Migraine is associated with considerable pain and disability. Accurate diagnosis that differentiates migraine from other primary and secondary headache disorders is needed, and clinicians should assess the patient’s risk of progressing to chronic migraine. Many patients will benefit from preventive treatment. Clinicians must know how to identify these patients and be familiar with the numerous preventive treatments. Finally, clinicians must select the safest, most effective treatment while keeping in mind individual characteristics such as coexisting conditions and patient preferences.
CME Objectives
After studying this educational activity, you should be able to:
- Use updated classifications and rating scales in the assessment of patients with headache disorders such as migraine
- Identify and educate candidates for current and future preventive migraine treatments
Financial Disclosure
The faculty for this CME activity and CME Institute staff were asked to complete a statement regarding all relevant personal financial relationships between themselves or their spouse/partner and any commercial interest. The CME Institute has resolved any conflicts of interest that were identified. No member of the CME Institute staff reported any relevant personal financial relationships.
Migraine is associated with considerable pain and disability. Accurate diagnosis that differentiates migraine from other primary and secondary headache disorders is needed, and clinicians should assess the patient’s risk of progressing to chronic migraine. Many patients will benefit from preventive treatment. Clinicians must know how to identify these patients and be familiar with the numerous preventive treatments. Finally, clinicians must select the safest, most effective treatment while keeping in mind individual characteristics such as coexisting conditions and patient preferences.
(Prim Care Companion CNS Disord 2018;20[suppl E1]:li17059su1c)
To cite: Lipton RB, Silberstein S. Migraine headache: diagnosis and current and emerging preventive treatments. Prim Care Companion CNS Disord. 2018;20(suppl E1):PCC.li17059su1c.
To share: https://doi.org/10.4088/PCC.li17059su1c
© Copyright 2018 Physicians Postgraduate Press, Inc.
From Montefiore Headache Center and Department of Neurology, Albert Einstein College of Medicine, Bronx, NY (Dr Lipton), and Jefferson Headache Center and Department of Neurology, Thomas Jefferson University, Philadelphia, PA (Dr Silberstein).
This Supplement is derived from the planning teleconference series “Migraine Headache: Diagnosis and Current and Emerging Preventive Treatments,” which was held in May and June 2018, and supported by an educational grant from Lilly.
Dr Lipton has received grant/research support from National Institutes of Health, Migraine Research Foundation, and National Headache Foundation; holds stock options in eNeura and Biohaven; serves as a consultant/advisory board member for or has received honoraria from American Academy of Neurology, Alder, Allergan, American Headache Society, Amgen, Autonomic Technologies, Avanir, Biohaven, Biovision, Boston Scientific, Dr Reddy’s , electroCore, Eli Lilly, eNeura, GlaxoSmithKline, Merck, Pernix, Pfizer, Supernus, Teva, Trigemina, Vector, and Vedanta; and receives royalties from Oxford University Press, Wiley, and Informa. Dr Silberstein is a consultant for Alder, Allergan, Amgen, Avanir, Curelator, Dr Reddy’s , eNeura, Eli Lilly, electroCore, Medscape, Supernus, Teva, Theranica, and Trigemina.
Corresponding authors: Richard B. Lipton, MD ([email protected]), and Stephen Silberstein, MD ([email protected]).
Far more serious than a simple headache, migraine is a neurovascular disorder that is associated with considerable pain and impairment. According to the 2016 Global Burden of Disease Study, migraine was the second greatest cause of disability, affecting more than 45 million people worldwide.1 The burden of migraine can be eased through accurate diagnosis and effective preventive treatment, but many patients who could benefit from preventive treatment do not receive it.2 One study of 775 patients with episodic migraine found that only 39.5% received a diagnosis and only 26.3% received appropriate acute treatment.3 Here, we present information that we hope helps clinicians diagnose and treat patients with migraine.
IMPROVING THE DIAGNOSIS OF MIGRAINE
A key point to remember when assessing any patient with headaches is that a headache is a symptom, not a diagnosis. Just as back pain or abdominal pain can have multiple causes, headaches can stem from numerous etiologies. The current edition of the International Classification of Headache Disorders (ICHD-3)4 documents over 300 types of headaches, which are broadly categorized as primary or secondary headache disorders. Primary headaches are those without an underlying cause, whereas secondary headaches have a distinct etiology (eg, hemorrhage, brain tumor, traumatic brain injury).5 Primary headaches can be placed into 4 broad categories: tension-type headache, migraine, trigeminal autonomic cephalalgias, or other primary headache disorders.4 Although tension-type headaches are the most common, migraine is the most debilitating of the primary headache disorders.5
Characteristics of Migraine
Migraine is a complex neurologic disease with identifiable characteristics. According to the ICHD-3,4 to be diagnosed with migraine, a patient must experience ≥ 5 attacks that last 4 to 72 hours if untreated; are accompanied by nausea and vomiting and/or light or sound sensitivity; and have at least 2 of the following characteristics: unilateral location, pulsating quality, moderate to severe pain intensity, or aggravation by routine physical activity.
Frequency/continuity of attacks. Diseases are typically classified as episodic or chronic. Episodic diseases are monophasic conditions such as a cold or the flu, and chronic diseases occur over an extended period of time and are often progressive, such as Alzheimers, epilepsy, or Parkinson disease. Migraine and most other primary headache disorders are different. They, like epilepsy, are chronic disorders with episodic manifestations, but additional confusion exists. This is because headache disorders occurring less than 15 days are called episodic, whereas those occurring 18 or more days a month are called chronic, even though they are both chronic disorders—they are chronic disorders with episodic attacks. Headache attacks are the most prominent manifestation of the disorder and occur as distinct episodes, but between episodes the person’s predisposition to headache endures, which is a state of neuronal hyperexcitability, similar to that which underlies epilepsy.6
Triggers. Frequently factors can be identified that trigger episodic attacks, and these triggers can serve as therapeutic targets. Common migraine triggers include stress, sleep deprivation, fatigue, and hormonal changes.7
Symptoms. The main symptom of migraine is a moderate to severe headache, which is often unilateral and/or has a pulsating quality.4 Migraine headaches are also commonly accompanied by nausea, vomiting, and sensitivity to light or sound.
