychiatric conditions was reviewed. The literature showed an increased prevalence of mild/moderate depression and sleep disturbance in this population. Also, when psychiatric disorders occur in patients with POTS, clinicians may face challenges with regard to selecting appropriate psychopharmacologic interventions.
Conclusions and Relevance: This review provides an evidence-based approach to treating common psychiatric conditions in those who suffer from POTS, with a particular emphasis on side effects that may worsen the associated symptoms. A list of the classes of psychopharmacologic treatment with a focus on adverse effects on heart rate and blood pressure is included, as is a case vignette of a patient with complex comorbid psychiatric conditions. It is of significant value to highlight the complexities associated with POTS; to raise awareness of the disorder, particularly in the context of psychiatric comorbidities; and to disseminate evidence-based information to aid clinicians in making informed medication choices with their patients.
Prim Care Companion CNS Disord 2023;25(1):22nr03243
To cite: Attard A, Attard S, Stanniland C, et al. Management of psychiatric conditions in patients with comorbid postural orthostatic tachycardia syndrome: a literature review and case vignette. Prim Care Companion CNS Disord. 2023;25(1):22nr03243.
To share: https://doi.org/10.4088/PCC.22nr03243
© 2023 Physicians Postgraduate Press, Inc.
aPharmacy Department, West London NHS Trust, London, United Kingdom
bCentral and North West London NHS Foundation Trust, London, United Kingdom
cSurrey and Borders Partnership NHS Foundation Trust, London, United Kingdom
dOxford University Hospital NHS Foundation Trust, London, United Kingdom
*Corresponding author: Stephen Attard, MBChB, Central and North West London NHS Foundation Trust, HMP Woodhill, Tattenhoe St, Milton Keynes MK4 4DA, United Kingdom ([email protected]).
Disorders of the autonomic nervous system present a challenge for the treating clinician. These syndromes have a significant impact on quality of life, offer very subtle diagnostic clues, and often mimic other disease processes, including certain psychiatric disorders. Pharmacologic treatment for psychiatric conditions in this group of patients can also be complicated by the pathophysiology of the various syndromes. It is necessary to shift one’s normal focus when considering the side effect profiles of potential pharmacologic interventions in these patients.
Postural orthostatic tachycardia syndrome (POTS) is the final common pathway of a heterogenous group of underlying disorders that display similar characteristics.1 The degree of functional impairment in patients with POTS has been reported to be similar to that in chronic obstructive pulmonary disease and congestive heart failure.2 POTS is not considered to be a distinct disease but rather a collection of overlapping pathophysiologic processes.3
A 2011 consensus statement outlined the diagnostic criteria for POTS as a sustained heart rate increment of at least 30 bpm within 10 minutes of standing or head-up tilt in the absence of orthostatic hypotension.3 For individuals aged 12 to 19 years, the increment should be at least 40 bpm.3 These criteria along with common symptoms are outlined in Table 1.3–5
Patients frequently report the onset of symptoms following acute stressors such as a traumatic event, surgery, or a viral illness.5,6 Symptoms can be worsened by simple activities of daily living such as standing still, exercise, food or alcohol ingestion, or heat exposure.5,6 A grading system exists for orthostatic intolerance, which focuses on functional severity in a manner similar to the classifications used by heart associations such as the New York Heart Association.
The objective of this article is to review the literature regarding the association between POTS and psychiatric conditions. A case vignette of a patient with complex comorbid psychiatric conditions is also presented. This article aims to provide an evidence-based approach to treating common psychiatric conditions in those who suffer from POTS, with a particular emphasis on side effects that may worsen the symptoms associated with the disorder.
LITERATURE REVIEW
Pathophysiology
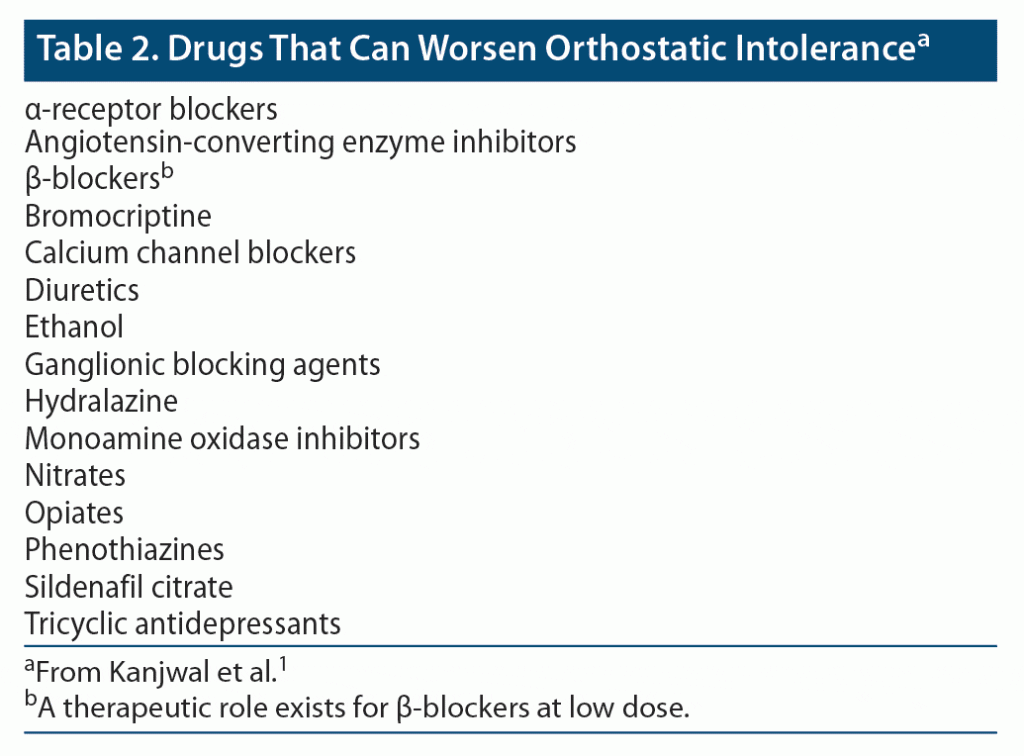
The pathophysiology underlying POTS remains incompletely understood but is thought to be multifactorial and variable across subgroups of POTS patients. It is a syndrome, not a disease. Factors such as moderate autonomic dysfunction, increased sympathetic tone, severe deconditioning, inadequate venous return, or excessive blood venous pooling may contribute to POTS symptoms.7 Common POTS phenotypes include neuropathic POTS, central hyperadrenergic POTS, norepinephrine transporter deficiency and blockers, mast cell activation, and hypovolemia and blood volume regulation.4 Work by Li et al8 further suggests considering POTS as a form of cardiovascular dysfunction that can have an effect on the receptors needed for a normal response to orthostasis. A proportion of patients, as indicated in work by Shibao and colleagues9 and Wang et al,10 have presentations suggestive of POTS being conceptualized as a cardiovascular disorder, whereby autoimmune and hyperadrenergic clinical states are a contributing factor. Although this conceptualization remains somewhat controversial and immunotherapy is rarely indicated, it further highlights the difficulty faced by clinicians in choosing pharmacologic treatment, as currently available agents have an effect on the receptors needed for a normal response to orthostasis. The novel syndrome of chronic hypoadrenergic orthostatic intolerance has been associated with mast cell activation and identified particularly in patients who experience flushing as a symptom, highlighting the importance of specific questioning by the clinician. One example of the significant challenges in prescribing medication in patients with POTS is that β-blockers, a common therapy used to treat POTS, may induce mass cell activation. In this subgroup, histamine blockers and central sympatholytics should be chosen in place of β-blockers.
