Objective: Orthostatic intolerance (OI) is an important health problem for children and adolescents. The onset and exacerbation of OI are strongly affected by psychosocial factors. Intestinal microbial deviations, which are affected by food and lifestyle factors, are an important risk factor for adult psychiatry patients, but their roles in pediatric patients are unclear. The objective of this study was to investigate the intestinal microbiota and its involvement in the mental health of children with OI.
Methods: Fifty-six fecal samples from pediatric OI patients and 9 samples from healthy children were examined with terminal-restriction fragment length polymorphism analysis from July 2016 to January 2018 at Nihon University Itabashi Hospital, Tokyo, Japan. Bacterial diversity was analyzed using the Shannon-Wiener index and the Simpson index. All OI patients were assessed using 2 different psychological scales: the Children’s Depression Inventory and the Children’s Manifest Anxiety Scale. The patients were then divided into the following subgroups: depression or nondepression and anxiety or nonanxiety.
Results: The mean proportion of Clostridium subcluster XIVa and/or Enterobacteriaceae (operational taxonomic unit [OTU] 940) in the OI patients was significantly higher than that in the healthy controls (P = .02). Among OI patients, Bifidobacterium (OTU 124) was less frequent in the depression group than in the nondepression group. However, depression and anxiety showed no correlation with bacterial diversity.
Conclusions: Pediatric OI patients showed deviations in the intestinal bacterial flora. The intestinal flora can serve as a novel therapeutic target for the mental health management of intractable pediatric OI patients.
ABSTRACT
Objective: Orthostatic intolerance (OI) is an important health problem for children and adolescents. The onset and exacerbation of OI are strongly affected by psychosocial factors. Intestinal microbial deviations, which are affected by food and lifestyle factors, are an important risk factor for adult psychiatry patients, but their roles in pediatric patients are unclear. The objective of this study was to investigate the intestinal microbiota and its involvement in the mental health of children with OI.
Methods: Fifty-six fecal samples from pediatric OI patients and 9 samples from healthy children were examined with terminal-restriction fragment length polymorphism analysis from July 2016 to January 2018 at Nihon University Itabashi Hospital, Tokyo, Japan. Bacterial diversity was analyzed using the Shannon-Wiener index and the Simpson index. All OI patients were assessed using 2 different psychological scales: the Children’s Depression Inventory and the Children’s Manifest Anxiety Scale. The patients were then divided into the following subgroups: depression or nondepression and anxiety or nonanxiety.
Results: The mean proportion of Clostridium subcluster XIVa and/or Enterobacteriaceae (operational taxonomic unit [OTU] 940) in the OI patients was significantly higher than that in the healthy controls (P = .02). Among OI patients, Bifidobacterium (OTU 124) was less frequent in the depression group than in the nondepression group. However, depression and anxiety showed no correlation with bacterial diversity.
Conclusions: Pediatric OI patients showed deviations in the intestinal bacterial flora. The intestinal flora can serve as a novel therapeutic target for the mental health management of intractable pediatric OI patients.
Prim Care Companion CNS Disord 2019;21(2):18m02401
To cite: Ishii W, Komine-Aizawa S, Takano C, et al. Relationship between the fecal microbiota and depression and anxiety in pediatric patients with orthostatic intolerance. Prim Care Companion CNS Disord. 2019;21(2):18m02401.
To share: https://doi.org/10.4088/PCC.18m02401
© Copyright 2019 Physicians Postgraduate Press, Inc.
aDepartment of Pediatrics and Child Health, Nihon University School of Medicine, Tokyo, Japan
bDivision of Microbiology, Department of Pathology and Microbiology, Nihon University School of Medicine, Tokyo, Japan
cCenter for Institutional Research and Medical Education, Nihon University School of Medicine, Tokyo, Japan
*Corresponding author: Shihoko Komine-Aizawa, MD, PhD, Division of Microbiology, Department of Pathology and Microbiology, Nihon University School of Medicine, 30-1, Oyaguchi-Kamicho, Itabashi-ku, Tokyo, 1738610 Japan ([email protected]).
Orthostatic intolerance (OI) is the most frequent pediatric psychosomatic disorder.1 OI is suspected to cause autonomic nerve dysfunction.2 Over- or underactivation of the autonomic nerve system causes various symptoms, including recurrent dizziness, chronic fatigue, headache, and syncope. OI often occurs with psychiatric disorders such as depression and anxiety. The susceptibility of children is influenced by genetic and environmental factors.2
A report3 suggested an important role of the intestinal microbiome in the function of the autonomic nervous system. Some molecules produced by intestinal bacteria, as well as some tissue products stimulated by bacterial substances, enter the systemic circulation and are delivered into target organs.4,5 These neurotransmitters impact brain function by acting on the enteric nervous system, although they do not enter the central nervous system through the blood-brain barrier.6 For instance, Bifidobacterium infantis has been shown to increase plasma tryptophan, which is an essential amino acid needed for the synthesis of central serotonin.7 In addition, Candida, Streptococcus, Escherichia, and Enterococcus species can synthesize serotonin.6 It is also known that many neurotransmitters are synthesized and released by various bacteria and fungi, including γ-aminobutyric acid (GABA) from Bifidobacterium and Lactobacillus species; norepinephrine from Escherichia, Bacillus, and Saccharomyces species; and dopamine from Bacillus species.6,8 Intestinal microbiota, such as Bifidobacterium species, also produce short-chain fatty acids, including butyrate, propionate, and acetate, by fermenting indigestible dietary components.9 Short-chain fatty acids modulate serotonin synthesis in enterochromaffin cells.10 Thus, the intestinal microbiota play an important role in bidirectional communication between the gut and brain. In this sense, this type of communication could be named the gut-microbiota-brain axis.6,11-13
This axis has potentially important roles in the pathophysiology of psychosocial stress-related diseases. Interestingly, microbial deviations have been reported in adult patients with depression and anxiety.14-16 In a murine model, the postnatal development of hypothalamic-pituitary-adrenal stress responses was strongly influenced by commensal microbiota.17 This finding supports clinical studies18-20 suggesting that probiotic administration can benefit mental health in adult patients. However, there have been few reports on microbial deviations in child and adolescent patients with psychiatric diseases and OI. In this study, we examined the fecal flora of pediatric patients with OI to determine the roles of the gut microbiota in OI pathophysiology.
