LESSONS LEARNED AT THE INTERFACE OF MEDICINE AND PSYCHIATRY
The Psychiatric Consultation Service at Massachusetts General Hospital sees medical and surgical inpatients with comorbid psychiatric symptoms and conditions. During their twice-weekly rounds, Dr Stern and other members of the Consultation Service discuss diagnosis and management of hospitalized patients with complex medical or surgical problems who also demonstrate psychiatric symptoms or conditions. These discussions have given rise to rounds reports that will prove useful for clinicians practicing at the interface of medicine and psychiatry.
Prim Care Companion CNS Disord 2022;24(1):21f02948
To cite: Van Alphen MU, Vyas CM, Paudel S, et al. Managing clozapine in primary care settings. Prim Care Companion CNS Disord. 2022;24(1):21f02948.
To share: https://doi.org/10.4088/PCC.21f02948
© Copyright 2022 Physicians Postgraduate Press, Inc.
aDepartment of Psychiatry, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts
bNorth Suffolk Mental Health Association, Boston, Massachusetts
cDepartment of Psychiatry, Duke University, Durham, North Carolina
‡Drs Van Alphen, Vyas, Paudel, Makhlouf, and Arjyal contributed equally to this work.
*Corresponding author: Manjola U. Van Alphen, MD, PhD, MBA, Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School, 25 Staniford St, Boston, Massachusetts ([email protected]).
Have you ever wondered whether and how you could manage a person with serious mental illness who takes clozapine? Have you been uncertain about whether and how you should safely monitor blood levels, the hemogram, and cardiac function? Have you been apprehensive about rechallenging your patients after having sustained a serious complication from clozapine? If you have, then the following case presentation and discussion should prove useful.
CASE VIGNETTE
Ms A, a 44-year-old married mother of 2, had persistent auditory hallucinations and delusions despite taking antipsychotic medications. She reported to her physician that she had been sad, crying, and not enjoying her life for several years. She endorsed low energy, increased sleeping, some difficulty focusing and concentrating, and feeling worried daily. However, her appetite was good, and despite feeling tired, she was pushing herself to exercise regularly and managing her household as usual. Moreover, she had no history of euphoria or irritability. Ms A thought she was being followed wherever she went and stated, “They inserted a camera into my eyes to watch my activities.” She heard voices that said mean things about her. However, she did not endorse having command hallucinations or thoughts about hurting herself or others; she felt safe and well supported by her family. She was a nonsmoker and did not abuse recreational drugs. Ms A was taking olanzapine (20 mg/night), haloperidol (5 mg twice/d), and sertraline (100 mg/d). She had noticed no easing of her depression, hallucinations, or delusions after 6 months of pharmacologic treatment. On examination, Ms A was calm, cooperative, affectively blunted, and fully oriented; had a linear thought process; and had no thoughts of suicide or violence. However, she described auditory hallucinations and paranoid delusions. After obtaining collateral information from her previous providers and her family and reviewing prior failed trials of antipsychotics, her physician proposed that she try clozapine to manage her treatment-resistant schizophrenia. Ms A went to the laboratory several days later to obtain a complete blood count (CBC), complete metabolic panel, and lipid profile, as well as hemoglobin A1c, high-sensitivity C-reactive protein (CRP), and high-sensitivity cardiac troponin (cTn) levels; all test results were within normal limits. After discussing the risks (including agranulocytosis and myocarditis) and benefits (alleviation of her hallucinations and delusions), Ms A agreed to take clozapine and to be monitored regularly.
Ms A received an initial dose of clozapine (12.5 mg the first night); subsequently, she started taking 25 mg each night for the first week. Her CBC, high-sensitivity CRP, and high-sensitivity cTn were monitored weekly, and her nightly clozapine dose was increased by 25 mg every week according to the plan. Fortunately, her psychotic symptoms (auditory hallucinations and delusions) lessened. However, after several weeks on clozapine trial, her serum CRP and cTn values trended up. Her physician called her immediately after reviewing the reports, discussed the results with her, and asked whether she was experiencing cardiac symptoms. Ms A reported worsening shortness of breath on walking and mild ongoing substernal chest pain without radiation. She had no fever, fatigue, or other physical symptoms. Suspecting myocarditis, Ms A was told to go to the emergency department (ED) without delay. Her family was informed about her symptoms and her abnormal laboratory results, and the ED was contacted to provide details of her history and course. In the ED, her high-sensitivity cTn and high-sensitivity CRP were found to be higher than they had been previously, and she had T-wave changes on her electrocardiogram. She was diagnosed with clozapine-induced myocarditis and was admitted for observation. Clozapine was discontinued immediately, and she recovered completely within 1 week. The consulting cardiologist recommended against rechallenging Ms A with clozapine.
WHAT IS CLOZAPINE AND HOW DOES IT WORK?
Clozapine is a tricyclic dibenzodiazepine derivative antipsychotic and the only US Food and Drug Administration (FDA)–approved medication for treatment-resistant schizophrenia (TRS) and reduction in the risk of recurrent suicidal behavior in schizophrenia and schizoaffective disorders.1 Clozapine was the first atypical antipsychotic medication introduced for the treatment of schizophrenia (in 1989).2 Clozapine was discovered in the late 1950s during trials with tricyclic antidepressants, which share a similar structure to clozapine, yet have unexpected antipsychotic properties.2,3 However, continued research into its use as an antipsychotic for schizophrenia was discarded since it did not induce extrapyramidal symptoms (EPS), which at the time were considered to be essential to the antipsychotics’ efficacy based on the “neuroleptic dogma.”4 This also earned clozapine the “atypical” label that is still used alongside “second-generation” antipsychotics (SGAs).5