Etiology
The largely nonspecific symptoms, the natural variability in heart rate responses to orthostasis in the general population, and a lack of specific biomarkers have made it difficult to establish the true prevalence of POTS. In a cross-sectional study from the United Kingdom11 assessing the frequency of symptoms and their associated variables, it was concluded that POTS patients are characteristically women, young, and well educated and have significant and debilitating symptoms that impact their quality of life. An expert consensus statement11 expressed that, while the natural history of POTS is unclear, it does not seem to increase the risk of mortality.
Due to the complex nature of POTS presentation (see Table 2 for a list of drugs that can worsen POTS), patients are often subjected to numerous and extensive unnecessary examinations, with fragmented clinical care by multiple specialists. Ultimately, therapy is primarily directed toward symptom control.
Treatment of POTS
No single therapy has proved to be uniformly successful, further highlighting the complex nature of the syndrome. Combinations of sometimes multiple nonpharmacologic and pharmacologic treatments are often necessary. As a consultant psychiatrist may refer patients with comorbid POTS, a multidisciplinary approach spanning physical and mental health services is recommended. Initially, efforts to reverse the causative factor, if identified, is the focus.
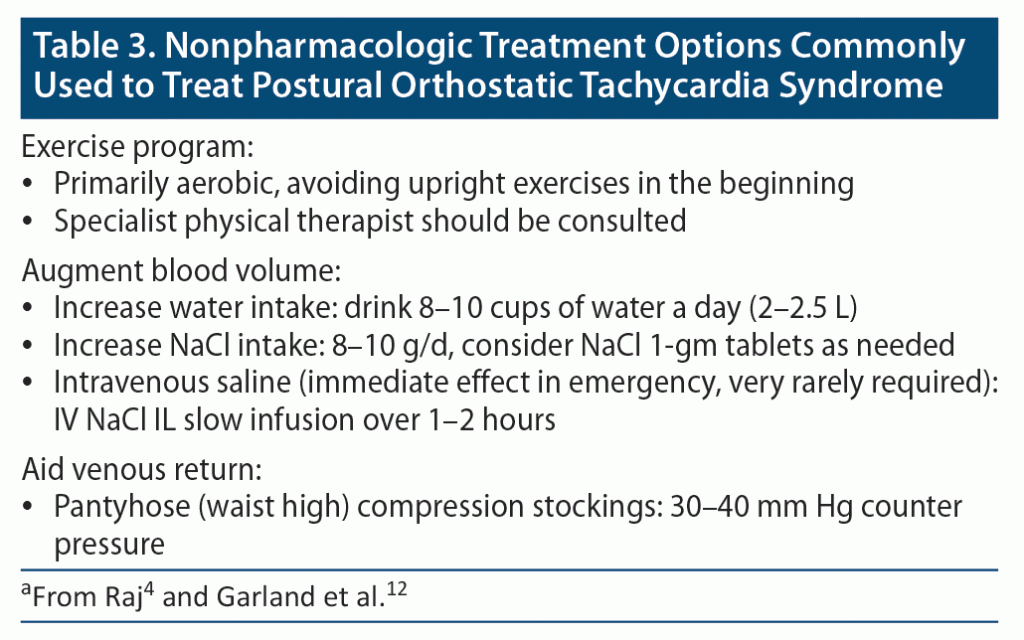
Table 3 summarizes the mainstay of nonpharmacologic therapy. Recommendations include ensuring that patients are well hydrated, increasing their sodium intake, and engaging in specialist exercise programs, bearing in mind that many POTS patients feel debilitated for days after exertion.4,12 Participation in exercise programs undoubtedly requires that the patient understands ways to ensure their symptoms are not aggravated. A structured 3-month exercise program delivered to 19 patients in one study1 showed improvements in blood volume, left ventricular mass, and stroke volume; reduced orthostatic tachycardia; and improved quality of life. That trial1 indicates the potential longer-term prognostic gains when exercise regimens are carefully introduced.