METHODS
Subjects
This study was approved by the ethical committee of Nihon University Itabashi Hospital (RK-160510-01) according to the revised version of the Declaration of Helsinki. Written informed consent was obtained from all subjects and their parents before collecting samples.
Patients with OI were recruited from the outpatient clinic of Nihon University Itabashi Hospital, Tokyo, Japan, between July 2016 and January 2018. All patients with OI were diagnosed in accordance with the Japanese Clinical Guidelines for Juvenile Orthostatic Dysregulation Version 12 according to the conventional Schellong orthostatic test using a noninvasive beat-to-beat blood pressure monitor. We excluded patients with other underlying diseases, such as inflammatory bowel disease, infectious enteritis, and eating disorders.
Healthy subjects with no diagnosed psychiatric disorders were enrolled as the control group after interviews with their parents. No subjects were treated with antimicrobial or probiotic medicine for at least 2 weeks before testing.
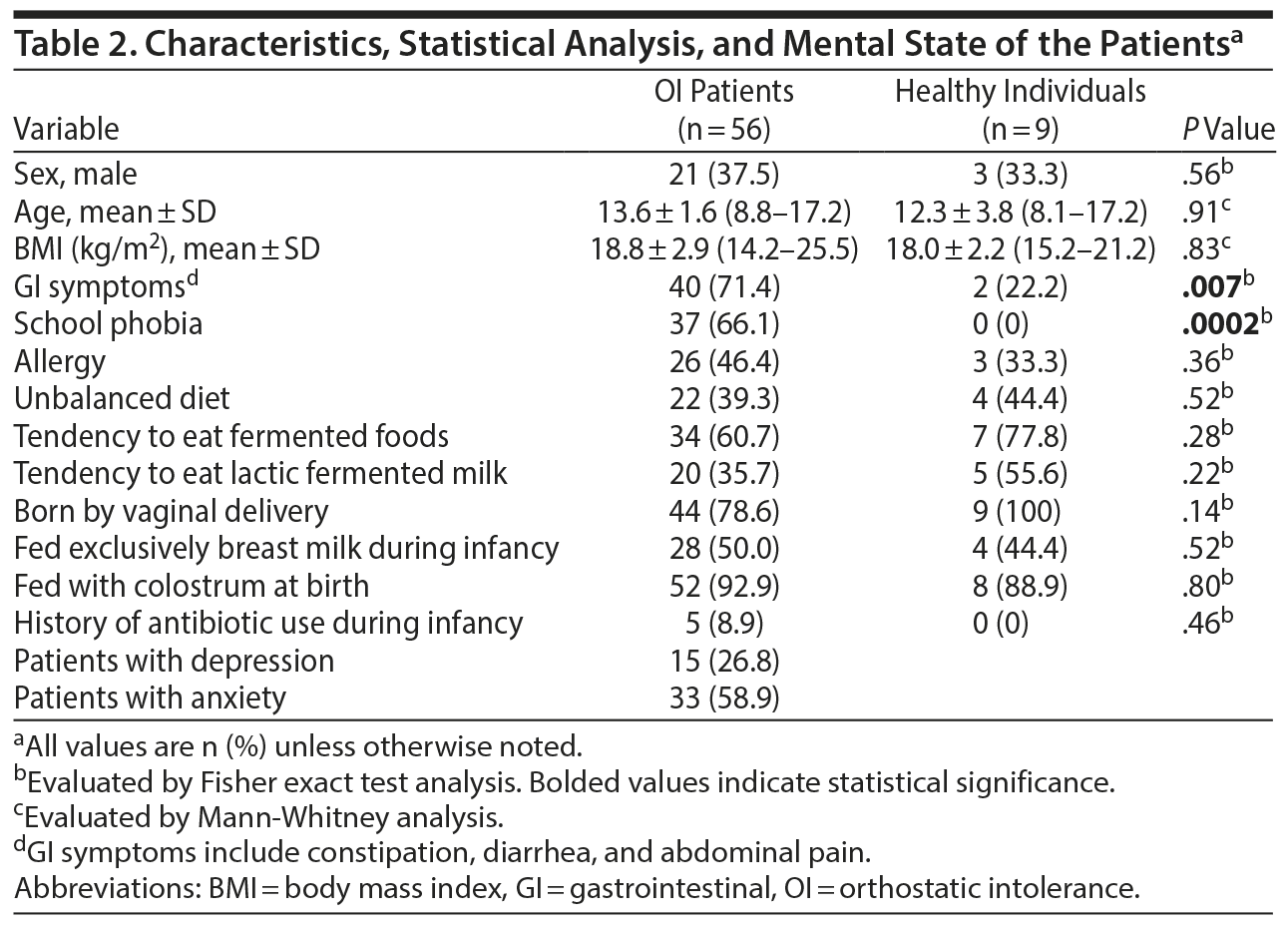
This study enrolled 56 patients with OI and 9 healthy children as controls. All subjects were < 18 years of age. The ages of the patients ranged from 8.8 to 17.2 (mean ± SD = 13.6 ± 1.6) years and those of the control children from 8.1 to 17.2 (mean ± SD = 12.3 ± 3.8) years. The OI group consisted of 21 boys and 35 girls, while the control group consisted of 3 boys and 6 girls.

- There is a possibility of an association between microbial deviation and the exacerbation of mental health symptoms in patients with pediatric orthostatic intolerance (OI).