Impairments. The impairments associated with migraine are considerable and affect multiple domains.8 During headache attacks, individuals with migraine experience impairments at school, work, home, and in social settings. Many require bedrest to manage their headaches, leading to reduced productivity. Migraines also lead to reduced productivity in the form of presenteeism. Furthermore, the burden of migraine is felt not only during episodic attacks; individuals with migraine often experience anxiety about when their next headache will hit. They may engage in phobic avoidance of certain activities out of fear of triggering an attack.8
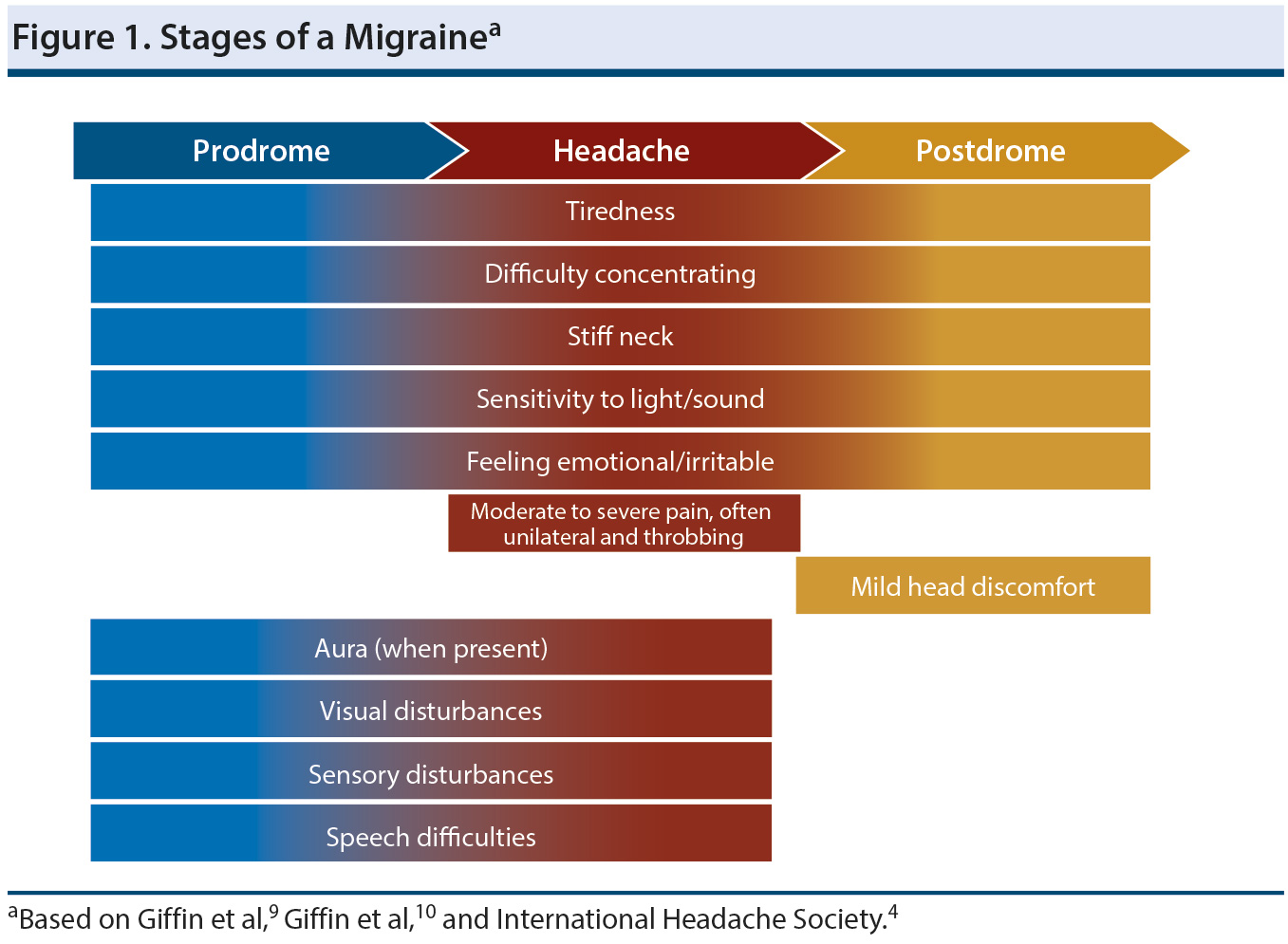
Stages. A migraine progresses through 3 stages (Figure 1),4,9,10 beginning with a premonitory or prodromal phase. During this phase, which precedes the onset of pain, the most commonly reported symptoms include tiredness, difficulty concentrating, stiff neck, and sensitivity to light or noise.9 During the prodromal phase, some patients will experience an aura, which is a complex of neurologic symptoms that may continue into the headache phase.4 Auras will often have both positive and negative features, and visual auras are most common. Thus, a person may experience positive features such as flashing lights or zig-zagging lines, followed by negative features such as greyed-out vision or a blind spot. Auras can also manifest as sensory disturbances such as numbness or a feeling of pins and needles, or as speech disturbance, which is typically aphasia.4
For those who experience an aura, it may continue into the headache stage, or it may not begin until the headache stage.4 Once the active headache phase begins, the pain will be moderate to severe and will often have a pulsing or throbbing quality. In addition, the pain will tend to be unilateral and is often worsened by physical activity, light, sound, and smells. Nausea and vomiting are common symptoms. Once the full headache resolves, mild head discomfort may persist into the postdrome.10 During this phase, a number of non-headache symptoms may continue for as long as 1 to 2 days. The most commonly reported postdromal symptoms include tiredness, difficulty concentrating, changes in mood, stiff neck, sensitivity to light and sound, and thirst.