Medication Used to Treat POTS
In a cross-sectional study13 from the United Kingdom assessing the frequency of POTS and its associated variables, it was determined that up to 33% of the clinic cohort were taking no medications for their POTS. This mirrors results of a larger US study from the Mayo Clinic.5 An initial treatment step is often the withdrawal of medication that has the potential to worsen hypovolemia such as drospirenone or oral contraceptives.4 Withdrawal of medication that can increase peripheral sympathetic tone and worsen tachycardia is also common, including tricyclic antidepressants and atomoxetine (used for attention-deficit/hyperactivity disorder [ADHD]).4
While no medications are specifically approved for POTS, commonly used treatments include β-blockers such as propranolol at low doses to reduce tachycardia. Also, α-1 agonists such as midodrine and methylphenidate, a long-acting α-agonist, have also been found to be effective by improving orthostatic tolerance (avoiding bedtime, as it can cause insomnia).14,15 Medications that increase blood volume such as fludrocortisone (monitoring for hypokalemia) and desmopressin (monitoring for hyponatremia) and pyridostigmine may increase parasympathetic tone and be beneficial.4,16 A retrospective study17 of 121 adolescent patients with a follow-up survey found that metoprolol had contributed to their progress in comparison to midodrine. Octreotide, a somatostatin analog with potent vasoconstrictive effects, can be used as an add-on therapy for resistant POTS.18 Once again, it is important to note that β-blockers are also known to worsen tachycardia as well as POTS secondary to mast cell activation in some subgroups.18 Ivabradine, a selective blocker of the If channel of the sinoatrial node that does not lower blood pressure, has also been used with some success.19
Although there is an absence of evidential data, there are some proponents for the use of selective serotonin reuptake inhibitors (SSRIs) and even serotonin-norepinephrine reuptake inhibitors (SNRIs) in the second-line treatment of some POTS patients, despite clear evidence of SNRIs in particular primarily exacerbating POTS symptoms.20 It has, for example, been considered that SSRIs could increase standing blood pressure and subsequently decrease reflex tachycardia through inhibition of catecholamine reuptake.20 However, in a study20 in which the primary endpoint was standing heart rate 4 hours post dose, sertraline had a modest effect on increasing blood pressure in POTS patients but did not translate to a reduced heart rate or improved POTS symptoms. Indeed, it was considered that sertraline may have exacerbated POTS symptoms.20 Further, in a nonrandomized retrospective chart review of 47 patients that evaluated for significant findings including reduced orthostasis and improvement of symptoms with use of bupropion XL 150–300 mg/d (a norepinephrine reuptake and dopamine reuptake inhibitor), bupropion was not associated with a statistically significant improvement in orthostatic vital signs.21
Some medications, such as the stimulant modafinil, are specifically utilized to target the cognitive symptoms of POTS, though this can also exacerbate tachycardia.22 In a placebo-controlled trial, modafinil was not found to worsen tachycardia on standing for 4 hours and did not worsen the POTS symptom burden scores, although a change was noticed in sitting and standing blood pressure.22
Common Concurrent Clinical Syndromes
Irritable bowel syndrome. Symptoms of irritable bowel syndrome, food intolerance, and allergic sinusitis are commonly reported in patients with POTS.16 Many patients will go through a process of restricting their diet in an attempt to identify triggers for orthostatic intolerance, although the benefits of a low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet remains uncertain.23 Interestingly, the treatment of POTS may improve irritable bowel syndrome symptoms, as seen in a cohort of children treated with fludrocortisone.24
Ehlers-Danlos syndrome. Hypermobility, often diagnosed by the standardized Brighton score tool, is overrepresented in patients with POTS.25,26 Connective tissue matrix weakness is one possible reason for the link between connective tissue hypermobility and POTS. Copresence of anxiety, interoceptive sensibilities, chronic fatigue syndrome (discussed later in this article), and either hypermobility or POTS has been reported.27,28
Vascular compression syndromes. The association of vascular compression syndromes such as median arcuate ligament syndrome, thoracic outlet syndrome, and pelvic compression syndrome with POTS is thought to be due to the reduction in venous return.29,30 The clinical implications of treating these individual syndromes to reduce POTS symptomatology is still unknown and is beyond the scope of this review.
COVID-19 and POTS
POTS is increasingly becoming recognized as an important component of long coronavirus disease 2019 (COVID-19) syndrome, and this is a developing area of active research.31 Proposed pathophysiologic mechanisms32–34 include the following:
- Hypovolemia, fever, anorexia, nausea, excessive nocturnal sweating, and prolonged bed rest, resulting in decreased blood volume and increased cardiac sympathetic noradrenergic system (SNS) outflow
- COVID-19 infection and destruction of extracardiac postganglionic SNS neurons, leading to increased cardiac SNS outflow
- COVID-19 invasion of the brain stem, affecting functions of the medullary centers and increasing central sympathetic outflows
- Autoimmunity
- COVID-19 interaction with the neuronal angiotensin converting enzyme 2 (ACE2) protein, disrupting the ACE2-mediated regulation of blood pressure
- Mast cell activation syndrome.
In a retrospective study of 27 patients whose symptoms met the criteria for long COVID, symptoms included lightheadedness (93%), orthostatic headache (22%), and syncope (11%).35 Orthostatic symptoms without tachycardia or hypotension was the most common scenario (41%), and 22% of the patients met the criteria for POTS.35
In the wake of the COVID-19 pandemic, there has been a burgeoning body of case reports and case series in the literature regarding people who have developed POTS after infection.36–40 The experience of a 50-year-old woman, who continued to meet the criteria for POTS and mast cell activation a year after the onset of COVID-19, revealed that post-COVID symptoms can be prolonged and possibly chronic.41 Although her POTS persisted, it was being effectively managed with amphetamine salts 5 mg daily.41 In a case series of 20 patients, 15 were diagnosed with POTS, 3 with neurocardiogenic syncope, and 2 with orthostatic hypotension; 85% of the patients reported residual symptoms 6–8 months after COVID-19 infection.42 All of the patients were advised to use nonpharmacologic management for their conditions. Pharmacotherapy, for the 16 patients who needed it, included β-blockers, fludrocortisone, midodrine, and ivabradine in addition to symptomatic management of comorbid conditions.42 In a case report,43 a 22-year-old woman with COVID-19–induced POTS that did not respond to nonpharmacologic interventions was successfully treated with ivabradine 5 mg twice/d. Another report44 of a 25-year-old woman found bisoprolol 2.5 mg/d was markedly effective for acute symptomatic improvement and reducing the standing heart rate.