- The relationship between intestinal microbiota and the mental health of patients with pediatric OI is still unknown.
- Probiotics containing Bifidobacteria may serve as a novel therapeutic intervention in the mental health treatment of patients with OI.
Evaluation of Depressive State and Anxiety
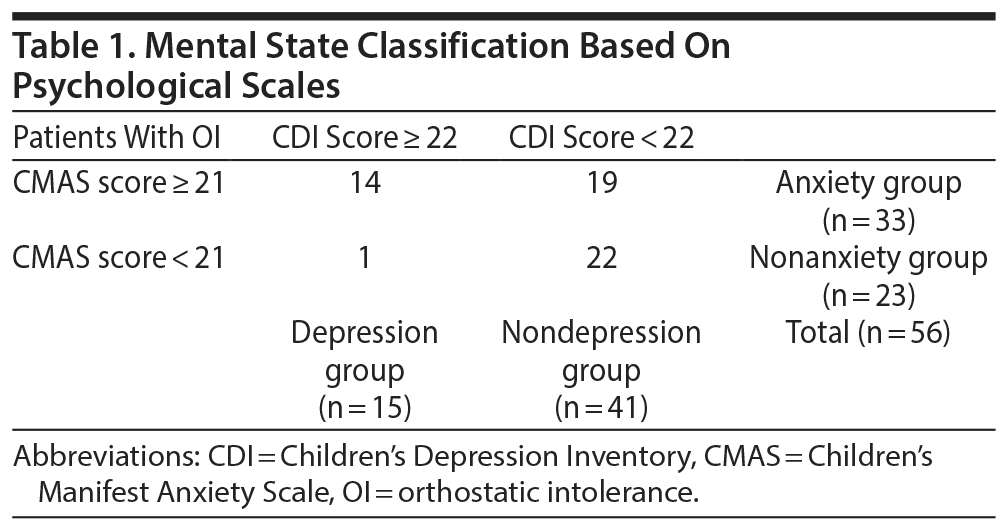
Mental states were assessed on the basis of the following 2 scales: a Japanese version of the Children’s Depression Inventory (CDI)21 and the Children’s Manifest Anxiety Scale (CMAS).22 The CDI consists of 27 items related to children’s depressive symptoms. Subjects provided a score of 0, 1, or 2 points for each item. Thus, a full score would be 54 points. The cutoff score for the Japanese test is set at 22 points. Therefore, a subject with more than 22 points was defined as having depressive tendencies. The CMAS consists of 42 short items that are used to determine the presence of anxiety-related symptoms in children, as well as 15 items to measure falsehoods. A subject with more than 21 points was defined as a high-anxiety patient, and a subject with more than 29 points was defined as a very high-anxiety patient. Therefore, the cutoff score was 21 points in this study.
Each classification is defined in Table 1. OI patients were classified according to the 2 scales as follows: patients with a CDI score ≥ 22 points were placed in the depression group, and patients with a CDI score < 22 points were placed in the nondepression group. Similarly, patients with a CMAS score ≥ 21 points were placed in the anxiety group, and patients with a CMAS score < 21 points were placed in the nonanxiety group.
Clinical Information
Clinical information was obtained for all subjects using a questionnaire designed for this study. The questionnaire included the following items: presence or absence of gastrointestinal (GI) symptoms, refusal to attend school, unbalanced diet, tendency to eat fermented foods and lactic fermented milk, perinatal histories including mode of delivery, and main source of nutrition during infancy including colostrum intake. In this study, we defined GI symptoms as constipation, diarrhea, and abdominal pain.
Fecal Samples
Fecal samples were collected from all 56 patients with OI and from 9 healthy children. Samples were prepared by the subjects at their homes, transported to the hospital immediately, and then stored at −80°C until DNA extraction. DNA was extracted from the fecal samples (180-220 mg from each stool sample) using the QIAamp DNA Stool Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
Terminal-Restriction Fragment Length Polymorphism
The intestinal microbiota were analyzed in the prepared DNA samples by terminal-restriction fragment length polymorphism (T-RFLP) (TechnoSuruga Laboratory, Shizuoka, Japan). The samples were analyzed based on the Nagashima method using a primer-enzyme combination targeting the 16S rRNA gene.23 Polymerase chain reaction was performed using a terminal fluorescence-labeled primer set. The purified polymerase chain reaction products were digested using BslI. Fluorescence-labeled T-RFs were divided into 29 operational taxonomic units (OTUs). These OTUs were quantified as the percentage values of the individual OTU per total OTU area, which were expressed as the percentage of the area under the curve. On the basis of our analyses of fecal bacterial communities using the BslI-digested T-RF patterns, the fecal microbial profiles were compared in terms of OTUs.
Statistical Analysis
All OTU values are expressed as mean ± SD. The diversity of the gut microbiome was analyzed using the Shannon-Wiener index and the Simpson index.24 Data were compared between each group using the Mann-Whitney test, and a value of P < .05 was considered significant. The Mann-Whitney test was used in addition to the Kruskal-Wallis test for analysis among 3 groups. A value of P < .017 was considered significant.
The detection rate in each group was compared using Fisher exact test, and a value of P < .05 was considered significant. All data were analyzed using Statcel 4 software (OMS Publishing Inc, Tokorozawa, Japan).
RESULTS
Clinical Profiles of OI Patients and Healthy Individuals
The clinical profiles of the participants and the results of the statistical analyses are presented in Table 2. Fisher exact test showed significant differences in GI symptoms and school phobia between the OI patients and the healthy controls. Depressive tendencies were observed in 26.8% of OI patients, and anxiety was found in 58.9% of OI patients. In OI patients, there was a positive correlation between GI symptoms and anxiety (P = .04); however, there was no correlation between GI symptoms and depressive tendencies (P = .13). There were no correlations of school phobia with either depressive tendencies or anxiety (P = .56 and P = .91, respectively).