Migraine is a cyclical disorder, and, therefore, in addition to the time spent in active phases of migraine, individuals with this disorder spend a large amount of time between episodes. Although symptoms may not be present during this state, much of the burden and distress associated with migraine may persist due to anxiety in anticipation of the next episode.8
 PATIENT PERSPECTIVES
PATIENT PERSPECTIVES
“I am a migraineur and have been off of work [for 5 months]. I tried to return twice but ended up home within the week. I miss my friends at work most of whom don’ t know why I’ m out. I follow and touch base with many of them thru Facebook but every time I post about my migraines I end up deleting the post. I am afraid to admit the reason for my absence (& their overtime) because I don’ t think many would understand that it’s not just a headache. It’s excruciating pain for days at a time followed by post-drome. It makes me feel lonely which, on top of the stress being out of work causes, only adds to the problem. According to my doc I may have to go on total disability. My husband is very supportive but even he doesn’ t understand the scope of the problem. The stigma of migraine can be as bad as the migraine itself.”11
Differential Diagnosis of Migraine
Headache is a frequent reason for people to seek medical care. Diagnosis is a 3-step process.
Step 1: Rule out headaches secondary to other causes. The first step is to determine if the patient is experiencing a primary headache disorder.12 All new headache patients should receive an initial assessment for indications that headaches are secondary to another condition. These signs can be remembered with the acronym SNOOP:
Systemic signs/symptoms (fever, weight loss) or disease (malignancy, acquired immune deficiency syndrome)
Neurologic symptoms or signs
Onset is abrupt (thunderclap headache)
Onset is after age 40 years
Pattern change (change in severity, frequency, or type)12
If any of these signs are identified, the clinician should suspect a secondary headache disorder and conduct appropriate evaluations, such as cranial imaging, lumbar puncture, or other diagnostic testing.
Step 2: Use frequency and duration of headaches to narrow possible diagnoses. The next step in diagnosing a primary headache disorder is to differentiate between chronic and episodic headaches based on average monthly frequency of the attacks.13 Individuals who experience 15 or more headache days per month are considered to have chronic headaches, whereas fewer headaches per month are considered episodic. Episodic headaches are further categorized as either short-duration, if the headache lasts for less than 4 hours, or long duration, if it lasts 4 hours or more. The frequency and duration of headaches can help clinicians include or exclude possible diagnoses. For example, per the ICHD-3,4 to receive a diagnosis of migraine, a patient’s headaches must have a minimum duration of 4 hours, so this diagnosis can be ruled out in all patients who experience headache durations under 4 hours.
Step 3: Match patient’s symptoms to the appropriate headache syndrome. The final step in diagnosing a primary headache disorder is to determine which of the 4 primary headache syndromes in the ICHD-3 best matches the patient’s symptoms.13 For patients experiencing headaches with a greater-than-4-hour duration, clinicians will primarily need to distinguish between the 2 most common types of primary headache: tension-type headache and migraine.4,5,13 Although these 2 headache disorders have some similarities, they can be differentiated from each other based on the location, quality, and intensity of pain. Migraine headaches are moderate to severe and tend to have a pulsating quality and unilateral location. In contrast, tension-type headaches are mild to moderate, and the associated pain tends to be bilateral and have a pressing or tightening quality. Furthermore, migraines are often aggravated by physical activity and accompanied by nausea or vomiting, but tension-type headaches do not have these features.
Obtaining an accurate history of a patient’s symptoms can be challenging. Patients’ self-reported symptoms can be inaccurate.3 The best approach is to ask simple, open-ended questions—”Tell me about your headaches and how they led to your coming here”—and allow the patient to talk.14 Patients should be asked about impairment as well as symptoms. Using this open-ended, directed approach actually makes visits shorter, not longer, contrary to the fear of many clinicians.8
Clinicians should ask patients the number of days per month they experienced a headache of any severity, and also the number of days that they were completely free of headaches.15 When these 2 numbers are added, they should roughly equal the total number of days in the month, but if the number is well below 30, the clinician must ask about the unaccounted-for days. Many patients report only severe headaches, and the missing days may be days on which they experienced mild headaches. Mild headache days must also be identified because the total number of headache days is necessary for an accurate migraine diagnosis.4,15,16
 CASE PRACTICE QUESTION
CASE PRACTICE QUESTION
Discussion of best responses can be found at the end of the activity
Case 1.1 Lee is a 25-year-old woman who reports a 4-year history of headaches. She experiences 2 headache attack days per month, and the headaches last 12 hours if untreated. The pain of these headaches is worse on the right side of her head, has a pulsating quality, and is severe. Lee has premonitory irritability but does not experience an aura. Her headaches worsen during her menstrual cycle and are accompanied by nausea and photophobia. She takes ibuprofen to treat these headaches. In addition to the headaches, Lee has mild depression and anxiety and is obese.
What should be the first step in establishing Lee’s headache diagnosis?
- Evaluate Lee for symptoms or signs that her headaches may be due to secondary causes
- Rule out migraine based on the duration of Lee’s headaches
- Determine a specific headache diagnosis based on Lee’s symptoms
Possible Trajectories of a Migraine Diagnosis and Risk Factors for Progression
Migraine is not static.17 Some individuals will cease experiencing symptoms over a prolonged period; their migraine diagnosis should be considered to be in remission. Other individuals will experience symptoms that remain stable over time and show no signs of progression. These individuals are exhibiting persistent migraine. In contrast, many individuals who begin with episodic migraine will experience a progression of symptoms, and their diagnosis will evolve to chronic migraine.17 The ICHD-34 diagnosis of chronic migraine requires that a patient experience headache on 15 or more days per month for 3 or more months, and the headaches on at least 8 of those days must have the features of a migraine. The rate of conversion from episodic to chronic migraine is estimated to be about 3% per year.18,19
Risk factors that contribute to the development of chronic migraine have been identified. Although many of these risk factors are not modifiable, several are remediable and may serve as targets to prevent disease progression (Table 1).17 Gender, race, and age are nonmodifiable risk factors; chronic migraine is more common in women and white people,20 and although the prevalence of migraine is highest in individuals aged 30 to 49 years and declines after 50 years, the proportion of patients with frequent headache days increases with age.21 Lifetime occurrence of a head or neck injury can increase an individual’s risk of developing chronic headaches.22 Low socioeconomic status23 and the experience of major or stressful life events24 are associated with chronicity.