Patients may experience disabling symptoms, although testing does not quite meet the threshold for POTS. In a cohort of 6 patients who reported symptoms typical of COVID-19–associated dysautonomia, testing revealed postural tachycardia in 4 patients, 1 patient with orthostatic hypotension and cardiovagal impairment, and 1 patient with postural hypertension and cardiovagal impairment.45 The American Autonomic Society published a statement including a review of the potential impact of long COVID POTS on health care systems and opportunities for further research.46
Common Psychiatric Comorbidities That Exist in POTS Patients
Patients with POTS commonly complain of symptoms including fatigue and difficulty with sleep. But when considering whether POTS patients objectively suffer from a poorer quality of life, with regard to psychological well-being, studies have produced varied results depending largely on the type of objective score used.47 A study2 carried out by the Mayo Clinic Autonomic Disorders Laboratory used the 36-item Short-Form Health Survey (SF-36) and found that patients with POTS had a diminished quality of life (a lower score in 6 of the 8 SF-36 domains) but did not score poorly on psychological domains. This study2 concluded that patients with POTS experience clear limitations across multiple domains of quality of life, including physical, social, and role functioning. The authors therefore suggest a focused treatment to address the multiple and varied impairments rather than aiming to globally improve quality of life.2
In a questionnaire-based assessment of symptoms, 107 patients aged 12–19 years with excessive orthostatic tachycardia completed the Orthostatic Intolerance Questionnaire, the Beck Depression Inventory-II, and the SF-36.48 The findings revealed that orthostatic intolerance symptoms, but not the maximal heart rate increment, correlated significantly with depression and diminished quality of life.48
In another study,49 the EuroQoL “health thermometer” was used to assess patients’ health. Mobility, self-care, usual activities, pain/discomfort, and anxiety/depression were significantly worse in patients with POTS.49 In terms of health-related quality of life, POTS patients scored significantly lower in physical health composite scores (26 ± 9 vs 54 ± 6, P < .0001), and mental health composite scores were also lower in POTS patients (43 ± 11 vs 52 ± 10, P = .002).49 An interesting point to consider, however, is that while sleep problems affected both physical and mental health domains, physical health was more diminished by sleep problems.
Sleep Disturbances
It is now commonly accepted that subjective poor sleep is associated with reduced physical performance, greater functional limitation, and increased risk of cardiovascular diseases and may even predict all-cause mortality.50–53 A study49 found that patients with POTS have poor sleep quality, excessive sleepiness, and excessive fatigue, and many suffered diminished quality of life. The reports of poor sleep quality are consistent with a reduction in sleep efficiency determined by actigraphy (a noninvasive method of monitoring human rest/activity cycles).53 To put this into clinical context, patients with POTS reported poor sleep adequacy, shortness of breath or headache with sleep, and sleep somnolence.52 POTS patients have also been shown to have excessive daytime somnolence (51% vs 16%, P = .0004 on the Epworth Sleepiness Scale) and greater fatigue (7.5 ± 2.0 vs 2.8 ± 2.5, P < .0001 on the Fatigue Visual Analog Scale) compared to control patients.49
Sleep disturbance on its own should be a clinical concern, as it is linked with an increased risk of suicidal ideation and suicide.50,54 Insomnia has further been linked independently with an increased risk of depression.55,56 The association between insomnia and suicide remains, even when controlling for depressive disorder, intensity of depression, and hopelessness.57,58 In a study57 using online surveys, including the Pittsburgh Sleep Quality Index Questionnaire-revised, with 705 POTS patients and 104 non-POTS patients, 98.4% of POTS patients had poor sleep quality compared to 69.4% in the control group. POTS patients reported frequent (> 3 times a week) sleep disturbances from pain (53%), failing to fall asleep within 30 minutes (59.9%), and waking in the middle of the night or early morning (68.9%) and had more disturbances from bad dreams and breathing problems.57
Chronic Fatigue Syndrome
Patients with POTS in one study59 reported a mean level of fatigue almost 3 times greater than normal. Chronic fatigue syndrome (CFS) is characterized by persistent or relapsing unexplained fatigue and related symptoms of at least 6 months duration. Like POTS, CFS is a clinical syndrome that is commonly diagnosed in females. There are data to suggest an overlap in the pathophysiology between POTS and CFS. Orthostatic hypotension, tachycardia, and subnormal erythrocyte volume, all characteristic features of CFS, are also present in patients with POTS.59 Additionally, a 27% incidence of POTS was found in well-characterized patients with CFS.60
Depression and Anxiety
The mechanisms underlying depression in POTS patients have not been well studied. Difficulties in concentration and chest discomfort were most strongly correlated with depression in POTS patients.48,61 Many studies have found an increased incidence of depression in patients with POTS. Anderson et al60 found that 87% of POTS patients met the threshold for clinician-rated mild to moderate depressive disorder compared with 3% of matched healthy subjects. Raj et al62 noted that, psychologically, POTS patients face challenges seen in many chronic illnesses, particularly in the early stages of adjusting to the disease, which include mild to moderate depression. Suicidal ideation, threatened suicide (37% vs 19%), and attempted suicide (16% vs 3%) have also been found to be higher in patients with POTS than in matched control subjects.57
Although patients with POTS commonly present with symptoms of anxiety, a structured evaluation using the DSM-IV criteria did not identify a higher incidence of anxiety disorder or substance abuse compared to the general population.60 Indeed, the tachycardia, palpitations, hyperventilation, and tremulousness consequential to POTS can be misinterpreted as symptoms of anxiety.63,64 However, while it is still thought that physical anxiety symptoms in POTS patients are largely driven by the physiologic process of the disease, studies64 suggest that POTS patients are more likely to experience anxiety than matched controls.
Memory Complaints or Cognitive Function
Patients with POTS often complain of “memory problems” and “mental clouding,” which might represent decreased attention and concentration.62,65 A comprehensive neuropsychological evaluation of 28 patients in a seated position found deficits in selective and cognitive processing as well as impaired executive functioning.62 Memory functioning in these patients, however, did not differ from healthy controls.62 Other studies60 have found impairments in working memory of a pattern similar to that found in chronic fatigue syndrome.
Treatment of Psychiatric Comorbidities
In addition to the increased prevalence of mild-moderate depression and sleep disturbance in this population, when psychiatric disorders occur in patients with POTS, clinicians can face challenges with regard to selecting appropriate psychopharmacologic interventions. The side effect profile of medications should be considered within the context of the clinical manifestations of POTS. The cardiovascular side effects of some commonly used psychotropic drugs are outlined in Table 4.
CASE VIGNETTE
A 24-year-old woman was referred to the NHS community home assessment and treatment team by her early intervention in psychosis care coordinator. She had an existing diagnosis of schizophrenia for which she was receiving quetiapine modified release 400 mg/d. Her symptoms of psychosis had been in remission for 2 years, but she had reported mild tachycardia since starting this medication. There was a positive family history of mood disorder and Ehlers-Danlos syndrome. For several months, she had not wanted to leave the house or attend college and had developed increasing symptoms of anxiety, sleep disturbance, reduced appetite, fatigue, and sudden onset of “panic” when in the community, such as at a bus stop or at college. She described these symptoms as including an unusually high heart rate, shallow breathing, dizziness, and chest pain, and she was unable to move when very severe. The symptoms worsened when she was sitting upright or trying to walk. As the symptoms emerged, she had begun to self-harm by cutting her arms. She was initially prescribed citalopram 20 mg, but her core symptoms remained unchanged. After 6 weeks, this medication was replaced by venlafaxine, and she was referred to the NHS community home assessment and treatment team due to lack of improvement in her presentation and the severity of her symptoms. At the same time, she was referred for a cardiology review, given her recurrent experience of tachycardia, chest pain, and dizziness.