Bacterial Flora of OI Patients and Healthy Individuals
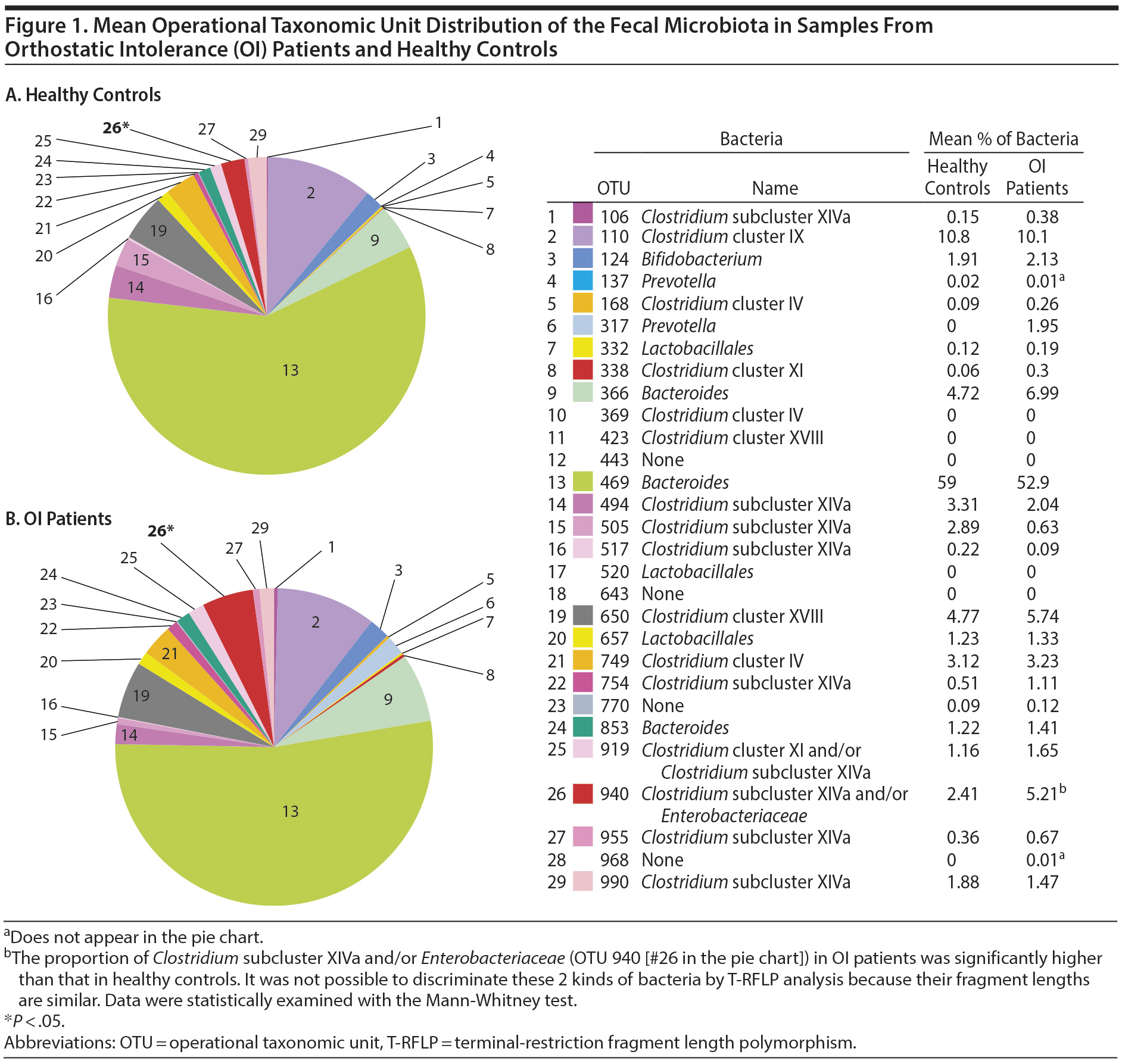
The gut bacterial populations of OI patients and healthy controls were investigated using the BslI-digested T-RFLP database. Twenty-nine OTUs were compared between OI patients and healthy controls. As shown in Figure 1, the proportion of Clostridium subcluster XIVa and/or Enterobacteriaceae (OTU 940) among all gut microbiota in OI patients (5.21 ± 6.40) was significantly higher than that in healthy controls (2.41 ± 4.54) (P = .02). There were no significant differences in the other 28 OTUs.