Risk factors that can be targeted to reduce a patient’s likelihood of developing chronic migraine include high caffeine consumption,25 psychiatric disorders such as depression and anxiety,26 and medical conditions such as obesity, arthritis, diabetes, and sleep disorders, particularly snoring.20,27 Clinicians should provide treatment for comorbid conditions and implement behavioral interventions such as weight loss strategies or caffeine reduction as needed to reduce the patient’s risk of progressing to chronic migraine.17
Certain headache and treatment characteristics have also been identified as risk factors for progression. Greater headache frequency and intensity and the experience of allodynia or nausea are all associated with progression to chronic migraine.20,26,28,29 One of the greatest risk factors for migraine progression is excessive use of symptomatic medications.18 The amount of a medication that is considered excessive depends on the agent, but for acetaminophen and nonsteroidal anti-inflammatory drugs, 15 or more days per month is considered overuse.4 For other agents used to treat migraines, such as triptans and opioids, 10 or more days per month is considered overuse. In headache clinics, more than 80% of patients with chronic migraine have been found to overuse symptomatic medication.18 Opiates and barbiturates are the treatments associated with the highest risk of progression to chronic migraine,18 and these agents should, therefore, be used with caution.
 CASE PRACTICE QUESTION
CASE PRACTICE QUESTION
Discussion of best responses can be found at the end of the activity
Case 1.2 Lee is now 38 years old. After her initial visit at age 25 years, she was diagnosed with episodic migraine and prescribed an oral triptan. Initially, this treatment was successful, but she discontinued the triptans after a year because of recurrence. At age 36 years, Lee and her husband entered a stressful divorce, and her headache frequency increased. She began acute treatment with butalbital, aspirin, and caffeine (BAC) combination. By the following year, Lee’s headache frequency had further increased to 10 to 14 days per month, and her depressive features had also worsened. Her headaches continued to worsen, and now Lee is experiencing severe headaches 9 days per month and moderate headaches 15 to 20 days per month. She treats only her severe headaches with BAC combination. Her severe headaches are accompanied by increased sensitivity and pain while brushing her hair, showering, or just resting her head on a pillow. You now diagnose her with chronic migraine.
Which of the following is NOT a risk factor that predisposed Lee to developing chronic migraine?
- Coexisting depression
- Use of butalbital, aspirin, and caffeine
- Experience of a stressful life event
- Presence of allodynia
- Discontinuing triptans
IMPROVING OUTCOMES THROUGH PREVENTIVE TREATMENT
The ultimate goal of headache treatment is to provide relief from the headache. Acute treatment includes not only medication but also strategies such as escaping noisy or smelly environments, resting, and using cold compresses. Acute medications are taken after an attack has begun to relieve pain and disability and stop progression, and preventive treatments are taken to reduce the frequency, severity, and duration of attacks.30 Effective preventive treatment has the additional benefits of improving responsiveness to acute treatments and reducing disability.31 Despite the pain and disability associated with migraine attacks and the benefits of preventive treatment, most patients rely on acute treatments to manage their headaches.2 The American Migraine Prevalence and Prevention study2 found that although 38.8% of patients with migraine are potential candidates for preventive treatment, only 13% are actually receiving it.
When to Consider Preventive Treatment
Not every patient is a candidate for preventive treatment. Guidelines are available to assist clinicians in selecting patients who are likely to benefit from this type of treatment.32 Candidates include those receiving acute treatment and continuing to experience headaches that are frequent or severe enough to interfere with functioning. A patient may also be experiencing side effects from or be in danger of overusing acute treatments, or the acute treatments may be ineffective, contraindicated, or cost-prohibitive. For patients with uncommon migraine conditions including hemiplegic migraine, basilar migraine, migraine with prolonged aura, or migrainous infarction, preventive treatment is necessary to avoid neurologic damage. Finally, some patients may prefer preventive treatment over acute treatment.32
 CASE PRACTICE QUESTION
CASE PRACTICE QUESTION
Discussion of best responses can be found at the end of the activity
Case 2 Jonathan is a 45-year-old bus driver who has 5 migraine attacks per month that interfere with his functioning despite acute treatment with sumatriptan. His headache profile is stable, and his medical and neurologic examinations are normal. What should you do?