The community home assessment and treatment team’s initial intervention included increasing her dose of venlafaxine 125 mg modified release and adding quetiapine up to 300 mg modified release. As the doses were increased, her symptoms of tachycardia and chest pain became progressively worse until she was effectively bedbound. Her level of distress correspondingly increased, as did the frequency and degree of her self-harming behavior. During cardiology review, her echocardiogram revealed no abnormalities, but the Holter monitor identified repeated episodes of tachycardia. The clinical picture was suggestive of POTS. The results of a subsequent head-up tilt test suggested grade 3 orthostatic intolerance. In liaison with the mental health team, her venlafaxine dose was reduced and then stopped (as this may have been contributing to the tachycardia), and she was started on propranolol 10 mg 3 times/d. Her physical symptoms began to improve. Close liaison between community mental health services, cardiology, and the primary care team was maintained over subsequent months. Her quetiapine dose was cautiously changed to aripiprazole 15 mg/d, as it is less likely to cause a postural hypotensive drop and changes in heart rate compared to quetiapine. An SSRI (sertraline 100 mg) was reinstated in place of venlafaxine, as venlafaxine can cause changes in heart rate and blood pressure. Melatonin was used to aid her sleep, as it has very few cardiac side effects and did not interact with any of her medication to add to her adverse effect burden. It was primarily used to counteract any stimulative/insomnia-related side effects from aripiprazole. Within 6 months, there was a significant improvement in her symptoms and a corresponding decrease in her experience of distress, and she was once again attending college.
Psychological Interventions
Psychological factors can amplify the experience of chronic health conditions such as POTS. Anxiety about symptom onset, a reduction in previously enjoyed activities and the capacity to work, and loss of relationships can all contribute to a person’s experience of distress and functional loss. Chronic health conditions can also have a significant impact on a person’s identity, sense of self, and their beliefs and hopes for the future.
There is limited evidence for the efficacy of specific psychological interventions in the treatment of POTS, with treatment recommendations generally growing out of experience of other chronic conditions such as pain. However, in general terms, physical and psychological symptoms of many chronic health conditions such as poor sleep, fatigue, and impaired cognitive functioning can be improved through psychological interventions.
Cognitive-behavioral therapy (CBT) is a mainstay of psychological intervention for POTS, centered on developing awareness of the interaction between physical responses, thoughts, and emotions with the goal of identifying and changing unhelpful patterns. Catastrophic thinking, for example, which can be associated with functional impairment in POTS, is a potential area of focus in CBT.2
There is increasing evidence in the literature for the efficacy of so-called “third-wave” CBT approaches, incorporating mindfulness, self-compassion, and acceptance principles in patients with chronic physical health conditions. Some recommend that, in the absence of treatment tailored specifically for POTS, it is reasonable to make appropriate adjustments to protocols with proven efficacy for other chronic conditions.64 The variability of presentation and broader context of each patient highlights the need for an individualized approach in this group.
Summary of Suggested Pharmacologic Choices of Common Psychiatric Conditions in POTS Patients
It is imperative that a multidisciplinary approach is taken where possible, involving cardiology, mental health services, physical health practitioners, and dietitians working together to coordinate a holistic treatment package and closely monitor any untoward side effects resulting from use of psychotropic medication in POTS patients. Patients are more sensitive to adverse effects related to their physical health.
The side effect profile of medication, particularly in relation to its potential to exacerbate the manifestations of POTS, should be a primary factor when considering pharmacologic interventions for mental health disorders in patients with POTS. Given the potential for side effects, which may exacerbate POTS symptoms, regular monitoring and review of medication should be maintained. Of late, much emphasis on the choice of medication for patients is placed on the safety in overdose and the cardiac safety profile of the medication including potential prolongation of QTc interval, development of Torsades de pointes, and arrhythmia. Choices are also primarily driven by the longer-term cardiometabolic syndrome impact and risk of dependency. However, when choosing a psychotropic for patients with POTS, the emphasis shifts somewhat. While obviously still weighing the above factors, more attention should be paid to the medication’s effect on the patient’s heart rate and blood pressure changes, ensuring that the side effects of any psychopharmacologic intervention do not have similar symptoms to POTS itself (for example worsening gastrointestinal symptoms or insomnia).
Depression and Anxiety
Issues such as toxicity in overdose and the antidepressant’s overall side effect profile need to be considered at all times. SSRIs are an appropriate first-line medication for the pharmacologic treatment of depression and anxiety in patients with POTS.18 Recommendations for drugs that should be used as second- or third-line treatments should be taken with caution, as there are conflicting case reports, and the body of evidence is lacking. Sometimes, clinicians may find that patients with depression, symptoms of ADHD, and fibromyalgia have been prescribed SNRIs. It is imperative to review this treatment given the potential negative impact of SNRIs on POTS symptoms, as highlighted in a randomized, placebo-controlled trial in which a 40-mg oral dose of the SNRI atomoxetine increased orthostatic tachycardia and worsened symptoms in POTS patients.70 Tricyclic antidepressants, such as amitriptyline and nortriptyline, should be used with caution in POTS patients, since they can exacerbate tachycardia and increase drowsiness and cognitive impairment. With treatment-resistant depression in the general population, the use of SNRIs alone or in combination is a well-established regimen; in the POTS population, however, extreme caution is recommended. The randomized, placebo-controlled trial of atomoxetine in POTS patients found that it increased heart rate and worsened symptoms.70 Another study by Schroeder and colleagues71 found that reboxetine, a highly selective norepinephrine inhibitor was associated with many healthy participants suffering from hyperadrenergic symptoms. SNRIs and tricyclic antidepressants can also negatively impact the cognitive dysfunction or brain fog commonly experienced by patients with POTS.72
Sleep Disturbance
Melatonin is an appropriate sleep aid in patients with POTS. In addition to decreasing the sympathetic response to orthostatic stress, supplementation may be particularly helpful in POTS patients prescribed β-blockers, as these medications can lower melatonin naturally.6,66 Z-hypnotics are also appropriate for use in patients with POTS, though their longer-term use is not recommended given their potential for dependence. The tricyclic amitriptyline has long been used as a sleep aid in low doses, particularly in primary care settings; however, this would not be recommended in POTS patients given the potential for symptom exacerbation.73 It is also not uncommon for antipsychotic medications, particularly quetiapine, to be prescribed for insomnia in primary care settings.74 Again, the use of such medication in patients with POTS could lead to an exacerbation of symptoms and is not recommended.