Clozapine binds to more receptors than possibly any other molecule studied by psychopharmacologists.6 The term atypical now refers to its unique profile that involves binding to dopamine receptors and its effects on various dopamine-mediated behaviors that differ from those exhibited by conventional antipsychotics, also known as first-generation antipsychotics (FGAs).2,3 Clozapine is a D2 receptor antagonist that occupies only 40%–60% of D2 receptors, as opposed to the stronger D2 receptor binding of FGAs, which bind 80% or more of D2 receptors.7 Clozapine binds much more strongly to many other receptors, including dopaminergic (ie, D4 with especially high affinity, D1, D5) and serotoninergic (5-hydroxytryptamine2A [5-HT2A, 5-HT2B, 5-HT2C]) receptors, also known as the dual D2 and 5-HT2A antagonism, which may explain why clozapine does not induce catalepsy.3 This evidence, coupled with the view that clozapine is preferentially more active at limbic than at striatal dopamine receptors, may explain the low rate of EPS caused by clozapine. Recent evidence that suggests clozapine’s potential agonist effect on GABAB receptors further supports the antispasmodic effects and efficacy in treating catatonia.8 Clozapine is also an antagonist at adrenergic, cholinergic, and histaminergic receptors, and it acts as a partial agonist at muscarinic receptors (m1, m2, m3). Clozapine’s high affinity for muscarinic receptors likely explains its ability to induce sedation, constipation, and ileus.3
Despite multiple theories, the exact mechanism of clozapine’s superior antipsychotic effect, as compared to FGAs or other SGAs, remains poorly understood.3 Clozapine’s higher D1︎:D2 ratio compared to other SGAs (eg, olanzapine, quetiapine), very high affinity to D4 receptors, combined activity at multiple receptor types, and antipsychotic effects of its metabolite, norclozapine, are among some of the most widely discussed theories; however, none have yet to be proven. The mechanism behind clozapine’s ability to reduce suicidal behavior also remains unknown, although it might be related to the enhancement of peripheral norepinephrine levels induced by clozapine.9 Clozapine’s actions on both the dopaminergic and noradrenergic systems may account for the decrease in use of alcohol and other substances in those with schizophrenia and co-occurring substance use disorders.2 Lastly, clozapine’s ability to treat catatonia has been linked to its proposed ability to increase GABA activity via agonistic binding to GABAB receptors.8,10
The mechanisms of action of the most serious clozapine side effects also remain unclear. Several hypotheses have attempted to explain clozapine-induced agranulocytosis, including immune regulation (patients on clozapine developing a hypersensitivity to the drug) and clozapine toxicity on neutrophils (via nitrenium clozapine ions, one of its metabolites).11 Recent genome-wide analysis studies have provided convincing evidence about the involvement of human leukocyte antigen locus (HLA-DQB1*0502 and HLA-B38/39/6), which have suggested that clozapine-induced agranulocytosis is most likely a complex, polygenic trait, leading to the development of a genetic test with clinical relevance.11
The etiology of clozapine-related myocarditis and cardiomyopathy remains unclear, with the most probable hypothesis being acute hypersensitivity (type 1, IgE-mediated) reaction, more likely to occur during rapid clozapine titration.12 Living in areas with high ozone concentrations (eg, Australia, New Zealand) linked to possible higher risk of m2 receptor blockade and cholinergic receptor dysfunction is another hypothesis, albeit yet to be proven.13 Clozapine’s propensity to cause increased appetite and weight gain, possibly leading to diabetes and metabolic syndrome, has been associated with polymorphisms in its serotoninergic and histaminergic systems as supported by a robust pharmacogenetics mechanism.14
Pharmacodynamically, clozapine is metabolized in the liver to its major metabolite, norclozapine or N-desmethylclozapine, by the cytochrome P450 (CYP) enzymes, CYP1A2 and CYP3A4.1,15 Norclozapine is not only a strong 5-HT1C receptor antagonist but also has a similar affinity to clozapine for D2 and 5-HT2 receptors.16 Plasma clozapine levels have been correlated with clinical effects. Nevertheless, due to its complex metabolism, there are significant inter- and intraindividual variations in serum clozapine levels for a given dose.17 Variables that affect serum levels of clozapine vary significantly from study to study, and predictors of the variability are inconclusive.18
WHAT SYMPTOM TYPES CAN BENEFIT FROM CLOZAPINE?
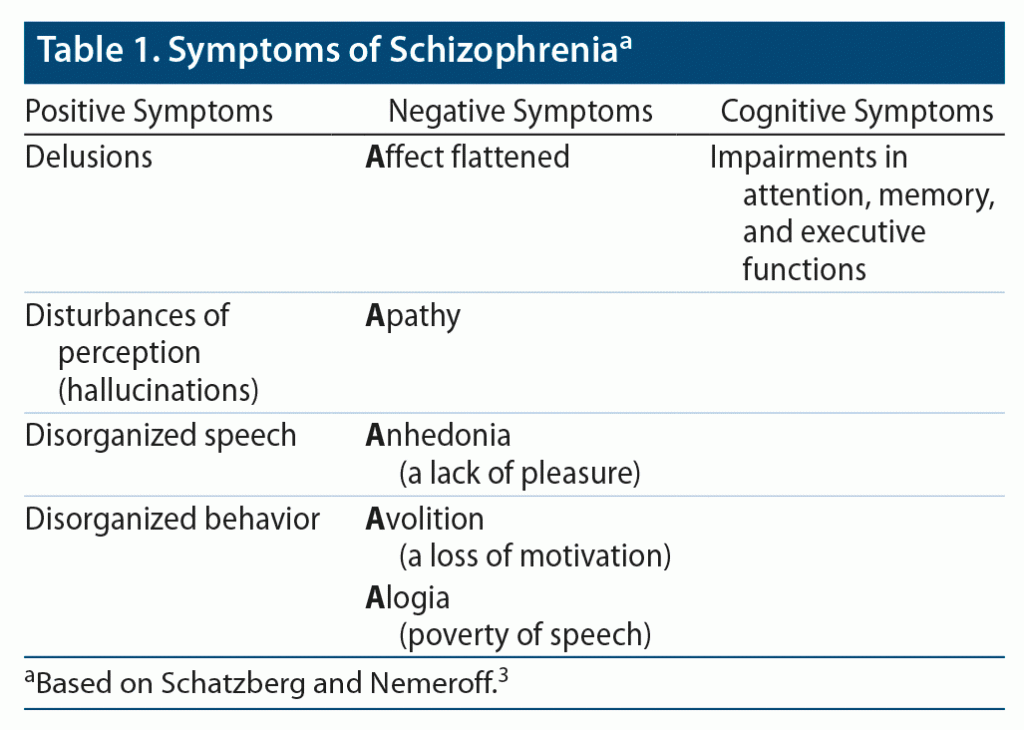
Schizophrenia is a chronic illness that affects 1% of the population. It is comprised of positive symptoms, negative symptoms, and impairment in cognitive function (Table 1).3 Antipsychotic medications are the first-line pharmacologic treatment for people with schizophrenia. Compared to use of FGAs and other SGAs, clozapine more effectively treats both positive and negative symptoms of schizophrenia.19 Furthermore, several studies20,21 have revealed improvements in hostility, aggression, and cognition (in areas such as memory, verbal learning, verbal fluency, and psychomotor speed).
Clozapine improves symptoms of depression in individuals with schizophrenia and reduces suicidal behavior.9 Use of alcohol and other substances has also decreased in those with schizophrenia and comorbid substance use disorders when treated with clozapine, but this has not been the case with other antipsychotics, which suggests another unique effect of clozapine.2 In addition to having the lowest chance of inducing tardive dyskinesia (TD) among FGAs and SGAs, clozapine has improved existing TD and the risk associated with ongoing antipsychotic use.22 A review23 found a negative correlation between TD severity and the duration of clozapine administration. Unlike all other antipsychotics, clozapine has been effective for catatonia related to an underlying psychotic and depressive illness as well as secondary to neurologic conditions.24–26 Overall, use of clozapine leads to a substantially lower risk of antipsychotic discontinuation, the need to switch to another medication, rehospitalization rates, suicide attempts, and death.27,28
WHO IS A GOOD CANDIDATE FOR CLOZAPINE?