- Reevaluate Jonathan for potential secondary headache causes
- Try a different triptan
- Discontinue acute treatment and start preventive medication
- Use combined acute and preventive treatments
Principles of Preventive Pharmacologic Therapy
A variety of medications is used for the preventive treatment of migraine, and consensus-based principles have been established that clinicians should follow regardless of the specific agent being used.32 Medication should be initiated at the lowest possible dose and slowly increased until efficacy is achieved or until side effects become a problem. Other medications that may interfere with the preventive treatment should be avoided, particularly overuse of acute migraine treatments. New treatments must be given an adequate trial, keeping in mind that 2 to 3 months may be needed before benefits become apparent. Patients should be educated about medication use and expectations. To accurately evaluate the effect of treatment, patients should keep a headache diary that includes information on the frequency, severity, duration, disability, and response to treatment of all attacks, as well as adverse effects. After patients have shown sustained improvement for 6 months,33 tapering and discontinuing treatment may be considered.32
Coexisting conditions must also be considered when initiating preventive treatment.32 Conditions known to occur more frequently in patients with migraine include cardiovascular disorders such as stroke, angina, and hypertension; psychiatric disorders such as depression, anxiety, and bipolar disorder; restless leg syndrome; asthma; and irritable bowel syndrome, among others.30,32,34 If a comorbid condition is present, the ideal treatment strategy is to select an agent that effectively treats both conditions, but if this is not possible, clinicians must select a preventive treatment that is not contraindicated for the other condition. Caution must also be taken to ensure that none of a patient’s medications will interact or exacerbate any symptoms. If a female patient is of childbearing age, clinicians must determine the possibility of her becoming pregnant because some preventive treatments have teratogenic effects.32
Selection of Preventive Treatment
The pathophysiology of migraine is complex and not fully understood. The pharmacologic options for the preventive treatment of this disorder, therefore, are numerous. These treatments include α antagonists, anticonvulsants, antidepressants, β blockers, a calcitonin gene-related peptide (CGRP) receptor antagonist, and calcium channel blockers, among others.32,35,36
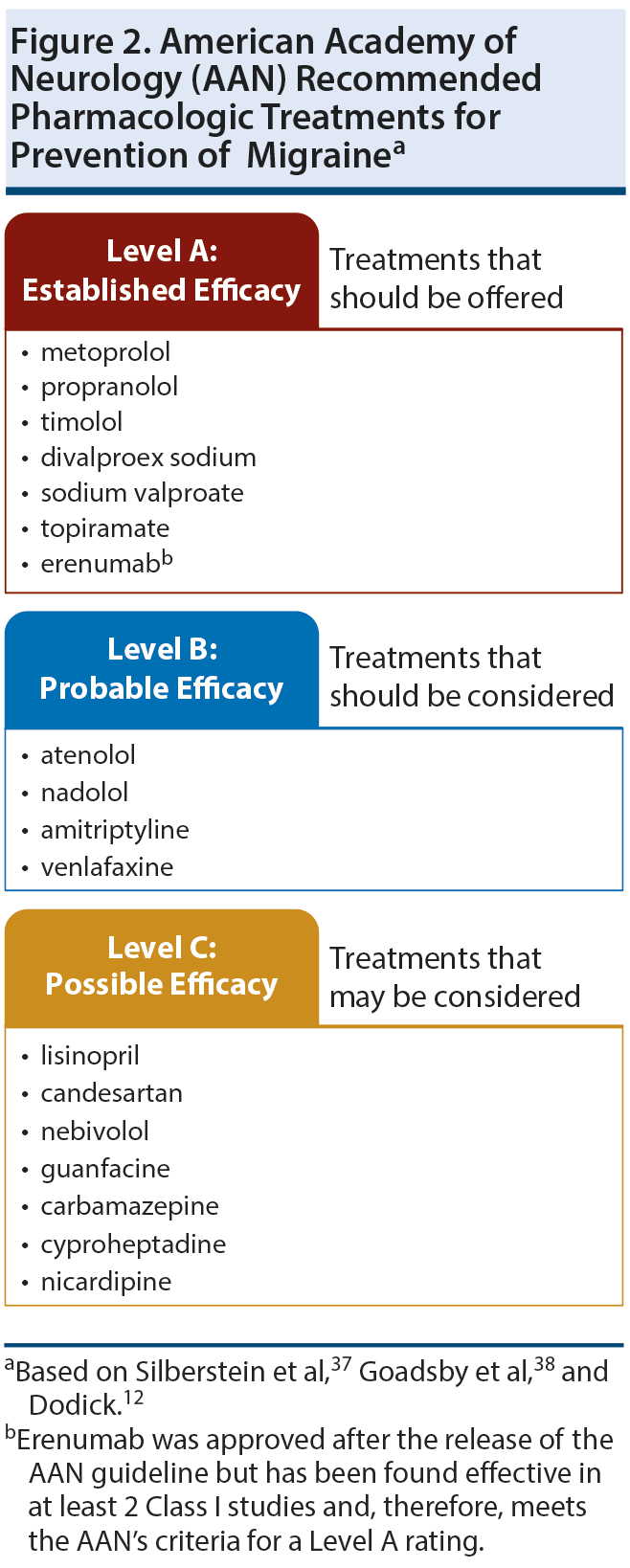
To select the best treatment for an individual patient, clinicians must select the agent that has the greatest evidence of efficacy and safety for the patient’s migraine type, but the treatment must also be agreeable to the patient and must not exacerbate any coexisting conditions.37 The American Academy of Neurology (AAN) and the American Headache Society (AHS) analyzed studies of preventive migraine treatments available in the United States that were published from June 1999 to May 2009 and developed guidelines that classified the treatments into different levels of efficacy depending on the strength of the evidence (Figure 2).12,37,38 Since that time, additional evidence and new treatments have become available.30,35,36
Treatments with established efficacy. The β-blockers metoprolol, propranolol, and timolol and the antiepileptic drugs divalproex sodium, valproate, and topiramate are considered by the AAN to be the treatments with the best evidence of efficacy and therefore should be considered first-line treatments for migraine prevention. Recently, a treatment with a novel mechanism was approved for the prevention of migraine.36 Erenumab is the only approved agent that was designed specifically for the treatment of migraine. This agent is a human monoclonal antibody (mAb) that acts as an antagonist of the CGRP receptor.39 The AAN recommends trying erenumab for patients who have not had success with other preventive therapies.40
β-blockers are a widely used treatment for migraine prophylaxis.30 An early hypothesis of migraine pathology held that the pain of migraines was caused by dilation of sensitive cranial blood vessels. Although this theory has been abandoned as too simplistic, vascular mechanisms are known to be involved in migraine pathology.41 The mechanisms by which β-blockers are able to reduce migraine frequency are not fully understood,42 but they may disrupt descending nociceptive signaling through aminergic-mediated modulation.43,44 Despite the efficacy and relative safety of these agents, they should not be used to treat migraine in patients with coexisting asthma, bradycardia, depression, or hypotension, although β-blockers may be beneficial in patients with coexisting hypertension.37
The anticonvulsants that have been found to be effective for migraine are thought to derive their therapeutic effect through different mechanisms.45 Valproate and divalproex sodium are thought to inhibit trigeminovascular and trigeminothalamic nociception and cortical hyperexcitability through modulation of GABA receptors. Topiramate is thought to inhibit trigeminovascular pain responses through potentiation of GABA inhibition, non-NMDA glutamate receptor inhibition, and modulation of voltage-gated sodium and calcium ion channels.43,45 Anticonvulsant agents should be the treatment of choice in patients with migraine and coexisting epilepsy. Valproate/divalproex sodium is associated with weight gain and therefore should be avoided in patients with obesity, but this side effect may make this treatment beneficial for patients with coexisting anorexia. Topiramate, however, should be avoided in patients with anorexia as well as those with cognitive complaints.37 Although topiramate has been suspected of increasing a patient’s risk of urolithiasis, evidence suggests that the risk is increased not by topiramate46 but rather by the experience of having migraines.47
Treatments with probable efficacy. The β-blockers atenolol and nadolol and the antidepressants amitriptyline and venlafaxine are treatments with probable efficacy that clinicians should consider for migraine prevention.37 Atenolol and nadolol lack as strong an evidence base as other agents in this class and therefore have been designated as probably effective.48 Amitriptyline is a tricyclic antidepressant that inhibits the reuptake of both norepinephrine and serotonin and has antagonistic effects at some 5HT2-receptors; venlafaxine is a serotonin-norepinephrine reuptake inhibitor.49 Abnormal serotonergic neurotransmission has been implicated in migraine,50 and the ability of these and other antidepressants to block serotonin receptors may disrupt pain signaling.43,44 Antidepressants are the ideal treatment choice in patients with coexisting depression.37
Treatments with possible efficacy. Agents with limited evidence of efficacy may be considered for migraine prevention.37 These include the angiotensin-converting-enzyme (ACE) inhibitor lisinopril, the angiotensin receptor blocker candesartan, the β-blocker nebivolol, the α-agonist guanfacine, the anticonvulsant carbamazepine, the antihistamine cyproheptadine, and the calcium channel blocker nicardipine.