Psychosis
The avoidance of antipsychotic medications that have a significant effect on heart rate and blood pressure is of paramount importance. Antipsychotics that have the greatest impact on changes to the heart rate or blood pressure may worsen POTS symptoms and cause an increase in anxiety and depression. It is fair to say that as a group, first-generation antipsychotics are best avoided; changes in heart rate, particularly tachycardia, are a common side effect in every first-generation antipsychotic currently. Similarly, orthostatic hypotension or hypotension in general is a common side effect.
Almost all second-generation antipsychotics can cause changes in heart rate. To fully inform a decision, the probability or significance of the side effect is imperative. The details in these differences are ultimately what best informs the choice of medication. Those antipsychotics that require a slow titration to avoid postural hypotension such as quetiapine or titration to prevent tachycardia such as clozapine should not be considered as first-line therapy in this cohort of patients.
Bipolar Affective Disorder
Some general considerations apply when choosing a mood stabilizer. For example, one must be acutely aware of the drug interaction profile of carbamazepine, which may inhibit or induce the cytochrome P450 enzymes in the liver and have a negative effect on other medications the patient may be taking. The risk of birth defects and developmental disorders in children born to women who take sodium valproate during pregnancy needs to be considered.
With regard to POTS patients, there is no evidence in the literature to suggest that mood stabilizers are likely to exacerbate symptoms of POTS. In terms of the potential effect on heart rate and blood pressure, lamotrigine and sodium valproate seem relatively benign in this regard. It may be worthwhile to consider sodium valproate as first-line treatment for women past childbearing age and for men. Lamotrigine could be considered as an alternative first-line treatment for women of childbearing age. When considering the use of antipsychotic medications to treat bipolar affective disorder, the same considerations given when selecting medication for the treatment of psychosis would apply in relation to POTS.
Summary
POTS is a complex multifactorial syndrome and a common symptom of long COVID. POTS has various phenotypes (which we cannot yet accurately test for), and its multidisciplinary management, involving nonpharmacologic therapy followed by a choice of pharmacology options dependent on the POTS phenotype, is a significant challenge to specialist physicians. There is a need for a better understanding and a closer working relationship among psychiatric and general medical physicians in the management and treatment of comorbid mental illness.
It is beyond the scope of this review to offer prescriptive guidance on psychotropic medication for the psychiatric comorbidities experienced by POTS patients due to the complexity and diversity of expression of this syndrome. A prime example of this is the use of SNRIs in combination with SSRIs in one phenotype, while recommending the complete avoidance of an SNRI in others.
However, it is of significant value to highlight these complexities; raise awareness of POTS, particularly in the context of psychiatric comorbidities; and to disseminate what we would hope is useful information to aid clinicians in making informed medication choices with their patients. A call for more research and guidance is without a doubt a reflective outcome from this writing.
Submitted: January 10, 2022; accepted May 25, 2022.
Published online: January 31, 2023.
Relevant financial relationships: None.
Funding/support: None.
Clinical Points
- POTS is moving increasingly into the limelight as its link with long COVID is explored.
- Common treatments for psychiatric disorders can exacerbate POTS symptoms.
- A multidisciplinary approach is often key to the effective care of POTS patients with psychiatric disorders.
References (74)

- Kanjwal Y, Kosinski D, Grubb BP. The postural orthostatic tachycardia syndrome: definitions, diagnosis, and management. Pacing Clin Electrophysiol. 2003;26(8):1747–1757. PubMed CrossRef
- Benrud-Larson LM, Dewar MS, Sandroni P, et al. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc. 2002;77(6):531–537. PubMed CrossRef
- Freeman R, Wieling W, Axelrod FB, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21(2):69–72. PubMed CrossRef
- Raj SR. Postural tachycardia syndrome (POTS). Circulation. 2013;127(23):2336–2342. PubMed CrossRef
- Thieben MJ, Sandroni P, Sletten DM, et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. 2007;82(3):308–313. PubMed CrossRef
- Raj SR. The postural tachycardia syndrome (POTS): pathophysiology, diagnosis & management. Indian Pacing Electrophysiol J. 2006;6(2):84–99. PubMed
- Grubb BP, Kosinski DJ, Boehm K, et al. The postural orthostatic tachycardia syndrome: a neurocardiogenic variant identified during head-up tilt table testing. Pacing Clin Electrophysiol. 1997;20(9 Pt 1):2205–2212. PubMed CrossRef
- Li H, Yu X, Liles C, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. 2014;3(1):e000755. PubMed CrossRef
- Shibao C, Arzubiaga C, Roberts LJ 2nd, et al. Hyperadrenergic postural tachycardia syndrome in mast cell activation disorders. Hypertension. 2005;45(3):385–390. PubMed CrossRef
- Wang XL, Chai Q, Charleswort MC, et al. Autoimmunoreactive IgGs from patients with PoTS. Proteomics Clin Appl. 2012;6(11–12):615–625. PubMed CrossRef
- Sheldon RS, Grubb BP 2nd, Olshansky B, et al. 2015 Heart Rhythm Society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12(6):e41–e63. PubMed CrossRef