Relationship Between the Fecal Microbiota and Depression and Anxiety Among OI Patients
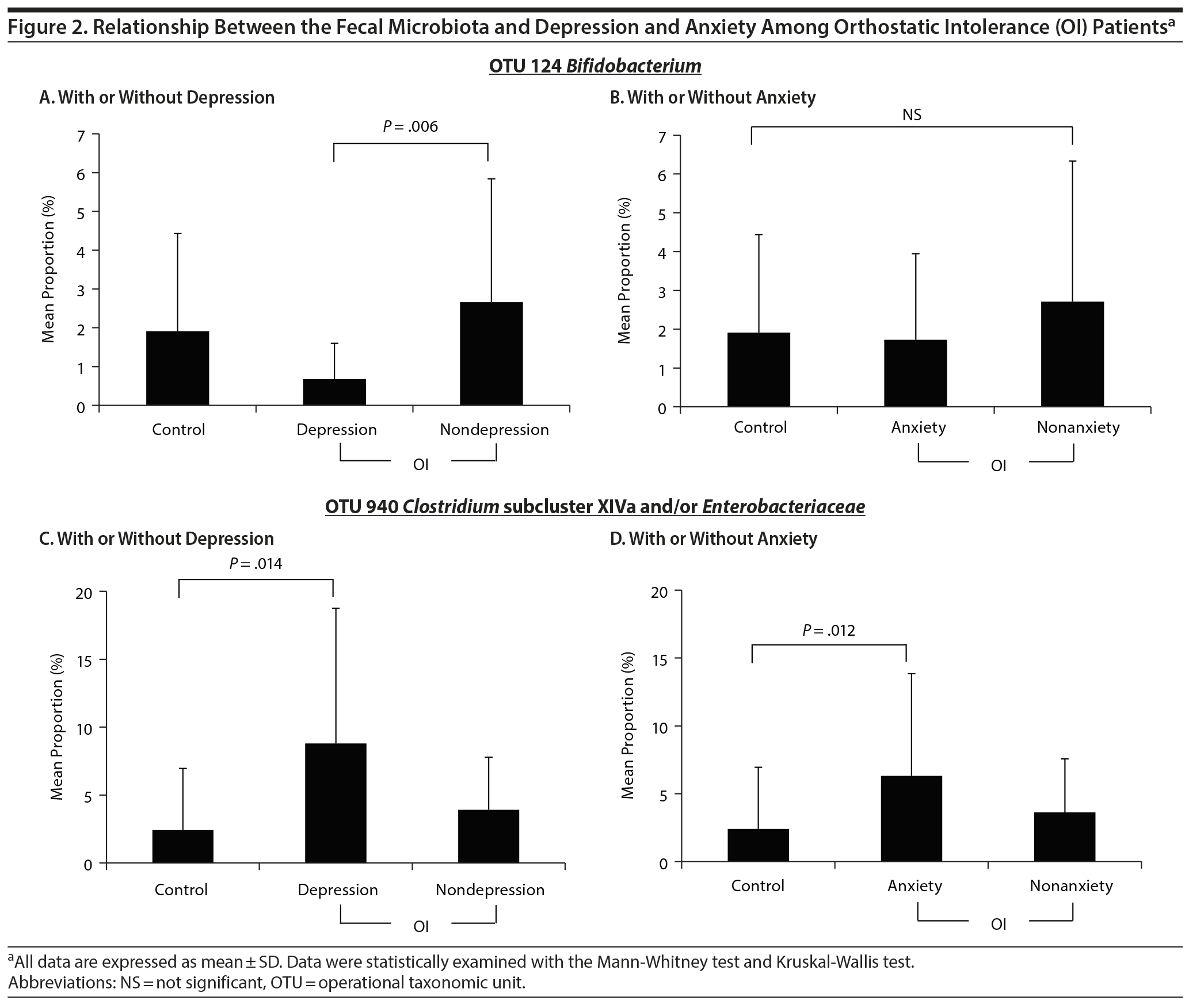
Next, we examined the frequency of OTUs among OI patients with or without depression or anxiety. As shown in Figure 2, significant changes were identified in 2 of the 29 OTUs. The mean proportion of Bifidobacterium (OTU 124) in the nondepression group (2.66 ± 3.18) was significantly higher than that in the depression group (0.67 ± 0.92, P = .006) (Figure 2A). However, there were no significant differences among the healthy control, anxiety, and nonanxiety groups (Figure 2B). The mean proportion of Clostridium subcluster XIVa and/or Enterobacteriaceae (OTU 940) in the healthy controls (2.41 ± 4.54) was significantly lower than that in the depression group (8.80 ± 9.95, P = .014) and the anxiety group (6.32 ± 7.53, P = .012) (Figure 2C and 2D). In addition, there was no significant difference in GI symptoms, as indicated by the proportion of Clostridium subcluster XIVa and/or Enterobacteriaceae (OTU 940) (data not shown).
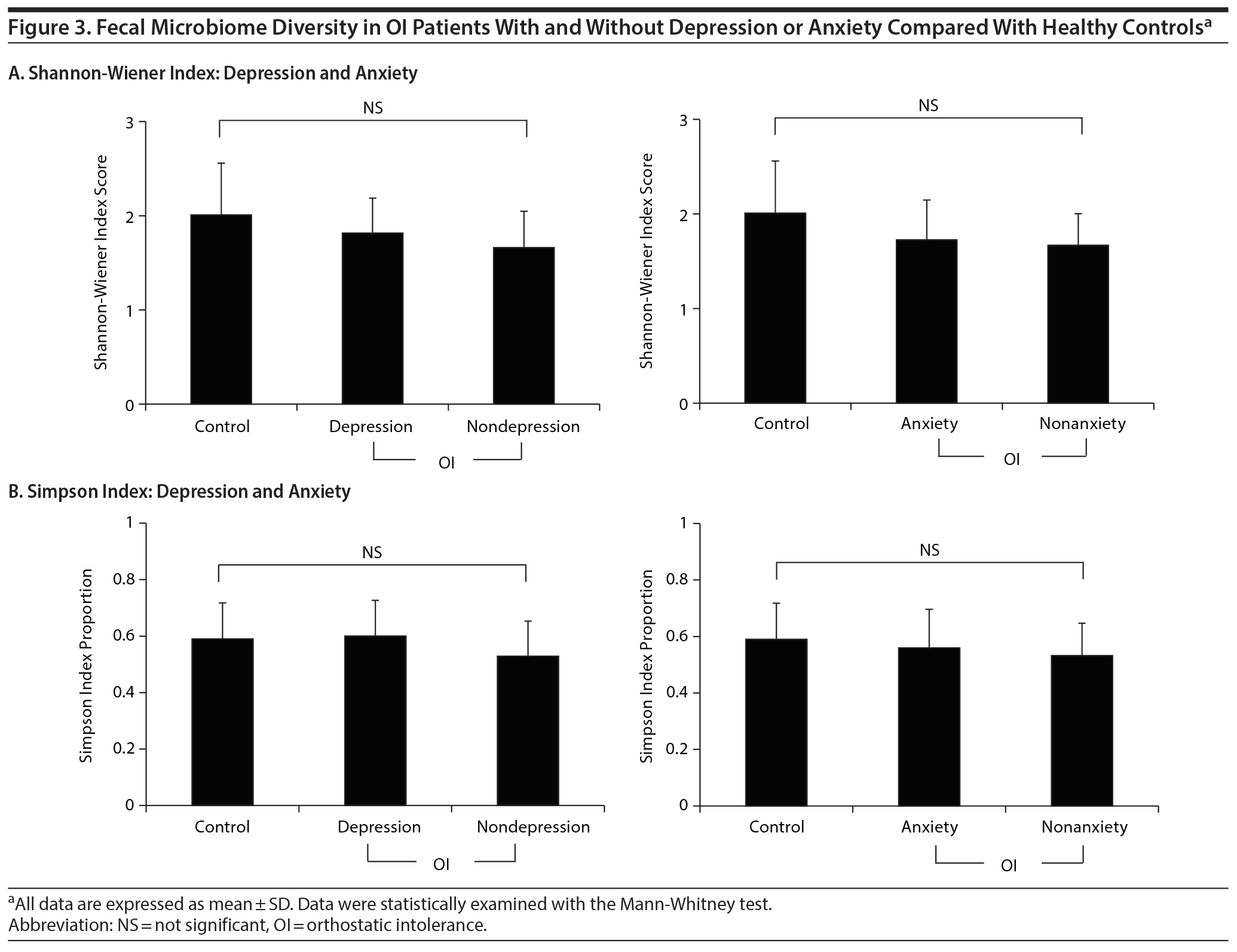
Fecal Microbiome Diversity
The bacterial diversity of the microbiota profiles from OI patients and healthy individuals was measured using the Shannon-Wiener index and the Simpson index. There were no significant differences in either index among the control, depression, and nondepression groups. There were also no significant differences between the anxiety and nonanxiety groups (Figure 3). In addition, our data showed that the microbiota diversity displayed no differences based on clinical information, including age, body mass index, diet, and history of antibiotic treatment (Supplementary Table 1).
DISCUSSION
OI is one of the most frequent health problems among children and adolescents. In this study, we found deviations in the intestinal flora in pediatric patients with OI. This finding suggests a possible role of intestinal dysbiosis as the cause of pediatric psychiatric disorders with OI. Pediatric OI cases are often complicated by depression and anxiety. The global prevalence of depression and anxiety among children and adolescents is 2.6% and 6.5%, respectively.25 In our study, the rates of depressive tendencies and anxiety were 26.8% and 58.9%, respectively; these data were consistent with previous reports.2
The possible importance of the intestinal microbiota has been reported in adult psychiatric diseases.26,27 Several researchers have reported changes in the composition of major gut bacterial groups in patients with depression and anxiety using exhaustive intestinal flora analysis based on the 16S rRNA gene. Naseribafrouei et al28 reported a significant increase in the order Bacteroidales and a decrease in the family Lachnospiraceae in patients with depression. Jiang et al16 observed similar findings: increased OTUs from the phyla Bacteroidetes and Proteobacteria and a decrease in the phylum Firmicutes in patients with depression. Aizawa et al29 also