Treatments with preliminary evidence of efficacy. OnabotulinumtoxinA has been shown to reduce chronic migraine by about 2 days per month, but the evidence for prevention of episodic migraine is limited.51 Additionally, acupuncture has evidence suggesting that it reduces migraine frequency when used to augment symptomatic treatment.52 A number of monoclonal antibodies have been developed in recent years to treat a variety of conditions ranging from asthma to multiple sclerosis.53 Natural antibodies are glycoproteins that target and destroy antigens.54 They do this by binding to specific sites called epitopes on the surface of antigens. They have a y-shaped structure comprising a heavy chain and a light chain, and within each chain are variable and constant regions. The variable regions are different in every antibody, and this variability allows the antibody to bind to specific epitopes. The constant region is the same for an entire family of antibodies, or isotype, and 5 different isotypes exist in humans. The predominant isotype is Ig γ (IgG) and accounts for about 85% of all natural antibodies.54 Furthermore, all therapeutic mAbs are IgG isotypes.
The earliest attempts to use therapeutic antibodies involved using a variety of antibodies from immunized animals in an attempt to transfer the immunity to humans, but the antibodies used had unknown specificities and were not well tolerated by humans.54 As this type of treatment has evolved, the antibodies used have come increasingly from human sources, and now are usually either > 90% human and referred to as humanized or 100% human and referred to as fully human. Another important development was to produce antibody clones from a hybrid cell called a hybridoma. The antibody clones produced from this process are called monoclonal because they are identical to the parent cell, and because they are identical, their specificities are more predictable than those of polyclonal antibodies. Furthermore, whereas natural antibodies can only target and destroy foreign pathogens, therapeutic mAbs can be created to bind to a target receptor or its associated ligand, and then either destroy the cell or alter the function of the target cell without destroying it.54
Because mAbs are proteins, they must be administered through injection, which may be a drawback for some patients. However, their large size confers some advantages. They cannot be excreted by the kidneys and are therefore broken down by the body into peptides and amino acids, thus avoiding the creation of toxic metabolites.54 Therapeutic mAbs also have a much longer half-life than small-molecule oral medications, which makes them ideal for preventive treatment. The average half-life of serum IgG antibodies is about 3 weeks. Additional advantages of mAbs over other types of pharmacotherapy include a greatly reduced risk of drug interactions and, because these agents are highly selective for their targets, off-target toxicity is unlikely.54
CGRP is known to be involved in the pathophysiology of migraine and, therefore, is an attractive target for mAb treatment.55-57 CGRP is abundant in the trigeminovascular system, with levels increasing during migraine, and is involved in vasodilation and nociception.56 Erenumab is thought to exert its therapeutic effect by blocking the CGRP signaling pathway by potently and selectively binding to CGRP receptors,58 and in randomized controlled trials, this agent was associated with a significantly greater reduction in migraine frequency compared with placebo (P < .001). Additional CGRP-related mAbs for the preventive treatment of migraine include eptinezumab, fremanezumab, and galcanezumab.59 Galcanezumab, for example, has shown prophylactic efficacy versus placebo among patients with episodic migraine who have not experienced treatment success with other preventive therapies.60 Clinical guidelines will hopefully be updated as more new therapies become approved and evidence becomes stronger, especially for long-term use.
 PATIENT PERSPECTIVES
PATIENT PERSPECTIVES
“I’ m a 51 year old woman and have suffered from migraines since I was a kid. As did my mother before me (and two of my 3 brothers). Between my siblings an[d] myself, we’ ve tried just about every migraine treatment there is out there. It’s only been the last few years that, with the help of a new neurologist, I have actually had any success in reducing the number of headaches I suffer, and getting through the ones I do suffer more quickly. . . . My new neurologist and I took a 3-pronged approach that has really made a difference in my quality of life. First, I got serious about eliminating food triggers from my diet. . . . Next I changed up the prescription medication I take if I do get a headache. . . . The third prong in our attack has been trying to prevent the headaches in the first place.”11
 DISCUSSION OF CASE PRACTICE QUESTIONS
DISCUSSION OF CASE PRACTICE QUESTIONS
Case 1.1: Preferred response is a. Evaluate Lee for symptoms or signs that her headaches are due to secondary causes.
Secondary headache causes must always be excluded first because they may be an indication of a more serious condition that requires further evaluation.
Case 1.2: Preferred response is b. Use of butalbital, aspirin, and caffeine.
Because Lee uses this treatment only on the days she experiences severe headaches, which is approximately 9 days per month, this would not constitute overuse. Furthermore, BAC combination is not one of the treatments associated with an increased risk of progression to chronic migraine.
Case 2: Preferred response is d. Use combined acute and preventive treatments.