- Garland EM, Celedonio JE, Raj SR. Postural tachycardia syndrome: beyond orthostatic intolerance. Curr Neurol Neurosci Rep. 2015;15(9):60. PubMed CrossRef
- McDonald C, Koshi S, Busner L, et al. Postural tachycardia syndrome is associated with significant symptoms and functional impairment predominantly affecting young women: a UK perspective. BMJ Open. 2014;4(6):e004127. PubMed CrossRef
- Grubb BP, Karas B, Kosinski D, et al. Preliminary observations on the use of midodrine hydrochloride in the treatment of refractory neurocardiogenic syncope. J Interv Card Electrophysiol. 1999;3(2):139–143. PubMed CrossRef
- Grubb BP, Kosinski D, Mouhaffel A, et al. The use of methylphenidate in the treatment of refractory neurocardiogenic syncope. Pacing Clin Electrophysiol. 1996;19(5):836–840. PubMed CrossRef
- Mathias CJ, Low DA, Iodice V, et al. Postural tachycardia syndrome–current experience and concepts. Nat Rev Neurol. 2011;8(1):22–34. PubMed CrossRef
- Lai CC, Fischer PR, Brands CK, et al. Outcomes in adolescents with postural orthostatic tachycardia syndrome treated with midodrine and beta-blockers. Pacing Clin Electrophysiol. 2009;32(2):234–238. PubMed CrossRef
- Agarwal AK, Garg R, Ritch A, et al. Postural orthostatic tachycardia syndrome. Postgrad Med J. 2007;83(981):478–480. PubMed CrossRef
- Gee ME, Watkins AK, Brown JN, et al. Ivabradine for the treatment of postural orthostatic tachycardia syndrome: a systematic review. Am J Cardiovasc Drugs. 2018;18(3):195–204. PubMed CrossRef
- Mar PL, Raj V, Black BK, et al. Acute hemodynamic effects of a selective serotonin reuptake inhibitor in postural tachycardia syndrome: a randomized, crossover trial. J Psychopharmacol. 2014;28(2):155–161. PubMed CrossRef
- Vyas R, Nesheiwat Z, Ruzieh M, et al. Bupropion in the treatment of postural orthostatic tachycardia syndrome (POTS): a single-center experience. J Investig Med. 2020;68(6):1156–1158. PubMed CrossRef
- Kpaeyeh J Jr, Mar PL, Raj V, et al. Hemodynamic profiles and tolerability of modafinil in the treatment of postural tachycardia syndrome: a randomized, placebo-controlled trial. J Clin Psychopharmacol. 2014;34(6):738–741. PubMed CrossRef
- Sampson HA, Gerth van Wijk R, Bindslev-Jensen C, et al. Standardizing double-blind, placebo-controlled oral food challenges: American Academy of Allergy, Asthma & Immunology-European Academy of Allergy and Clinical Immunology PRACTALL consensus report. J Allergy Clin Immunol. 2012;130(6):1260–1274. PubMed CrossRef
- Krogsgaard LR, Lyngesen M, Bytzer P. Systematic review: quality of trials on the symptomatic effects of the low FODMAP diet for irritable bowel syndrome. Aliment Pharmacol Ther. 2017;45(12):1506–1513. PubMed CrossRef
- Fortunato JE, Wagoner AL, Harbinson RL, et al. Effect of fludrocortisone acetate on chronic unexplained nausea and abdominal pain in children with orthostatic intolerance. J Pediatr Gastroenterol Nutr. 2014;59(1):39–43. PubMed CrossRef
- De Wandele I, Rombaut L, Leybaert L, et al. Dysautonomia and its underlying mechanisms in the hypermobility type of Ehlers-Danlos syndrome. Semin Arthritis Rheum. 2014;44(1):93–100. PubMed CrossRef
- Bulbena A, Baeza-Velasco C, Bulbena-Cabré A, et al. Psychiatric and psychological aspects in the Ehlers-Danlos syndromes. Am J Med Genet C Semin Med Genet. 2017;175(1):237–245. PubMed CrossRef
- Bulbena A, Gago J, Pailhez G, et al. Joint hypermobility syndrome is a risk factor trait for anxiety disorders: a 15-year follow-up cohort study. Gen Hosp Psychiatry. 2011;33(4):363–370. PubMed CrossRef
- Kaymak B, Ozçakar L, Oğuz AK, et al. A novel finding in thoracic outlet syndrome: tachycardia. Joint Bone Spine. 2004;71(5):430–432. PubMed CrossRef
- Özçakar L, Ertan H, Kaymak B. Two cases and two particular signs of thoracic outlet syndrome: tremor and tachycardia. Rheumatol Int. 2008;29(2):227–228. PubMed CrossRef
- Touyz RM, Boyd MOE, Guzik T, et al. Cardiovascular and renal risk factors and complications associated with COVID-19. CJC Open. 2021;3(10):1257–1272. PubMed CrossRef
- Goldstein DS. The possible association between COVID-19 and postural tachycardia syndrome. Heart Rhythm. 2021;18(4):508–509. PubMed CrossRef
- Chilazi M, Duffy EY, Thakkar A, et al. COVID and cardiovascular disease: what we know in 2021. Curr Atheroscler Rep. 2021;23(7):37. PubMed CrossRef
- Yong SJ. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect Dis (Lond). 2021;53(10):737–754. PubMed CrossRef
- Shouman K, Vanichkachorn G, Cheshire WP, et al. Autonomic dysfunction following COVID-19 infection: an early experience. Clin Auton Res. 2021;31(3):385–394. PubMed CrossRef
- Miglis MG, Prieto T, Shaik R, et al. A case report of postural tachycardia syndrome after COVID-19. Clin Auton Res. 2020;30(5):449–451. PubMed CrossRef
- Umapathi T, Poh MQW, Fan BE, et al. Acute hyperhidrosis and postural tachycardia in a COVID-19 patient. Clin Auton Res. 2020;30(6):571–573. PubMed CrossRef
- Kanjwal K, Jamal S, Kichloo A, et al. New-onset postural orthostatic tachycardia syndrome following coronavirus disease 2019 infection. J Innov Card Rhythm Manag. 2020;11(11):4302–4304. PubMed CrossRef
- Johansson M, Ståhlberg M, Runold M, et al. Long-haul post-COVID-19 symptoms presenting as a variant of postural orthostatic tachycardia syndrome: The Swedish Experience. JACC Case Rep. 2021;3(4):573–580. PubMed
- Petracek LS, Suskauer SJ, Vickers RF, et al. Adolescent and young adult ME/CFS after confirmed or probable COVID-19. Front Med (Lausanne). 2021;8:668944. PubMed CrossRef
- Schofield JR. Persistent antiphospholipid antibodies, mast cell activation syndrome, postural orthostatic tachycardia syndrome and post-COVID Syndrome: 1 year on. Eur J Case Rep Intern Med. 2021;8:002378. PubMed CrossRef