reported decreased proportions of Bifidobacterium and Lactobacillus in patients with major depressive disorders. These findings suggest the possible involvement of intestinal dysbiosis in the pathophysiology of adult depressive disorders. However, adult and pediatric psychological disorders have different pathological and clinical backgrounds. Thus, the roles of the intestinal flora in pediatric psychological disorders must be clarified. To clarify these roles, we analyzed clinical fecal samples collected from pediatric patients and compared them with those of healthy subjects.
First, we analyzed intestinal bacterial flora by T-RFLP. In the patients with OI, a significantly higher rate of Clostridium subcluster XIVa and/or Enterobacteriaceae was observed. This finding suggests the possible role of Clostridium subcluster XIVa and/or Enterobacteriaceae in the pathophysiology of pediatric mental health with OI. However, we could not identify specific roles of each bacterial family for technical limit. Although Clostridium and Enterobacteriaceae genomes are similar in 16S rRNA coding genes, they possess very different biological natures including Gram stainability, aerobic/anaerobic metabolism, and cytokine induction. In this study, we could not differentiate them with T-RFLP analysis targeting this area. More precise bacterial analysis at the species level employing next-generation sequencers is needed.
Next, we analyzed intestinal flora among OI patients with or without depression or anxiety. Focusing on depression, increased frequency of Bifidobacterium was observed in the OI patient with depression compared with the OI patient without depression as shown in Figure 2A. Furthermore, the proportions of Clostridium subcluster XIVa and/or Enterobacteriaceae were also significantly higher in the patients with depression than in the healthy controls as shown in Figure 2C. On the other hand, focusing on anxiety, only proportions of Clostridium subcluster XIVa and/or Enterobacteriaceae were significantly higher in the patients with anxiety than in the healthy controls as shown in Figure 2D. No significant difference was observed in the other 27 OTUs.
Among bacterial families with positive correlations, Bifidobacterium is a major representative beneficial bacterium.8 Bifidobacterium species are reported to produce GABA, which acts as an inhibitory neurotransmitter in the brain.6 A recent report by Jang et al30 found that the presence of Bifidobacterium adolescentis IM38 reduced the expression of stress-related cytokines, including tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6) and that this species potentially attenuated anxiety through the modulation of benzodiazepine sites on the GABA receptor in a mouse model. Probiotics and prebiotics containing Bifidobacterium have been shown to improve the psychological states of humans and rats.18,19 These results support the hypothesis that Bifidobacterium plays a potentially important role in mental health.
Clostridium subcluster XIVa was previously classified under Lachnospiraceae and is known to include strains that produce high concentrations of butyric acid, an important short-chain fatty acid, in the human intestine.31 Fusicatenibacter saccharivorans, a member of Clostridium subcluster XIVa, induces the expression of IL-10 in the lamina propria and inhibits inflammation of the intestinal tract in patients with ulcerative colitis.32 Although Clostridium subcluster XIVa is generally considered beneficial for humans, we observed that the proportion of Clostridium subcluster XIVa was significantly higher in the depression and anxiety groups than in the healthy controls. Many aspects of the impact of Clostridium subcluster XIVa on hosts and its function in the human intestine are unknown and need further elucidation. On the other hand, Enterobacteriaceae are not major bacteria in normal human intestinal flora, though they had been regarded as the primary bacteria at the culture-based period. These bacteria actually constitute less than 1% of the total gut flora.33 However, they may play strong biological roles including potent immunostimulatory effects as well as production of bioactive substances. Jiang et al16 observed that the proportion of Enterobacteriaceae was increased in patients with depressive disorders compared to that of healthy controls.
OI patients tend to spend long periods of time in a supine position because the symptoms of OI are often exacerbated when standing. Bedrest often weakens intestinal motor functions.34 In this study, OI patients showed a significantly higher frequency of GI symptoms than healthy subjects. This finding is consistent with previous reports.35 The severity of GI symptoms showed a correlation with anxiety in OI patients, but bacterial changes with and without GI symptoms were not identified in this study.
Finally, we examined bacterial diversity in OI patients with or without depression or anxiety. However, in our data, depression and anxiety showed no significant correlations with bacterial diversity. Even in adult patients, the role of bacterial diversity in depressive disorders is controversial. Jiang et al16 reported that the intestinal bacterial diversity of the depressive group was higher than that of the nondepressed group in adults. Naseribafrouei et al28 reported that there was no significant difference in bacterial diversity between depressed patients and nondepressed patients. Generally, high bacterial diversity is considered beneficial for human health. However, the role of intestinal microbiota diversity in the function of the central nervous system has not been fully elucidated. The degree of intestinal microbiota diversity is known to be affected by age, diet, history of antibiotic treatment, and pregnancy.36 However, our data did not show that microbiota diversity was affected by any of these clinical parameters.
There were several limitations of this study that should be mentioned. First, our study did not include controlled age-matched data, and the number of healthy subjects was small. Second, the QIAamp DNA Stool Mini Kit has difficulty detecting Gram-positive bacteria, which have relatively thick cell walls.37 Another problem is the equivalency between the real intestinal microflora and the microflora in fecal samples. Endoscopic sampling of the mucosal flora may reflect such possible discrepancies, but this procedure is not indicated. In addition, single-time stool sampling might be affected by the patient’s dietary composition and lifestyle. Repeated longitudinal sampling must be carried out to eliminate daily or conditional flora changes. Finally, due to the limitations of T-RFLP analysis, the bacterial classification obtained using this method is not comparable to that obtained with next-generation sequencing. Due to the advantages of T-RFLP, including its low cost and simple technique, we are planning a longitudinal study with the same patients to compare clinical features over time. Moreover, quantitative analysis of bacterial concentrations may improve our understanding.
In summary, this study suggests the involvement of intestinal microbial dysbiosis in the pathophysiology of pediatric OI patients. Bacterial components or bioreactive substances produced by the intestinal mucosa may affect the autonomic nervous system. Therefore, normalization of the intestinal environment can serve as a novel therapeutic intervention for pediatric psychiatric disorders in OI patients who are resistant to conventional drug therapy or psychotherapy.
Submitted: October 25, 2018; accepted January 14, 2019.
Published online: April 11, 2019.
Potential conflicts of interest: None.
Funding/support: None.
Acknowledgments: The authors would like to express special thanks to Yoshitaka Kaneita, MD, PhD (Department of Public Health, Nihon University School of Medicine, Tokyo, Japan) for his support in data analysis. The authors also appreciate Eric Hajime Jego, MA (Center for Institutional Research and Medical Education, Nihon University School of Medicine, Tokyo, Japan) for his assistance with the English editing. They also thank Momoko Takahashi, MA (clinical child psychologist, Nihon University Itabashi Hospital, Tokyo, Japan) for her advice on the evaluation of psychological states and Tatsuo Fuchigami, MD, PhD; Ayumi Fukuda, MD, PhD; Emiko Momoki, MD; Kaori Kimura, MD; Sonoko Kubota, MD; Tadayasu Kawaguchi, MD; and Yuki Kasuga, MD (Department of Pediatrics and Child Health, Nihon University School of Medicine, Tokyo, Japan) for recruiting patients. The acknowledged individuals report no conflicts of interest related to the subject of this article.
Supplementary material: See accompanying pages.
REFERENCES
1. Okuni M. Orthostatic dysregulation in childhood with special reference to the standing electrocardiogram. Jpn Circ J. 1963;27(2):200-204. PubMed CrossRef
2. Tanaka H, Fujita Y, Takenaka Y, et al; Task Force of Clinical Guidelines for Child Orthostatic Dysregulation, Japanese Society of Psychosomatic Pediatrics. Japanese Clinical Guidelines for Juvenile Orthostatic Dysregulation Version 1. Pediatr Int. 2009;51(1):169-179. PubMed CrossRef
3. Bonaz B, Bazin T, Pellissier S. The vagus nerve at the interface of the microbiota-gut-brain axis. Front Neurosci. 2018;12:49. PubMed CrossRef
4. Foster JA, Rinaman L, Cryan JF. Stress & the gut-brain axis: regulation by the microbiome. Neurobiol Stress. 2017;7:124-136. PubMed CrossRef
5. Huo R, Zeng B, Zeng L, et al. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front Cell Infect Microbiol. 2017;7:489. PubMed CrossRef
6. Dinan TG, Cryan JF. Brain-gut-microbiota axis and mental health. Psychosom Med. 2017;79(8):920-926. PubMed CrossRef
7. Desbonnet L, Garrett L, Clarke G, et al. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience. 2010;170(4):1179-1188. PubMed CrossRef
8. Lyte M. Microbial endocrinology and the microbiota-gut-brain axis. In: Cohen IR, Lajtha A, Lambris JD, et al, eds. Advances in Experimental Medicine and Biology. New York, NY: Springer; 2014:3-24.
9. Fukuda S, Toh H, Hase K, et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature. 2011;469(7331):543-547. PubMed CrossRef
10. Yano JM, Yu K, Donaldson GP, et al. Indigenous bacteria from the gut microbiota regulate host serotonin biosynthesis. Cell. 2015;161(2):264-276. PubMed CrossRef
11. Rhee SH, Pothoulakis C, Mayer EA. Principles and clinical implications of the brain-gut-enteric microbiota axis. Nat Rev Gastroenterol Hepatol. 2009;6(5):306-314. PubMed CrossRef
12. Petra AI, Panagiotidou S, Hatziagelaki E, et al. Gut-microbiota-brain axis and its effect on neuropsychiatric disorders with suspected immune dysregulation. Clin Ther. 2015;37(5):984-995. PubMed CrossRef
13. Martin CR, Osadchiy V, Kalani A, et al. The brain-gut-microbiome axis. Cell Mol Gastroenterol Hepatol. 2018;6(2):133-148. PubMed CrossRef
14. Luna RA, Foster JA. Gut brain axis: diet microbiota interactions and implications for modulation of anxiety and depression. Curr Opin Biotechnol. 2015;32:35-41. PubMed CrossRef
15. Foster JA, McVey Neufeld KA. Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci. 2013;36(5):305-312. PubMed CrossRef
16. Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186-194. PubMed CrossRef
17. Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(pt 1):263-275. PubMed CrossRef
18. Messaoudi M, Lalonde R, Violle N, et al. Assessment of psychotropic-like properties of a probiotic formulation (Lactobacillus helveticus R0052 and Bifidobacterium longum R0175) in rats and human subjects. Br J Nutr. 2011;105(5):755-764. PubMed CrossRef
19. Benton D, Williams C, Brown A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr. 2007;61(3):355-361. PubMed CrossRef
20. Vlainić JV, Šuran J, Vlainić T, et al. Probiotics as an adjuvant therapy in major depressive disorder. Curr Neuropharmacol. 2016;14(8):952-958. PubMed CrossRef
21. Kovacs M. Rating scales to assess depression in school-aged children. Acta Paedopsychiatr. 1981;46(5-6):305-315. PubMed
22. Castaneda A, McCandless BR, Palermo DS. The children’s form of the Manifest Anxiety Scale. Child Dev. 1956;27(3): 317-326. PubMed CrossRef
23. Nagashima K, Hisada T, Sato M, et al. Application of new primer-enzyme combinations to terminal restriction fragment length polymorphism profiling of bacterial populations in human feces. Appl Environ Microbiol. 2003;69(2):1251-1262. PubMed CrossRef
24. Hill MO. Diversity and evenness: a unifying notation and its consequences. Ecology. 1973;54(2):427-432. CrossRef
25. Wozney L, McGrath PJ, Gehring ND, et al. Mental healthcare technologies for anxiety and depression in childhood and adolescence: systematic review of studies reporting implementation outcomes. JMIR Ment Health. 2018;5(2):e48. PubMed CrossRef
26. MacQueen G, Surette M, Moayyedi P. The gut microbiota and psychiatric illness. J Psychiatry Neurosci. 2017;42(2):75-77. PubMed CrossRef
27. Mangiola F, Ianiro G, Franceschi F, et al. Gut microbiota in autism and mood disorders. World J Gastroenterol. 2016;22(1):361-368. PubMed CrossRef
28. Naseribafrouei A, Hestad K, Avershina E, et al. Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil. 2014;26(8):1155-1162. PubMed CrossRef
29. Aizawa E, Tsuji H, Asahara T, et al. Possible association of Bifidobacterium and Lactobacillus in the gut microbiota of patients with major depressive disorder. J Affect Disord. 2016;202:254-257. PubMed CrossRef
30. Jang HM, Jang SE, Han MJ, et al. Anxiolytic-like effect of Bifidobacterium adolescentis IM38 in mice with or without immobilisation stress. Benef Microbes. 2018;9(1):123-132. PubMed CrossRef
31. Barcenilla A, Pryde SE, Martin JC, et al. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl Environ Microbiol. 2000;66(4):1654-1661. PubMed CrossRef
32. Takeshita K, Mizuno S, Mikami Y, et al. A single species of Clostridium subcluster XIVa decreased in ulcerative colitis patients. Inflamm Bowel Dis. 2016;22(12):2802-2810. PubMed CrossRef
33. Mitsuoka T. Establishment of intestinal bacteriology. Biosci Microbiota Food Health. 2014;33(3):99-116. PubMed CrossRef
34. Camilleri M, Malagelada JR, Stanghellini V, et al. Gastrointestinal motility disturbances in patients with orthostatic hypotension. Gastroenterology. 1985;88(6):1852-1859. PubMed CrossRef
35. Sullivan SD, Hanauer J, Rowe PC, et al. Gastrointestinal symptoms associated with orthostatic intolerance. J Pediatr Gastroenterol Nutr. 2005;40(4):425-428. PubMed CrossRef
36. Lozupone CA, Stombaugh JI, Gordon JI, et al. Diversity, stability and resilience of the human gut microbiota. Nature. 2012;489(7415):220-230. PubMed CrossRef
37. Mirsepasi H, Persson S, Struve C, et al. Microbial diversity in fecal samples depends on DNA extraction method: easyMag DNA extraction compared to QIAamp DNA stool mini kit extraction. BMC Res Notes. 2014;7(1):50. PubMed CrossRef
Please sign in or purchase this PDF for $40.00.
Save
Cite