Jonathan is an appropriate candidate for the addition of preventive treatment because he is continuing to experience numerous headaches and associated impairment despite acute treatment.
Clinical Points
- Assess whether patients’ headaches are secondary to another condition prior to diagnosing a primary headache disorder.
- Use attack frequency, duration, and symptoms to diagnose primary headache disorders.
- Be alert for risk factors for or signs of progression from episodic to chronic migraine.
- Consider preventive treatment for patients whose migraines remain frequent and severe despite acute treatment.
- Select the safest, most effective preventive treatment that will not exacerbate coexisting conditions.
CONCLUSION
Many individuals with migraine experience ongoing attacks and substantial impairment. Clinicians can ease this burden beginning with an accurate diagnosis and evaluation of risk factors for progression from episodic to chronic status. Some risk factors can be moderated through simple strategies, such as avoiding triggers and limiting use of acute medications. Clinicians must also recognize patients who would benefit from preventive therapy, keeping in mind that this type of treatment reduces not only the frequency but also the severity of attacks and can make acute medications more effective. Once candidates for preventive therapy are identified, clinicians need to educate patients about available treatments, the efficacy and safety profiles of each, and ways in which any selected treatment could potentially affect a patient’s coexisting conditions or other medications.
Disclosure of off-label usage: The authors have determined that butalbital/aspirin/caffeine combination is not approved by the US Food and Drug Administration for the treatment of migraine. Dr Lipton emphasized that although off-label usage was discussed, the faculty do not recommend this agent for the treatment of migraine.
Find more articles on this and other psychiatry and CNS topics:
The Journal of Clinical Psychiatry
The Primary Care Companion for CNS Disorders
REFERENCES
1. Vos T, Abajobir AA, Abate KH, et al; GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1211-1259. PubMed CrossRef
2. Lipton RB, Bigal ME, Diamond M, et al; AMPP Advisory Group. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68(5):343-349. PubMed CrossRef
3. Lipton RB, Serrano D, Holland S, et al. Barriers to the diagnosis and treatment of migraine: effects of sex, income, and headache features. Headache. 2013;53(1):81-92. PubMed CrossRef
4. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1-211. PubMed CrossRef
5. Robbins MS, Lipton RB. The epidemiology of primary headache disorders. Semin Neurol. 2010;30(2):107-119. PubMed CrossRef
6. Haut SR, Bigal ME, Lipton RB. Chronic disorders with episodic manifestations: focus on epilepsy and migraine. Lancet Neurol. 2006;5(2):148-157. PubMed CrossRef
7. Park J-W, Chu MK, Kim J-M, et al. Analysis of trigger factors in episodic migraineurs using a smartphone headache diary applications. PLoS One. 2016;11(2):e0149577. PubMed CrossRef
8. Buse DC, Rupnow MFT, Lipton RB. Assessing and managing all aspects of migraine: migraine attacks, migraine-related functional impairment, common comorbidities, and quality of life. Mayo Clin Proc. 2009;84(5):422-435. PubMed CrossRef
9. Giffin NJ, Ruggiero L, Lipton RB, et al. Premonitory symptoms in migraine: an electronic diary study. Neurology. 2003;60(6):935-940. PubMed
10. Giffin NJ, Lipton RB, Silberstein SD, et al. The migraine postdrome: an electronic diary study. Neurology. 2016;87(3):309-313. PubMed CrossRef
11. Migraine community. www.Migraine.com. July 2018.
12. Dodick DW. Clinical clues and clinical rules: primary vs secondary headache. Adv Stud Med. 2003;3(6C):S550-S555.
13. Bigal ME, Lipton RB. The differential diagnosis of chronic daily headaches: an algorithm-based approach. J Headache Pain. 2007;8(5):263-272. PubMed CrossRef
14. Lipton RB, Hahn SR, Cady RK, et al. In-office discussions of migraine: results from the American Migraine Communication Study. J Gen Intern Med. 2008;23(8):1145-1151. PubMed CrossRef
15. Starling AJ, Dodick DW. Best practices for patients with chronic migraine: burden, diagnosis, and management in primary care. Mayo Clin Proc. 2015;90(3):408-414. PubMed CrossRef
16. Lipton RB. Chronic migraine, classification, differential diagnosis, and epidemiology. Headache. 2011;51(suppl 2):77-83. PubMed
17. Lipton RB. Tracing transformation: chronic migraine classification, progression, and epidemiology. Neurology. 2009;72(suppl):S3-S7. PubMed CrossRef
18. Bigal ME, Serrano D, Buse D, et al. Acute migraine medications and evolution from episodic to chronic migraine: a longitudinal population-based study. Headache. 2008;48(8):1157-1168. PubMed CrossRef
19. Lipton RB, Fanning KM, Serrano D, et al. Ineffective acute treatment of episodic migraine is associated with new-onset chronic migraine. Neurology. 2015;84(7):688-695. PubMed CrossRef
20. Scher AI, Stewart WF, Ricci JA, et al. Factors associated with the onset and remission of chronic daily headache in a population-based study. Pain. 2003;106(1-2):81-89. PubMed CrossRef
21. Bigal ME, Liberman JN, Lipton RB. Age-dependent prevalence and clinical features of migraine. Neurology. 2006;67(2):246-251. PubMed CrossRef
22. Couch JR, Lipton RB, Stewart WF, et al. Head or neck injury increases the risk of chronic daily headache: a population-based study. Neurology. 2007;69(11):1169-1177. PubMed CrossRef
23. Buse DC, Manack A, Serrano D, et al. Sociodemographic and comorbidity profiles of chronic migraine and episodic migraine sufferers. J Neurol Neurosurg Psychiatry. 2010;81(4):428-432. PubMed CrossRef
24. Scher AI, Stewart WF, Buse D, et al. Major life changes before and after the onset of chronic daily headache: a population-based study. Cephalalgia. 2008;28(8):868-876. PubMed CrossRef
25. Scher AI, Stewart WF, Lipton RB. Caffeine as a risk factor for chronic daily headache: a population-based study. Neurology. 2004;63(11):2022-2027. PubMed
26. Ashina S, Serrano D, Lipton RB, et al. Depression and risk of transformation of episodic to chronic migraine. J Headache Pain. 2012;13(8):615-624. PubMed CrossRef
27. Scher AI, Lipton RB, Stewart WF. Habitual snoring as a risk factor for chronic daily headache. Neurology. 2003;60(8):1366-1368. PubMed
28. Lipton RB, Bigal ME, Ashina S, et al; American Migraine Prevalence Prevention Advisory Group. Cutaneous allodynia in the migraine population. Ann Neurol. 2008;63(2):148-158. PubMed CrossRef
29. Reed ML, Fanning KM, Serrano D, et al. Persistent frequent nausea is associated with progression to chronic migraine: AMPP study results. Headache. 2015;55(1):76-87. PubMed CrossRef
30. Silberstein SD. Preventive migraine treatment. Continuum (Minneap Minn). 2015;21(4 Headache):973-989. PubMed CrossRef
31. Silberstein SD. Practice parameter: evidence-based guidelines for migraine headache (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55(6):754-762. PubMed
32. Morey SS. Guidelines on migraine: part 4. General principles of preventive therapy. Am Fam Physician. 2000;62(10):2359-2360, 2363. PubMed
33. Silberstein SD. Preventive migraine treatment. Continuum (Minneap Minn). 2015;21(4 Headache):973-989. PubMed CrossRef
34. Wang S-J, Chen P-K, Fuh J-L. Comorbidities of migraine. Front Neurol. 2010;1:16. PubMed
35. Jackson JL, Cogbill E, Santana-Davila R, et al. A comparative effectiveness meta-analysis of drugs for the prophylaxis of migraine headache. PLoS One. 2015;10(7):e0130733. PubMed CrossRef
36. Reinke T. Aimovig for migraine prevention: the new kid may have trouble fitting in. Manag Care. 2018;27(7):10-11. PubMed
37. Silberstein SD, Holland S, Freitag F, et al; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1337-1345. PubMed CrossRef
38. Goadsby PJ, Reuter U, Hallström Y, et al. A controlled trial of erenumab for episodic migraine. N Engl J Med. 2017;377(22):2123-2132. PubMed CrossRef
39. Aimovig [package insert]. Thousand Oaks, CA: Amgen Inc, 2018.
40. When others fail, new migraine treatment may work [press release]. American Academy of Neurology. https://www.aan.com/PressRoom/Home/PressRelease/1641. Published April 17, 2018. Accessed August 17, 2018.
41. Brennan KC, Charles A. An update on the blood vessel in migraine. Curr Opin Neurol. 2010;23(3):266-274. PubMed CrossRef
42. Wideroe TE, Vigander T. Propranolol in the treatment of migraine. BMJ. 1974;2(5921):699-701. PubMed
43. Welch KMA. Contemporary concepts of migraine pathogenesis. Neurology. 2003;61(suppl 4):S2-S8. PubMed
44. Ramadan NM. Prophylactic migraine therapy: mechanisms and evidence. Curr Pain Headache Rep. 2004;8(2):91-95. PubMed
45. Hoffmann J, Akerman S, Goadsby PJ. Efficacy and mechanism of anticonvulsant drugs in migraine. Expert Rev Clin Pharmacol. 2014;7(2):191-201. PubMed CrossRef
46. Shen A-L, Lin H-L, Tseng Y-F, et al. Topiramate may not increase risk of urolithiasis: a nationwide population-based cohort study. Seizure. 2015;29:86-89. PubMed CrossRef
47. Tsai M-J, Chen Y-T, Ou S-M, et al. Increased risk of urinary calculi in patients with migraine: a nationwide cohort study. Cephalalgia. 2015;35(8):652-661. PubMed CrossRef
48. Morey SS. Guidelines on migraine, part 5: recommendations for specific prophylactic drugs. Am Fam Physician. 2000;62(11):2535-2539. PubMed
49. Koch HJ, J×¼rgens TP. Antidepressants in long-term migraine prevention. Drugs. 2009;69(1):1-19. PubMed CrossRef
50. Panconesi A. Serotonin and migraine: a reconsideration of the central theory. J Headache Pain. 2008;9(5):267-276. PubMed CrossRef
51. Herd CP, Tomlinson CL, Rick C, et al. Botulinum toxins for the prevention of migraine in adults. Cochrane Database Syst Rev. 2018;6:CD011616. PubMed CrossRef
52. Linde K, Allais G, Brinkhaus B, et al. Acupuncture for the prevention of episodic migraine. Cochrane Database Syst Rev. 2016;(6):CD001218. PubMed CrossRef
53. McAllister P. Monoclonal antibodies: what the neurologist needs to know. Pract Neurol. 2017;(May):18-22.
54. Silberstein S, Lenz R, Xu C. Therapeutic monoclonal antibodies: what headache specialists need to know. Headache. 2015;55(8):1171-1182. PubMed CrossRef
55. Shi L, Lehto SG, Zhu DXD, et al. Pharmacologic characterization of AMG 334, a potent and selective human monoclonal antibody against the calcitonin gene-related peptide receptor. J Pharmacol Exp Ther. 2016;356(1):223-231. PubMed CrossRef
56. Silberstein SD. Emerging target-based paradigms to prevent and treat migraine. Clin Pharmacol Ther. 2013;93(1):78-85. PubMed CrossRef
57. Dodick DW, Ashina M, Brandes JL, et al. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026-1037. PubMed CrossRef
58. Tepper S, Ashina M, Reuter U, et al. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol. 2017;16(6):425-434. PubMed CrossRef
59. Edvinsson L, Haanes KA, Warfvinge K, et al. CGRP as the target of new migraine therapies – successful translation from bench to clinic. Nat Rev Neurol. 2018;14(6):338-350. PubMed CrossRef
60. Zhang Q, Ruff DD, Pearlman EM, et al. Efficacy of galcanezumab in patients who failed to respond to preventives previously: results from EVOLVE-1, EVOLVE-2 and REGAIN studies (S20.004). Neurology. 2018;90(suppl).
This PDF is free for all visitors!
Save
Cite