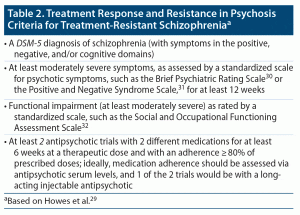
The ideal candidate for a clozapine trial is a patient with TRS, as defined by the Treatment Response and Resistance in Psychosis Working Group, when the benefits of being on clozapine outweigh the risks of developing side effects (Table 2).29–32 Patients should have an absolute neutrophil count (ANC) of at least 1,500 cells/microliter as well as the physical and cognitive capacities (ideally with family/caregiver support) to adhere to the medication regimen that has been prescribed and to follow monitoring guidelines. Patients with TRS and hostile and aggressive behavior in secluded settings, such as inpatient units or forensic facilities, might also be good candidates for clozapine, as it can lead to a decrease in rates of seclusion and use of restraints.3
In addition to FDA-approved indications for those with schizophrenia or schizoaffective disorder (including TRS and recurrent suicidal behavior), clozapine has off-label uses in treatment-resistant bipolar disorder and Parkinson’s disease (when dopamine-induced psychosis has emerged).33,34 As discussed previously, the presence of TD or alcohol and other substance use disorders in individuals with schizophrenia suggests that clozapine is a good option.2,23 Relatively young age, presence of few negative symptoms, and the paranoid type of schizophrenia have been associated with a better clozapine response; thus, patients with these demographic and clinical features appear to be good candidates for use of clozapine.35
WHY IS CLOZAPINE CONSIDERED MORE EFFICACIOUS THAN OTHER ANTIPSYCHOTICS?
Antipsychotic medications are the mainstay of schizophrenia management, but antipsychotics vary in their efficacy and side effect profiles. Clozapine use reduces psychotic symptoms more than use of other SGAs; however, measuring antipsychotic efficacy solely by use of symptom scales is insufficient to reflect the benefits of antipsychotic treatment for schizophrenia in real-world clinical practices.36,37 Instead, time to all-cause medication discontinuation can be a compelling proxy for antipsychotic effectiveness, as it integrates perspectives of patients and clinicians regarding drug safety, efficacy, and tolerability.38 A nationwide cohort study28 of 29,823 patients with schizophrenia revealed that clozapine was associated with the lowest rates of treatment discontinuation as compared to the most widely used medications. Moreover, long-term clozapine use has been associated with lower rates of hospitalization and mortality than other commonly used FGAs and SGAs.28,39 Although rates of clozapine use are low and variable in different geographic regions,40 clinical trials confirm that clozapine has a unique efficacy among those with TRS and reduces suicidal behaviors in individuals with schizophrenia in community-based practices.27,28,41–43 In addition, clozapine reduces aggression and violence among patients with schizophrenia.44 TRS remains costly and contributes to a significant loss in patients’ quality of life.45 However, a cost utility analysis46 suggested that clozapine therapy was more cost-effective than other antipsychotics (such as haloperidol and chlorpromazine) in patients with TRS.
WHICH CLINICAL ASSESSMENTS AND LABORATORY TESTS SHOULD BE OBTAINED BEFORE BEGINNING CLOZAPINE?
Although clozapine is more efficacious than other antipsychotics, the risk of side effects is daunting. While there is a considerable variation in approaches to defining TRS, treatment nonadherence of long-acting antipsychotics should be ruled out before initiating clozapine.29 Prescribers are required to enroll their patients into the Clozapine Risk Evaluation and Mitigation Strategy (REMS) program.47 The primary purpose of the Clozapine REMS program is to ensure that the benefits of clozapine pharmacotherapy outweigh the risks of inducing agranulocytosis.
Clozapine REMS is a shared program that incorporates the requirements to prescribe, dispense, and receive clozapine for both prescribers and pharmacies who must be enrolled and certified. While the REMS program provides extensive training, prescribing guidelines, and regulatory information to clozapine prescribers and pharmacies, it lacks educational material for nonpsychiatric providers, such as primary care providers (PCPs) or the patients and their families who are already taking clozapine or are interested in seeking more information. Furthermore, the REMS program lacks a centralized database of clozapine prescribers that makes it challenging for PCPs to identify providers when they need consultation. This might be a good opportunity for the clozapine providers and pharmacies to advocate with the REMS program so that a centralized database can be built and made available to the public, while PCPs might try to reach out to pharmacies or psychiatric care settings for further information.
Patients with schizophrenia should have baseline assessments before clozapine is started. Routine clinical assessments include vital signs (including documentation of orthostatic hypotension), an electrocardiogram (ECG), and tests to detect abnormal motor movements (eg, using the Abnormal Involuntary Movement Scale).48
Several clinical “red flags” should be identified before initiating clozapine. First, physicians should obtain a smoking history, as clozapine’s metabolism is induced by cigarette smoking, a factor that often leads to needing double the dose in smokers than in nonsmokers. Second, baseline bowel habits should be determined, as knowledge of this may lead to strategies to prevent constipation and intestinal obstruction induced by clozapine. Third, use of certain medications (such as ciprofloxacin, fluvoxamine, and carbamazepine) need to be identified to prevent serious drug-drug interactions. In addition, the medical history needs to be obtained; chronic medical conditions, a personal or family history of cardiovascular disease, and a history of seizures should be established.
Laboratory tests should include a CBC with a differential, a fasting blood sugar level (or HbA1c), lipid levels (non–high-density lipoprotein cholesterol if a nonfasting level is drawn), measures of inflammation (eg, CRP), markers of cardiac muscle damage (eg, troponin levels), anticonvulsant serum levels (if prescribed for seizures), liver enzymes, a basic metabolic panel, and a pregnancy test (in women of child-bearing potential).
HOW SHOULD LABORATORY TESTS AND PHYSICAL FINDINGS BE MONITORED DURING CLOZAPINE TREATMENT?
Laboratory Tests
Complete blood count with differential. The Clozapine REMS program requires regular monitoring and reporting of the neutrophil count for all patients taking clozapine.47 The highest risk of agranulocytosis occurs within 3 months of clozapine’s initiation. Therefore, routine neutrophil monitoring should be performed weekly from initiation of clozapine to the 6-month mark of treatment, then every 2 weeks from months 6 to 12, and then monthly starting 12 months after clozapine’s initiation (for the duration of the treatment). Moreover, a ratio of clozapine to norclozapine levels may hold value in predicting or optimizing clozapine treatment in relation to psychopathology, cognition, and adverse cardiometabolic effects.49,50 For example, the opposite receptor effects of clozapine versus norclozapine, especially at muscarinic sites, may explain the clinical application of the ratio of clozapine:norclozapine to assess cognitive effects.51–54 Moreover, a growing body of evidence suggests that a lower clozapine:norclozapine ratio was associated with better cognitive performance.55–58
Myocarditis panel. Clozapine-induced myocarditis can progress rapidly into a cardiomyopathy and congestive heart failure. Therefore, all patients should be monitored for myocarditis (with levels of troponin 1, creatine kinase [CK], CK-MB, sedimentation rate, or CRP) weekly during the first month after clozapine initiation. While ECG changes can be nonspecific early in a cardiomyopathy, obtaining a follow-up ECG is often useful. A comparison of the baseline ECG changes and follow-up tracings (specifically, looking at ST-segment elevations or T-wave inversions) should be conducted.13,59 An additional round of myocarditis laboratories can be ordered in the second month after clozapine initiation if a high index of suspicion for myocarditis remains.
Lipid levels, fasting blood sugar, or HbA1C. To detect clozapine-induced hyperglycemia or diabetes mellitus/insulin resistance, all patients should have plasma blood sugar levels and a lipid profile monitored 12 weeks after clozapine initiation. Metabolic laboratories can be obtained annually after 1 year.
Physical Findings
All patients should see a physician every month after beginning clozapine therapy. Vital signs and subjective signs of distress should be assessed at each clinical encounter. During the first 8 weeks of clozapine treatment, the early signs and symptoms of myocarditis (such as chest pain, dyspnea, fever, edema, and fatigue) should be evaluated. In cases of suspected myocarditis, echocardiography should be ordered to assess ventricular function. Patients should be monitored on a regular basis for weight gain; behavioral interventions can be recommended as required. A routine clinical examination can also be carried out to assess constipation, especially when clozapine is used in combination with an anticholinergic drug or when a patient has a comorbid medical illness. Additionally, individuals who have recently been immobilized, have genetic factors (mainly the factor V Leiden mutation, or a high concentration of factor VIII), or have had recent surgery should be evaluated carefully at regular follow-up visits for early signs of thromboembolic disorders; the mortality rate in the setting of blood clots in those receiving clozapine is considerably higher.60
WHICH SIDE EFFECTS OF CLOZAPINE ARE PROBLEMATIC AND WHAT CAN BE DONE TO MANAGE THEM?
Unfortunately, clozapine has myriad side effects that can be challenging to manage. The most common side effects include weight gain, diabetes, dyslipidemia, sedation, sialorrhea (excessive salivation), constipation, dizziness, tachycardia, dose-dependent seizures, and urinary incontinence, although management strategies can be recommended for these side effects.61 Specifically, sialorrhea is distressing after clozapine initiation and can lead to nonadherence and treatment discontinuation. Reducing the clozapine dose is often inadequate; however, concomitant use of glycopyrrolate (2–4 mg at bedtime) can be recommended.62 Chronic use of clozapine is associated with a greater risk of obesity, diabetes, hypertension, and hyperlipidemia63; close clinical monitoring and interventions are required when these problems arise.
Clozapine conveys a significant (albeit low) risk of agranulocytosis, myocarditis, neuroleptic malignant syndrome, seizures, and paralytic ileus, but these complications can be fatal. The estimated rate of clozapine-induced agranulocytosis ranges from 1% to 2%, but this rate can be reduced to ~0.4% with regular blood monitoring over 5 years.64 Additionally, recommendation for routine immunizations and smoking cessation may prove beneficial to prevent serious infections (eg, pneumonia) among patients receiving clozapine.65
The incidence of clozapine-induced myocarditis is estimated at 0.2%–3%, with most cases of myocarditis occurring within the first month of clozapine initiation.66 Therefore, a correlation between the findings of the clinical examination and results of laboratory tests should be determined during the first month of therapy. If myocarditis is suspected, a diagnostic workup, followed by the prompt discontinuation of clozapine, more frequent follow-up visits, and referral to cardiology, is recommended.
Gastrointestinal hypomotility is a highly prevalent (~30%) and potentially dangerous side effect of clozapine, as it may rapidly progress to a paralytic ileus.67,68 Furthermore, the prevalence rate of gastrointestinal hypomotility remained higher in hospitalized patients.69,70 Moreover, clozapine-induced gastrointestinal hypomotility is often underreported, which may underestimate the true prevalence.71 Given the high mortality rate among patients with severe clozapine-induced gastrointestinal hypomotility (15%–27.5%),67,72 close monitoring of patients’ defecation patterns and the concurrent use of laxatives is required.71 Clozapine also appears to be associated with a higher risk of seizures at therapeutic doses compared to other antipsychotics.73 Moreover, the seizure risk is associated with dosage escalation and rapid dose titration; seizures occur in 4.4% of patients taking ≥ 600 mg/d.74 When seizures develop, the clozapine dose should be reduced and antiepileptic medications should be administered.75
WHICH DRUG-DRUG INTERACTIONS CAN LEAD TO TOXICITY OR LACK OF EFFICACY?
Clozapine is primarily metabolized by the CYP1A2 enzyme and to a lesser extent by CYP2C, CYP2D6, and CYP3A3/4 enzymes in the liver. This metabolism leads to a variety of drug-drug interactions that can affect serum levels of clozapine; the most significant of which are agents that affect the CYP1A2 enzymes. CYP1A2 enzyme inducers, such as tobacco smoke, can lower serum concentrations of clozapine.76 Conversely, smoking cessation may increase serum clozapine concentrations by 50% or more.77 However, CYP1A2 enzyme inhibitors, such as ciprofloxacin (which also inhibits CYP3A4) and fluvoxamine (which also inhibits CYP3A3/4), can increase serum clozapine levels and lead to toxic levels.78 Recently, severe systemic inflammation, including severe respiratory illnesses such as COVID-19, has been recognized as a contributor to increased clozapine levels via cytokine-mediated inhibition of CYP1A2.79
Regular monitoring of clozapine levels when smoking status changes, when medications that interact with the CYP system are added or discontinued, and when systemic inflammation develops will help to avoid potentially fatal elevations in clozapine levels. In general, a therapeutic response in patients with schizophrenia is associated with serum clozapine levels between 200 and 450 ng/mL, whereas toxicity is associated with levels > 1,000 ng/mL.80
WHEN SHOULD CLOZAPINE BE DISCONTINUED AND WHY?
Clozapine should be immediately discontinued when rare yet potentially fatal side effects occur, including agranulocytosis, myocarditis or cardiomyopathy, a QTc interval > 500 ms, ileus, neuroleptic malignant syndrome, venous thromboembolism, diabetic ketoacidosis, or hyperosmolar coma.81 Other side effects such as neutropenia, leukocytosis, seizures, orthostatic hypotension, severe constipation, weight gain, and metabolic abnormalities can be medically managed while potentially adjusting the dose of clozapine. While neutropenia is defined as having an ANC < 1,500/µL, agranulocytosis is defined by severe neutropenia, with an ANC < 500/µL—it is a medical emergency. When agranulocytosis arises, in addition to close monitoring of the ANC, hematology consultation is recommended. If the ANC falls between 500 and 999/µL, clozapine treatment should be stopped; however, it can be continued with more rigorous monitoring of the ANC and benign ethnic neutropenia (BEN). When patients have an ANC of 1,000–1,499/µL, clozapine can be continued with monitoring of the ANC 3 times/week; however, there is no need to alter the treatment protocol for those with BEN.82
Similarly, myocarditis is another potentially fatal adverse effect associated with use of clozapine, and it should be monitored closely.12 A study of clozapine-induced myocarditis that developed during the first 12 weeks of clozapine treatment found that shortness of breath, fever, and tachycardia were the major clinical features.83 In addition, increased levels of CK and troponin in the blood and left ventricular dysfunction as detected on an echocardiogram were the key laboratory markers of myocarditis.84 Of the 2 tests, troponin is considered the more sensitive marker for guiding the discontinuation of clozapine.85 Clozapine-induced myocarditis tends to occur during the first few weeks of treatment, and most cases occur in the first 8–12 weeks of treatment.83,86 Weekly monitoring of troponin and CRP during the first 8–12 weeks and clinical monitoring for myocarditis are both important to avert serious complications of clozapine.
Except for a few side effects, including sedation, hypotension, and seizure activity, studies87 have found little correlation between serum clozapine levels and side effects, thus limiting their utility in their monitoring and possible prevention. The risk of seizures has been shown to increase with plasma clozapine levels > 600 µg/L or rapid upward titration. Onset of the first seizure must be managed by a 50% clozapine dose reduction, potentially in combination with use of antiepileptic medications, while ruling out other factors that lower seizure threshold.81
WHAT SHOULD I KNOW BEFORE DISCONTINUING THE CLOZAPINE TREATMENT?
Abrupt discontinuation of clozapine has been associated with a significant risk of rebound psychosis, suicidal ideation, and anticholinergic symptoms within the first week of discontinuation in more than half of patients.10,88,89 Rebound psychosis refers to a fast return of prominent psychotic symptoms to preclozapine severity of symptoms or more intense symptoms (eg, illusions, hallucinations, delusions). Withdrawal symptoms are related to clozapine’s cholinergic activity, and manifestations may include agitation, anxiety, insomnia, restlessness, TD, dystonia, altered consciousness, confusion, nausea, vomiting, diarrhea, diaphoresis, and catatonia.90,91 When rebound and withdrawal symptoms and disorders persist for ≥ 6 months following clozapine discontinuation, they are called “persistent” and indicate treatment resistance. Dopamine, serotonin, and GABA supersensitivity, in addition to cholinergic rebound, appear to be involved in clozapine’s severe withdrawal syndrome.89
When deciding when and how to discontinue or decrease the dose of clozapine to address side effects, providers should be aware of the potential for severe withdrawal and rebound symptoms and respective prevention or treatment strategies. Education of patients and caregivers about possible withdrawal symptoms and close monitoring should be instituted early in the treatment process.92 When possible, a gradual taper of clozapine over several weeks to months, rather than abrupt discontinuation, should be chosen. However, when an abrupt discontinuation is unavoidable, overlapping “plateau” antipsychotic switch strategies and temporary prescription of benzodiazepines or anticholinergic drugs should be adopted.93 Cholinergic rebound and withdrawal symptoms, including dystonia and dyskinesia, can be mitigated by adding an anticholinergic medication.84 Rebound psychosis, however, is more challenging to address by switching to another antipsychotic medication, since clozapine-treated patients typically have failed to respond to several antipsychotics. While olanzapine appears to be favored, supporting evidence is still insufficient,94 whereas use of adjunctive agents that tend to have long half-lives, such as long-acting injectable SGAs, has been proposed.95
Use of electroconvulsive therapy (ECT) primarily as augmentation in patients with TRS, including those partially responsive to clozapine or those who are clozapine intolerant, has been shown to be safe and highly effective, with a clinical response in 47%–72% of patients in both retrospective and prospective studies.96,97 Enhancement of the ECT technology and better control of the cognitive side effects have increased its use in this population in recent years.96 Finally, ECT and benzodiazepines can be used for treatment of clozapine-associated withdrawal catatonia.10
WHEN CAN CLOZAPINE BE REINITIATED AFTER ONSET OF A SERIOUS SIDE EFFECT?
Reinitiating or rechallenging with clozapine is the attempt to restart clozapine after a patient recovers from agranulocytosis or myocarditis. The decision to restart clozapine after serious side effects should be made only when there is no satisfactory alternative; the risk:benefit ratio must be considered in those with TRS. The risk of agranulocytosis is high in patients who have been rechallenged with clozapine.98 Rechallenge has been less successful in those who developed clozapine-associated myocarditis.99 Unfortunately, guidelines on the reinitiation of clozapine are lacking following the onset of clozapine-induced myocarditis; however, close monitoring for symptoms of heart failure, twice-a-week monitoring of CRP and troponin levels, gradual titration of clozapine with smaller doses, and an echocardiogram are recommended.12,85
WHEN SHOULD I CALL A PSYCHIATRIST?
PCPs are well-positioned to support patients with schizophrenia by either starting clozapine when it is indicated or managing patients already on clozapine. Reaching out to psychiatric providers in clozapine clinics should never be delayed when there is a question related to use of clozapine. These situations can include an inability to establish a rapport with a patient because of pervasive psychiatric symptoms, a lack of time, development of refractory or potentially fatal side effects that might require discontinuation of clozapine, a subtherapeutic response to clozapine, and the need for augmentation strategies, rechallenge, polypharmacy (especially when more than 1 antipsychotic medication is used), and comorbidity of other psychiatric disorders (eg, substance use disorders). Initial consultation prior to starting clozapine, as well periodic consultations such as yearly visits, could be helpful to PCPs, especially when they have been managing patients with clozapine for a long time.
CASE FOLLOW-UP
After discharge from the hospital, Ms A was started on risperidone, and the dose was optimized to 6 mg/d over 4 weeks. As she still had significant auditory hallucinations and persecutory delusions and was also concerned about weight gain on risperidone, aripiprazole was added to risperidone, and the dose was optimized to 30 mg/d. She received some benefits from the treatment; however, she continued to report hallucinations and delusions. ECT was offered; however, she did not want to consider ECT, citing potential side effects. She continued to take aripiprazole and risperidone along with sertraline. Ms A’s family members felt that she was doing a little better on the medication regimen and was able to function well at home. She continued to engage in her regular daily activities and was attempting to find a job despite ongoing symptoms.
CONCLUSION
Clozapine is an SGA mostly used in patients with TRS. Clozapine is often prescribed as a last resort in patients with TRS owing to its potential for serious and potentially life-threatening side effects (though rare), the most serious of which are agranulocytosis, myocardiopathy, seizures, and paralytic ileus. Close monitoring, particularly in the first few months of treatment, is essential to reduce the occurrence of such serious adverse reactions. Given the increased prevalence of TRS, PCPs should be aware of rare but serious complications associated with clozapine therapy. Our report highlights clozapine-induced myocarditis and provides critical steps for early detection and management of such life-threatening complications. Among patients with a high index of suspicion for myocarditis, PCPs should obtain laboratory testing (such as troponin and CRP) and echocardiography within 8–12 weeks of commencing clozapine. Further clinical testing and referral to a cardiologist should be considered.
Submitted: February 11, 2021; accepted August 12, 2021.
Published online: February 17, 2022.
Potential conflicts of interest: None.
Funding/support: None.
Clinical Points
- The most common side effects of clozapine include weight gain, diabetes, dyslipidemia, sedation, sialorrhea, constipation, dizziness, tachycardia, dose-dependent seizures, and urinary incontinence.
- Clozapine-induced myocarditis can progress rapidly into a cardiomyopathy and congestive heart failure.
- Abrupt discontinuation of clozapine has been associated with a significant risk of rebound psychosis, suicidal ideation, and anticholinergic symptoms within the first week of discontinuation in more than half of patients.
References (99)

- Clozapine [package insert]. East Hanover, NJ: Novartis Pharmaceutical Corporation; 2010. Accessed February 5, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/019758s062lbl.pdf
- Khokhar JY, Henricks AM, Sullivan EDK, et al. Unique effects of clozapine: a pharmacological perspective. Adv Pharmacol. 2018;82:137–162. PubMed CrossRef
- Schatzberg AF, Nemeroff CB. The American Psychiatric Association Publishing Textbook Of Psychopharmacology. Fifth Edition. Arlington, VA: American Psychiatric Association Publishing; 2017.
- Hippius H. A historical perspective of clozapine. J Clin Psychiatry. 1999;60(suppl 12):22–23. PubMed
- Burns MJ. The pharmacology and toxicology of atypical antipsychotic agents. J Toxicol Clin Toxicol. 2001;39(1):1–14. PubMed CrossRef
- Stahl SM. Stahl’s Essential Psychopharmacology. Cambridge: Cambridge University Press; 2014.
- Wenthur CJ, Lindsley CW. Classics in chemical neuroscience: clozapine. ACS Chem Neurosci. 2013;4(7):1018–1025. PubMed CrossRef
- Nair PC, McKinnon RA, Miners JO, et al. Binding of clozapine to the GABAB receptor: clinical and structural insights. Mol Psychiatry. 2020;25(9):1910–1919. PubMed CrossRef
- Nakajima S, Takeuchi H, Fervaha G, et al. Comparative efficacy between clozapine and other atypical antipsychotics on depressive symptoms in patients with schizophrenia: analysis of the CATIE phase 2E data. Schizophr Res. 2015;161(2–3):429–433. PubMed CrossRef
- Lander M, Bastiampillai T, Sareen J. Review of withdrawal catatonia: what does this reveal about clozapine? Transl Psychiatry. 2018;8(1):139. PubMed CrossRef
- de With SAJ, Pulit SL, Staal WG, et al. More than 25 years of genetic studies of clozapine-induced agranulocytosis. Pharmacogenomics J. 2017;17(4):304–311. PubMed CrossRef
- Knoph KN, Morgan RJ 3rd, Palmer BA, et al. Clozapine-induced cardiomyopathy and myocarditis monitoring: a systematic review. Schizophr Res. 2018;199:17–30. PubMed CrossRef
- Kanniah G, Kumar S. Clozapine associated cardiotoxicity: issues, challenges and way forward. Asian J Psychiatr. 2020;50:101950. PubMed CrossRef
- Zimbron J, Khandaker GM, Toschi C, et al. A systematic review and meta-analysis of randomized controlled trials of treatments for clozapine-induced obesity and metabolic syndrome. Eur Neuropsychopharmacol. 2016;26(9):1353–1365. PubMed CrossRef
- Eiermann B, Engel G, Johansson I, et al. The involvement of CYP1A2 and CYP3A4 in the metabolism of clozapine. Br J Clin Pharmacol. 1997;44(5):439–446. PubMed CrossRef
- Kuoppamäki M, Syvälahti E, Hietala J. Clozapine and N-desmethylclozapine are potent 5-HT1C receptor antagonists. Eur J Pharmacol. 1993;245(2):179–182. PubMed CrossRef
- Spina E, Avenoso A, Facciolà G, et al. Relationship between plasma concentrations of clozapine and norclozapine and therapeutic response in patients with schizophrenia resistant to conventional neuroleptics. Psychopharmacology (Berl). 2000;148(1):83–89. PubMed CrossRef
- Perry PJ, Bever KA, Arndt S, et al. Relationship between patient variables and plasma clozapine concentrations: a dosing nomogram. Biol Psychiatry. 1998;44(8):733–738. PubMed CrossRef
- Siskind D, McCartney L, Goldschlager R, et al. Clozapine v. first- and second-generation antipsychotics in treatment-refractory schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2016;209(5):385–392. PubMed CrossRef
- Krakowski MI, Czobor P, Citrome L, et al. Atypical antipsychotic agents in the treatment of violent patients with schizophrenia and schizoaffective disorder. Arch Gen Psychiatry. 2006;63(6):622–629. PubMed CrossRef
- McGurk SR. The effects of clozapine on cognitive functioning in schizophrenia. J Clin Psychiatry. 1999;60(suppl 12):24–29. PubMed
- Tarsy D, Lungu C, Baldessarini RJ. Epidemiology of tardive dyskinesia before and during the era of modern antipsychotic drugs. Handb Clin Neurol. 2011;100:601–616. PubMed CrossRef
- Pardis P, Remington G, Panda R, et al. Clozapine and tardive dyskinesia in patients with schizophrenia: a systematic review. J Psychopharmacol. 2019;33(10):1187–1198. PubMed CrossRef
- Chattopadhyay S, Saha I, Dan A, et al. Clozapine responsive catatonia: a series of five cases. Ind Psychiatry J. 2012;21(1):66–68. PubMed CrossRef
- Dursun SM, Hallak JE, Haddad P, et al. Clozapine monotherapy for catatonic schizophrenia: should clozapine be the treatment of choice, with catatonia rather than psychosis as the main therapeutic index? J Psychopharmacol. 2005;19(4):432–433. PubMed CrossRef
- Rommel O, Tegenthoff M, Widdig W, et al. Organic catatonia following frontal lobe injury: response to clozapine. J Neuropsychiatry Clin Neurosci. 1998;10(2):237–238. PubMed CrossRef
- Meltzer HY, Alphs L, Green AI, et al; International Suicide Prevention Trial Study Group. Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry. 2003;60(1):82–91. PubMed CrossRef
- Tiihonen J, Mittendorfer-Rutz E, Majak M, et al. Real-world effectiveness of antipsychotic treatments in a nationwide cohort of 29 823 patients with schizophrenia. JAMA Psychiatry. 2017;74(7):686–693. PubMed CrossRef
- Howes OD, McCutcheon R, Agid O, et al. Treatment-resistant schizophrenia: Treatment Response and Resistance in Psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. Am J Psychiatry. 2017;174(3):216–229. PubMed CrossRef
- Overall JA, Gorham DR. The Brief Psychiatric Rating Scale. Psychol Rep. 1962;10(3):799–812. CrossRef
- Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. PubMed CrossRef
- Morosini PL, Magliano L, Brambilla L, et al. Development, reliability and acceptability of a new version of the DSM-IV Social and Occupational Functioning Assessment Scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand. 2000;101(4):323–329. PubMed CrossRef
- Pollak P, Tison F, Rascol O, et al. Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry. 2004;75(5):689–695. PubMed CrossRef
- Suppes T, Webb A, Paul B, et al. Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry. 1999;156(8):1164–1169. PubMed
- Okhuijsen-Pfeifer C, Sterk AY, Horn IM, et al. Demographic and clinical features as predictors of clozapine response in patients with schizophrenia spectrum disorders: A systematic review and meta-analysis. Neurosci Biobehav Rev. 2020;111:246–252. PubMed CrossRef
- Soares-Weiser K, Béchard-Evans L, Lawson AH, et al. Time to all-cause treatment discontinuation of olanzapine compared to other antipsychotics in the treatment of schizophrenia: a systematic review and meta-analysis. Eur Neuropsychopharmacol. 2013;23(2):118–125. PubMed CrossRef
- Tandon R, Fleischhacker WW. Comparative efficacy of antipsychotics in the treatment of schizophrenia: a critical assessment. Schizophr Res. 2005;79(2-3):145–155. PubMed CrossRef
- Swartz MS, Perkins DO, Stroup TS, et al. Assessing clinical and functional outcomes in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial. Schizophr Bull. 2003;29(1):33–43. PubMed CrossRef
- Stroup TS, Gerhard T, Crystal S, et al. Comparative effectiveness of clozapine and standard antipsychotic treatment in adults with schizophrenia. Am J Psychiatry. 2016;173(2):166–173. PubMed CrossRef
- Stroup TS, Gerhard T, Crystal S, et al. Geographic and clinical variation in clozapine use in the United States. Psychiatr Serv. 2014;65(2):186–192. PubMed CrossRef
- Lewis SW, Barnes TR, Davies L, et al. Randomized controlled trial of effect of prescription of clozapine versus other second-generation antipsychotic drugs in resistant schizophrenia. Schizophr Bull. 2006;32(4):715–723. PubMed CrossRef
- McEvoy JP, Lieberman JA, Stroup TS, et al; CATIE Investigators. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. Am J Psychiatry. 2006;163(4):600–610. PubMed CrossRef
- Tiihonen J, Wahlbeck K, Lönnqvist J, et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in community care after first hospitalisation due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ. 2006;333(7561):224. PubMed CrossRef
- Frogley C, Taylor D, Dickens G, et al. A systematic review of the evidence of clozapine’s anti-aggressive effects. Int J Neuropsychopharmacol. 2012;15(9):1351–1371. PubMed CrossRef
- Kennedy JL, Altar CA, Taylor DL, et al. The social and economic burden of treatment-resistant schizophrenia: a systematic literature review. Int Clin Psychopharmacol. 2014;29(2):63–76. PubMed CrossRef
- Oh PI, Iskedjian M, Addis A, et al. Pharmacoeconomic evaluation of clozapine in treatment-resistant schizophrenia: a cost-utility analysis. Can J Clin Pharmacol. 2001;8(4):199–206. PubMed
- Clozapine REMS Program. 2015. Clozapine REMS website. Accessed January 18, 2021. https://www.clozapinerems.com
- Abnormal Involuntary Movement Scale. (117-AIMS). In: Guy W, ed. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health; 1976:534–537.
- Costa-Dookhan KA, Agarwal SM, Chintoh A, et al. The clozapine to norclozapine ratio: a narrative review of the clinical utility to minimize metabolic risk and enhance clozapine efficacy. Expert Opin Drug Saf. 2020;19(1):43–57. PubMed CrossRef
- Rajji TK, Mulsant BH, Davies S, et al. Prediction of working memory performance in schizophrenia by plasma ratio of clozapine to N-desmethylclozapine. Am J Psychiatry. 2015;172(6):579–585. PubMed CrossRef
- Cools R, Sheridan M, Jacobs E, et al. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. J Neurosci. 2007;27(20):5506–5514. PubMed CrossRef
- Shekhar A, Potter WZ, Lightfoot J, et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am J Psychiatry. 2008;165(8):1033–1039. PubMed CrossRef
- Veselinović T, Vernaleken I, Janouschek H, et al. Effects of anticholinergic challenge on psychopathology and cognition in drug-free patients with schizophrenia and healthy volunteers. Psychopharmacology (Berl). 2015;232(9):1607–1617. PubMed CrossRef
- Vinogradov S, Fisher M, Warm H, et al. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166(9):1055–1062. PubMed CrossRef
- McArdle PA, De Mel V, DeMonte V, et al. An investigation into the relationship between clozapine treatment and cognitive performance in patients with treatment resistant schizophrenia. Schizophr Res. 2019;206:450–451. PubMed CrossRef
- Molins C, Carceller-Sindreu M, Navarro H, et al. Plasma ratio of clozapine to N-desmethylclozapine can predict cognitive performance in treatment-resistant psychotic patients. Psychiatry Res. 2017;258:153–157. PubMed CrossRef
- Rajji TK, Uchida H, Ismail Z, et al. Clozapine and global cognition in schizophrenia. J Clin Psychopharmacol. 2010;30(4):431–436. PubMed CrossRef
- Thornton AE, Procyshyn RM, Barr AM, et al. Cognition and plasma ratio of clozapine to N-desmethylclozapine in patients with clozapine-resistant schizophrenia. Am J Psychiatry. 2015;172(12):1259. PubMed CrossRef
- Sackey BK, Moore TA, Cupples NL, et al. Clozapine-induced myocarditis: two case reports and review of clinical presentation and recognition. Ment Health Clin. 2018;8(6):303–308. PubMed CrossRef
- Paciullo CA. Evaluating the association between clozapine and venous thromboembolism. Am J Health Syst Pharm. 2008;65(19):1825–1829. PubMed CrossRef
- Newman BM, Newman WJ. Rediscovering clozapine: adverse effects develop – what should you do now? Curr Psychiatr. 2016;15(8):40–46, 48–49.
- Chen SY, Ravindran G, Zhang Q, et al. Treatment strategies for clozapine-induced sialorrhea: a systematic review and meta-analysis. CNS Drugs. 2019;33(3):225–238. PubMed CrossRef
- Henderson DC, Nguyen DD, Copeland PM, et al. Clozapine, diabetes mellitus, hyperlipidemia, and cardiovascular risks and mortality: results of a 10-year naturalistic study. J Clin Psychiatry. 2005;66(9):1116–1121. PubMed CrossRef
- Honigfeld G, Arellano F, Sethi J, et al. Reducing clozapine-related morbidity and mortality: 5 years of experience with the Clozaril National Registry. J Clin Psychiatry. 1998;59(suppl 3):3–7. PubMed
- Ponsford MJ, Pecoraro A, Jolles S. Clozapine-associated secondary antibody deficiency. Curr Opin Allergy Clin Immunol. 2019;19(6):553–562. PubMed CrossRef
- Cook SC, Ferguson BA, Cotes RO, et al. Clozapine-induced myocarditis: prevention and considerations in rechallenge. Psychosomatics. 2015;56(6):685–690. PubMed CrossRef
- Every-Palmer S, Ellis PM. Clozapine-induced gastrointestinal hypomotility: A 22-year bi-national pharmacovigilance study of serious or fatal ‘slow gut’ reactions, and comparison with international drug safety advice. CNS Drugs. 2017;31(8):699–709. PubMed CrossRef
- Shirazi A, Stubbs B, Gomez L, et al. Prevalence and predictors of clozapine-associated constipation: a systematic review and meta-analysis. Int J Mol Sci. 2016;17(6):863. PubMed CrossRef
- Centorrino F, Baldessarini RJ, Kando JC, et al. Clozapine and metabolites: concentrations in serum and clinical findings during treatment of chronically psychotic patients. J Clin Psychopharmacol. 1994;14(2):119–125. PubMed CrossRef
- Hayes G, Gibler B. Clozapine-induced constipation. Am J Psychiatry. 1995;152(2):298a. PubMed CrossRef
- Cohen D. Clozapine and gastrointestinal hypomotility. CNS Drugs. 2017;31(12):1083–1091. PubMed CrossRef
- Palmer SE, McLean RM, Ellis PM, et al. Life-threatening clozapine-induced gastrointestinal hypomotility: an analysis of 102 cases. J Clin Psychiatry. 2008;69(5):759–768. PubMed CrossRef
- Alper K, Schwartz KA, Kolts RL, et al. Seizure incidence in psychopharmacological clinical trials: an analysis of Food and Drug Administration (FDA) summary basis of approval reports. Biol Psychiatry. 2007;62(4):345–354. PubMed CrossRef
- Devinsky O, Honigfeld G, Patin J. Clozapine-related seizures. Neurology. 1991;41(3):369–371. PubMed CrossRef
- Williams AM, Park SH. Seizure associated with clozapine: incidence, etiology, and management. CNS Drugs. 2015;29(2):101–111. PubMed CrossRef
- Özdemir V, Kalow W, Posner P, et al. CYP1A2 activity as measured by a caffeine test predicts clozapine and active metabolite steady-state concentrationin patients with schizophrenia. J Clin Psychopharmacol. 2001;21(4):398–407. PubMed CrossRef
- Meyer JM. Individual changes in clozapine levels after smoking cessation: results and a predictive model. J Clin Psychopharmacol. 2001;21(6):569–574. PubMed CrossRef
- Thorn CF, Müller DJ, Altman RB, et al. PharmGKB summary: clozapine pathway, pharmacokinetics. Pharmacogenet Genomics. 2018;28(9):214–222. PubMed CrossRef
- Dotson S, Hartvigsen N, Wesner T, et al. Clozapine toxicity in the setting of COVID-19. Psychosomatics. 2020;61(5):577–578. PubMed CrossRef
- Freudenreich O. Clozapine drug levels guide dosing. Curr Psychiatr. 2009;8:78–79.
- Nielsen J, Correll CU, Manu P, et al. Termination of clozapine treatment due to medical reasons: when is it warranted and how can it be avoided? J Clin Psychiatry. 2013;74(6):603–613, quiz 613. PubMed CrossRef
- Manu P, Sarvaiya N, Rogozea LM, et al. Benign ethnic neutropenia and clozapine use: a systematic review of the evidence and treatment recommendations. J Clin Psychiatry. 2016;77(7):e909–e916. PubMed CrossRef
- Bellissima BL, Tingle MD, Cicović A, et al. A systematic review of clozapine-induced myocarditis. Int J Cardiol. 2018;259:122–129. PubMed CrossRef
- Khan AA, Ashraf A, Baker D, et al. Clozapine and incidence of myocarditis and sudden death: long term Australian experience. Int J Cardiol. 2017;238:136–139. PubMed CrossRef
- Patel RK, Moore AM, Piper S, et al. Clozapine and cardiotoxicity: a guide for psychiatrists written by cardiologists. Psychiatry Res. 2019;282:112491. PubMed CrossRef
- Curto M, Girardi N, Lionetto L, et al. Systematic review of clozapine cardiotoxicity. Curr Psychiatry Rep. 2016;18(7):68. PubMed CrossRef
- Stark A, Scott J. A review of the use of clozapine levels to guide treatment and determine cause of death. Aust N Z J Psychiatry. 2012;46(9):816–825. PubMed CrossRef
- Seppälä N, Kovio C, Leinonen E. Effect of anticholinergics in preventing acute deterioration in patients undergoing abrupt clozapine withdrawal. CNS Drugs. 2005;19(12):1049–1055. PubMed CrossRef
- Green A, Stephenson T, Whiskey E, et al. Closure beyond clozapine: successfully averting rebound symptoms in a patient with schizoaffective disorder and agranulocytosis. BJPsych Open. 2019;5(3):e43. PubMed CrossRef
- Verghese C, DeLeon J, Nair C, et al. Clozapine withdrawal effects and receptor profiles of typical and atypical neuroleptics. Biol Psychiatry. 1996;39(2):135–138. PubMed CrossRef
- Bilbily J, McCollum B, de Leon J. Catatonia secondary to sudden clozapine withdrawal: a case with three repeated episodes and a literature review. Case Rep Psychiatry. 2017;2017:2402731. PubMed CrossRef
- Shiovitz TM, Welke TL, Tigel PD, et al. Cholinergic rebound and rapid onset psychosis following abrupt clozapine withdrawal. Schizophr Bull. 1996;22(4):591–595. PubMed CrossRef
- Correll CU. From receptor pharmacology to improved outcomes: individualising the selection, dosing, and switching of antipsychotics. Eur Psychiatry. 2010;25(suppl 2):S12–S21. PubMed CrossRef
- Taylor D, Young A, Barnes TE. The Maudsley Prescribing Guidelines in Psychiatry. Thirteenth Edition. Wiley-Blackwell; 2018.
- Chouinard G, Samaha AN, Chouinard VA, et al. Antipsychotic-induced dopamine supersensitivity psychosis: Pharmacology, criteria, and therapy. Psychother Psychosom. 2017;86(4):189–219. PubMed CrossRef
- Kim JH, Youn T, Choi JG, et al. Combination of electroconvulsive therapy and clozapine in treatment-resistant schizophrenia. Psychiatry Investig. 2018;15(8):829–835. PubMed CrossRef
- Petrides G, Malur C, Braga RJ, et al. Electroconvulsive therapy augmentation in clozapine-resistant schizophrenia: a prospective, randomized study. Am J Psychiatry. 2015;172(1):52–58. PubMed CrossRef
- Dunk LR, Annan LJ, Andrews CD. Rechallenge with clozapine following leucopenia or neutropenia during previous therapy. Br J Psychiatry. 2006;188(3):255–263. PubMed CrossRef
- Manu P, Lapitskaya Y, Shaikh A, et al. Clozapine rechallenge after major adverse effects: Clinical guidelines based on 259 cases. Am J Ther. 2018;25(2):e218–e223. PubMed CrossRef
Please sign in or purchase this PDF for $40.
Save
Cite