- Blitshteyn S, Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol Res. 2021;69(2):205–211. PubMed CrossRef
- O’Sullivan JS, Lyne A, Vaughan CJ. COVID-19-induced postural orthostatic tachycardia syndrome treated with ivabradine. BMJ Case Rep. 2021;14(6):e243585. PubMed CrossRef
- Ishibashi Y, Yoneyama K, Tsuchida T, et al. Post-COVID-19 postural orthostatic tachycardia syndrome. Intern Med. 2021;60(14):2345. PubMed CrossRef
- Goodman BP, Khoury JA, Blair JE, et al. COVID-19 dysautonomia. Front Neurol. 2021;12:624968. PubMed CrossRef
- Raj SR, Arnold AC, Barboi A, et al; American Autonomic Society. Long-COVID postural tachycardia syndrome: an American Autonomic Society statement. Clin Auton Res. 2021;31(3):365–368. PubMed CrossRef
- Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc. 2012;87(12):1214–1225. PubMed CrossRef
- Moon J, Kim D-Y, Byun J-I, et al. Orthostatic intolerance symptoms are associated with depression and diminished quality of life in patients with postural tachycardia syndrome. Health Qual Life Outcomes. 2016;14(1):144. PubMed CrossRef
- Bagai K, Song Y, Ling JF, et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med. 2011;7(2):204–210. PubMed CrossRef
- Bernert RA, Kim JS, Iwata NG, et al. Sleep disturbances as an evidence-based suicide risk factor. Curr Psychiatry Rep. 2015;17:554. PubMed CrossRef
- Mallien J, Isenmann S, Mrazek A, et al. Sleep disturbances and autonomic dysfunction in patients with postural orthostatic tachycardia syndrome. Front Neurol. 2014;5:118–124. PubMed CrossRef
- Xu X. Huang H, Sethi S, et al. A survey based on sleep disturbance in postural tachycardia syndrome. J Neurol Sci. 2016;365:199–202. PubMed CrossRef
- Bagai K, Wakwe CI, Malow B, et al. Estimation of sleep disturbances using wrist actigraphy in patients with postural tachycardia syndrome. Auton Neurosci. 2013;177(2):260–265. PubMed CrossRef
- McCall WV, Black CG. The link between suicide and insomnia: theoretical mechanisms. Curr Psychiatry Rep. 2013;15(9):389–398. PubMed CrossRef
- Cukrowicz KC, Otamendi A, Pinto JV, et al. The impact of insomnia and sleep disturbances on depression and suicidality. Dreaming. 2006;16(1):1–10. CrossRef
- Ribeiro JD, Pease JL, Gutierrez PM, et al. Sleep problems outperform depression and hopelessness as cross-sectional and longitudinal predictors of suicidal ideation and behavior in young adults in the military. J Affect Disord. 2012;136(3):743–750. PubMed CrossRef
- Pederson CL, Blettner Brook J. Sleep disturbance linked to suicidal ideation in postural orthostatic tachycardia syndrome. Nat Sci Sleep. 2017;9:109–115. PubMed CrossRef
- Pigeon WR, Pinquart M, Conner K. Meta-analysis of sleep disturbance and suicidal thoughts and behaviors. J Clin Psychiatry. 2012;73(9):e1160–e1167. PubMed CrossRef
- Streeten DH, Thomas D, Bell DS. The roles of orthostatic hypotension, orthostatic tachycardia, and subnormal erythrocyte volume in the pathogenesis of the chronic fatigue syndrome. Am J Med Sci. 2000;320(1):1–8. PubMed CrossRef
- Anderson JW, Lambert EA, Sari CI, et al. Cognitive function, health-related quality of life, and symptoms of depression and anxiety sensitivity are impaired in patients with the postural orthostatic tachycardia syndrome (POTS). Front Physiol. 2014;5:230. PubMed CrossRef
- Ojha A, Chelimsky TC, Chelimsky G. Comorbidities in pediatric patients with postural orthostatic tachycardia syndrome. J Pediatr. 2011;158(1):20–23. PubMed CrossRef
- Raj V, Haman KL, Raj SR, et al. Psychiatric profile and attention deficits in postural tachycardia syndrome. J Neurol Neurosurg Psychiatry. 2009;80(3):339–344. PubMed CrossRef
- Masuki S, Eisenach JH, Johnson CP, et al. Excessive heart rate response to orthostatic stress in postural tachycardia syndrome is not caused by anxiety. J Appl Physiol (1985). 2007;102(3):896–903. PubMed CrossRef
- Raj V, Opie M, Arnold AC. Cognitive and psychological issues in postural tachycardia syndrome. Auton Neurosci. 2018;215:46–55. PubMed CrossRef
- Arnold AC, Haman K, Garland EM, et al. Cognitive dysfunction in postural tachycardia syndrome. 2015;128:39–45.
- Green EA, Black BK, Biaggioni I, et al. Melatonin reduces tachycardia in postural tachycardia syndrome: a randomized, crossover trial. Cardiovasc Ther. 2014;32(3):105–112. PubMed CrossRef
- Taylor DM, Barnes TRE, Young AH. The Maudsley Prescribing Guidelines In Psychiatry. 13th ed. Wiley Blackwell; 2018.
- Ray CA. Melatonin attenuates the sympathetic nerve responses to orthostatic stress in humans. J Physiol. 2003;551(Pt 3):1043–1048. PubMed CrossRef
- Sasaki N, Fujiwara S, Ozono R, et al. Lower blood pressure and smaller pulse pressure in sleeping pill users. 2017;96(42):e8272.
- Green EA, Raj V, Shibao CA, et al. Effects of norepinephrine reuptake inhibition on postural tachycardia syndrome. J Am Heart Assoc. 2013;2(5):e000395. PubMed CrossRef
- Schroeder C, Tank J, Boschmann M, et al. Selective norepinephrine reuptake inhibition as a human model of orthostatic intolerance. Circulation. 2002;105(3):347–353. PubMed CrossRef
- Ross AJ, Medow MS, Rowe PC, et al. What is brain fog? an evaluation of the symptom in postural tachycardia syndrome. Clin Auton Res. 2013;23(6):305–311. PubMed CrossRef
- Bakker MH, Hugtenburg JG, van Straten A, et al. Effectiveness of low-dose amitriptyline and mirtazapine for insomnia disorder: study protocol of a randomised, double-blind, placebo-controlled trial in general practice (the DREAMING study). BMJ Open. 2021;11(9):e047142. PubMed CrossRef
- Thompson W, Quay TAW, Rojas-Fernandez C, et al. Atypical antipsychotics for insomnia: a systematic review. Sleep Med. 2016;22:13–17. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite