Prim Care Companion CNS Disord 2021;23(5):21l02856
To cite: Simões I, Silva C, Pestana PC, et al. Marchiafava-Bignami disease: a case of success. Prim Care Companion CNS Disord. 2021;23(5):21l02856.
To share: https://doi.org/10.4088/PCC.21l02856
© Copyright 2021 Physicians Postgraduate Press, Inc.
aDepartment of Neurosciences and Mental Health, Psychiatry Department, Santa Maria Hospital, Lisbon, Portugal
*Corresponding author: Inês Simões, MD, Department of Neurosciences and Mental Health, Psychiatry Department, Santa Maria Hospital, Lisbon, Portugal, Av Prof Egas Moniz, 1649-035 Lisboa ([email protected]).
Marchiafava-Bignami disease (MBD) is a rare sequelae of alcohol use disorder and malnutrition characterized by demyelination of the corpus callosum and the near subcortical white matter.1 We report a case of a man with chronic alcohol consumption diagnosed with MBD. This case highlights the unspecific presentation of MBD, its challenging diagnosis, and how a high-dose course of parenteral thiamine might be required to achieve clinical improvement.
Case Report
A 52-year-old man with severe alcohol use disorder (per DSM-5 criteria) was referred to our institution to undergo treatment for his addiction. At admission, he displayed agitation, severe impairment of attention, imbalance, postural tremor, a wide-based gait, and disorientation in time, space, and person. He reported zoopsias, initial insomnia, and paresthesias of the lower limbs. Neither ophthalmoplegia nor nystagmus was present. There was no history of seizures or fever.
Laboratory results showed decreased hemoglobin (11.9 g/dL), increased mean globular volume (115.1fL), increased γ-glutamyl transferase (69U/L), and decreased vitamin B12 (174pg/mL). The computed tomography scan was unremarkable.
Given this presentation, severe alcohol withdrawal syndrome was considered the likeliest diagnostic hypothesis. The patient subsequently received intravenous hydration, folic acid 5 mg/d, haloperidol 10 mg/d, diazepam 10 mg/d, and parenteral thiamine 500 mg/d.
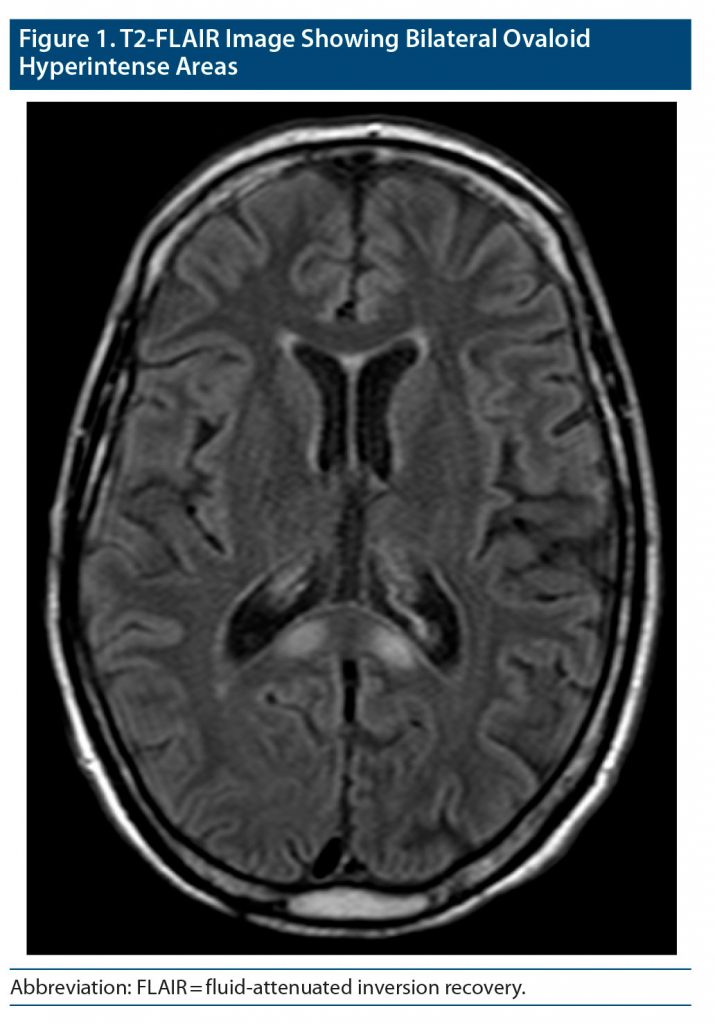
Over the course of 8 days, the patient did not improve. He was examined by neurology specialists, who ruled out Wernicke’s encephalopathy. The magnetic resonance imaging (MRI) solicited at admission was then conducted, revealing bilateral ovaloid hyperintense areas on T2-weighted images and hypointense areas on T1-weighted images in the splenium of the corpus callosum (Figure 1). There was also a discrete atrophy in the posterior body of the callosal commissure. These features were highly suggestive of MBD.
Given the likely diagnosis of MBD, the patient’s therapy was adjusted. He was given a 7-day course of parenteral thiamine 1,500 mg/d, oral B-complex vitamins, and intramuscular (IM) cyanocobalamin 1 mg/d.
We witnessed a gradual improvement of the patient’s clinical status following this therapeutic adjustment. His agitation ceased, and he displayed progressively more adequate behavior. His attention, orientation, speech, gait, and tremor improved, and the visual hallucinations disappeared.
Following the 7-day course of high-dose parenteral thiamine, the patient was switched to oral thiamine 300 mg/d and discharged. At a follow-up visit 6 months later, the patient was clinically well and abstinent. He was still taking vitamin supplementation. An MRI will be done to establish if the previously documented alterations remitted. Neuropsychological tests will also be performed to have a cognitive baseline assessment.
Discussion
MBD remains a challenging diagnosis from a clinical standpoint, but recent advances in neuroimaging facilitate its diagnosis. However, the disease classification is still mainly clinical.1 Type A MBD is characterized by coma, stupor, and pyramidal tract symptoms. On the other hand, type B presents a less severe but broader range of symptoms such as cognitive impairment, gait disturbances, behavioral abnormalities, visual hallucinations, dysarthria, apraxia, and even delusions.1,2 Our patient fits this subtype.
The symptoms of MBD are nonspecific and common in other alcohol-related diseases, namely alcohol withdrawal syndrome, Wernicke’s encephalopathy,3,4 delirium, and dementia.3,4 They can also be found in other medical conditions such as epilepsy, encephalitis, and vascular disturbances.5 The differential diagnosis is therefore wide ranging. The distinctive element of MBD lies on the characteristic MRI presentation consisting of T1-hypointense and T2-hyperintense bisymmetric lesions involving the central portion of the body of the corpus callosum.1 Sometimes lesions extend into the genu and splenium, as was the case in our patient.
Alcohol consumption is probably the most relevant risk factor for MBD. However, the etiology of the disease remains unclear. Some research6 suggests a role of alcohol-induced neurotoxicity and deficiency of B-complex vitamins. Given the uncertain underlying pathophysiology, there is no specific therapy for this disease and no management guidelines to date. Case reports5,7,8 have shown a favorable response to high-dose parenteral thiamine (1,500 mg/d) and oral B-complex vitamins. High-dose corticosteroids and amantadine have also been shown to be effective.9
In conclusion, early diagnosis and adequate treatment may provide a favorable outcome for a potentially fatal disease. Therefore, MBD should always be included in the differential diagnosis of patients with heavy alcohol consumption presenting with behavioral abnormalities, hallucinatory phenomena, and gait disturbances, as seen in our patient. Although therapy significantly overlaps with that of Wernicke-Korsakoff syndrome or alcohol withdrawal syndrome, one should always bear in mind that a high-dose thiamine course might be required, as illustrated by our case. We thus conclude that it is of the utmost importance to consider this diagnosis and to seek its confirmation through neuroimaging, so that we may acquire more reliable data regarding the incidence, treatment, and prognosis of this condition.
Published online: August 26, 2021.
Potential conflicts of interest: Dr Câmara Pestana reports nonfinancial support from Janssen-Cilag Farmacêutica, Lundbeck Portugal-Produtos Farmacêuticos Lda, Servier Portugal-Especialidades Farmacêuticas Lda, and Sanofi-Produtos Farmacêuticos, Lda outside the submitted work. Drs Simões, Silva, Gonçalves, and Ismail report no conflicts of interest related to the subject of this report.
Funding/support: None.
Patient consent: Consent was received from the patient to publish the case report, and information has been de-identified to protect anonymity.
References (9)

- Heinrich A, Runge U, Khaw AV. Clinicoradiologic subtypes of Marchiafava-Bignami disease. J Neurol. 2004;251(9):1050–1059. PubMed CrossRef
- Naaz A, Rizvi A, Usmani MA. Marchiafava-bignami disease presenting as acute psychosis. Indian J Psychol Med. 2018;40(5):494–496. PubMed CrossRef
- Zuccoli G, Pipitone N. Neuroimaging findings in acute Wernicke’s encephalopathy: review of the literature. AJR Am J Roentgenol. 2009;192(2):501–508. PubMed CrossRef
- Sullivan EV, Marsh L, Mathalon DH, et al. Relationship between alcohol withdrawal seizures and temporal lobe white matter volume deficits. Alcohol Clin Exp Res. 1996;20(2):348–354. PubMed CrossRef
- Helenius J, Tatlisumak T, Soinne L, et al. Marchiafava-Bignami disease: two cases with favourable outcome. Eur J Neurol. 2001;8(3):269–272. PubMed CrossRef
- Fernandes LMP, Bezerra FR, Monteiro MC, et al. Thiamine deficiency, oxidative metabolic pathways and ethanol-induced neurotoxicity: how poor nutrition contributes to the alcoholic syndrome, as Marchiafava-Bignami disease. Eur J Clin Nutr. 2017;71(5):580–586. PubMed CrossRef
- Nemlekar SS, Mehta RY, Dave KR, et al. Marchiafava: Bignami disease treated with parenteral thiamine. Indian J Psychol Med. 2016;38(2):147–149. PubMed CrossRef
- Garcia-Santibanez R. Marchiafava-Bignami disease presenting as acute dysarthria and ataxia. Alcohol Alcohol. 2015;50(2):256–257. PubMed CrossRef
- Staszewski J, Macek K, Stepień A. Reversible demyelinisation of corpus callosum in the course of Marchiafava-Bignami disease. Neurol Neurochir Pol. 2006;40(2):156–161. PubMed
Please sign in or purchase this PDF for $40.
Save
Cite